Abstract
SETTING:
North Carolina, United States.
OBJECTIVE:
To investigate the demographic and behavioral risk factors associated with death among tuberculosis (TB) patients in North Carolina.
DESIGN:
Retrospective cohort of all TB patients reported in North Carolina, 1993–2003 (inclusive). A surveillance dataset based upon Report of Verified Case of Tuberculosis (RVCT) records was cross-linked with the National Death Index (NDI) to confirm date of death and capture additional deaths.
RESULTS:
Among 5311 TB patients, 181 died before initiation of TB treatment, and 540 died before completion of TB treatment. Increasing age, miliary/meningeal disease, and human immunodeficiency virus (HIV) infection were associated with increased risk of death before treatment, during early treatment (initial 8 weeks) and later in TB treatment. In addition to these factors, excess alcohol use (HR 1.62, 95%CI 1.13–2.32) and residence in a nursing home (HR 1.65, 95%CI 1.20– 2.29) were associated with a significantly increased risk of death during the first 8 weeks of treatment.
CONCLUSION:
Many of the deaths in TB patients occurred in the most vulnerable populations, such as the elderly or those with HIV infection, and may be attributable to delayed diagnosis and poor functional status.
Keywords: tuberculosis, mortality, risk factors
Despite effective therapy, tuberculosis (TB) associated mortality remains relatively high, with 8.3 million new TB cases and 1.8 million TB deaths worldwide in 2000.1 Many of those patients died prior to TB diagnosis or during treatment.2 Frequently reported predictors of mortality during TB treatment include TB-HIV (human immunodeficiency virus) co-infection,3-8 previous TB treatment,9-11 low weight,12,13 older age8,9,11,14-17 and alcoholism.8,10,11,15 More recently, diabetes14,18 and tobacco smoking19 have been associated with TB mortality. Causes of TB-related mortality vary widely, with deaths reported to be from malignancy,8 pneumonia20,21 and respiratory failure,21 in addition to death attributed directly to TB itself.14,16,20
The state of North Carolina is in the southeastern United States, an area with the highest percentage of households with incomes below the federal poverty level of any region in the country.22 It differs from urban populations usually studied in the United States, as it comprises a predominantly rural population whose source of health care is geographically scattered. We set out to determine the demographic and behavioral risk factors associated with death prior to or during TB treatment among TB patients in North Carolina during 1993–2003.
STUDY POPULATION AND METHODS
We performed a retrospective cohort study associating patient characteristics with risk of death in TB patients reported to the North Carolina State TB Control Program between 1 January 1993 and 31 December 2003.
The project received approval from the Institutional Review Boards of Duke University Health System, the University of North Carolina–Chapel Hill’s School of Public Health, and the North Carolina Department of Health and Human Services’ Division of Public Health, and approval to query the US National Death Index (NDI) was granted by the National Center for Health Statistics.
The study includes data routinely collected on all patients diagnosed with active TB via the Centers of Disease Control and Prevention (CDC) data collection instrument, the Report of Verified Case of TB (RVCT). This instrument captures all TB cases within the State of North Carolina, including cases diagnosed after death via autopsy or culture specimens that grow Mycobacterium tuberculosis after the patient has died, and cases that are based on a clinical diagnosis. The North Carolina TB Control Program dataset was used in conjunction with information obtained from the NDI, a national mortality database that contains identifying data from state-mandated death certificates from all 50 states, New York City, the District of Colombia, Puerto Rico and the Virgin Islands.
Subjects who died prior to, during or after anti-tuberculosis therapy were initially identified in the RVCT records. The NDI was used to confirm date of death for these subjects, as well as to capture deaths that were not identified by RVCT records. The RVCT and NDI data were linked using subjects’ Social Security number, full name, date of birth, race, sex and last known state of residence. Any discrepancies in dates or status of patients between the databases were resolved in favor of the North Carolina TB Control Program’s database.
Patients who were alive at treatment initiation but who had no documented treatment start date were excluded from the study. All remaining patients reported to the North Carolina TB Control Program were included in the analyses. At the time of this study, death records up to 31 December 2004 were available from the NDI for all patients, so all deaths occurring up to this date were included.
We analyzed three separate outcomes based on time of death. The endpoint ‘death prior to initiation of treatment’ comprises all reported TB patients who were diagnosed with TB but died before any treatment was started; this subgroup includes patients who were diagnosed at autopsy. The endpoint ‘death early in TB treatment’ comprises all TB patients who began anti-tuberculosis therapy but died within the initial 8 weeks of treatment. The endpoint ‘death later in TB treatment’ comprises all TB patients who initiated anti-tuberculosis therapy, survived past 8 weeks but died prior to completion of treatment.
The elements reported in the RVCT were used to examine which patient characteristics predicted death in the different subgroups. The demographic, clinical and social history characteristics were based on either documented patient medical history or by patient self-report, as recorded by public health nurses via the RVCT form. HIV status was collapsed into two groups: ‘positive’ and ‘negative/unknown.’
Statistical analysis
Bivariate analyses were used to examine the relationship between patient characteristics and the time of death relative to treatment, as well as the relationship between patient characteristics. Because the number of HIV-infected persons in some race/ethnicity groups was very small, race/ethnicity and HIV status were analyzed using all combinations of race/ethnicity and HIV status available in the dataset. There was only one HIV-infected Asian person in the dataset, and this individual was deleted for purposes of this analysis. Differences in proportions were assessed using the χ2 test or Fisher’s exact test, as appropriate. Differences in survival among groups with different demographic and risk profiles were examined using the Kaplan-Meier method with survival functions compared with the log-rank test.
Unconditional logistic regression was used to evaluate factors potentially associated with death prior to initiation of TB treatment as well as to calculate adjusted odds ratios (aORs) for risk factors of allcause mortality. Cox proportional hazards (PH) modeling was used to examine survival after initiation of TB treatment. Extended Cox PH modeling, incorporating time-dependent interaction terms, revealed significant interaction between time period and the hazards ratios (HRs) associated with several potential predictors. Piecewise Cox PH modeling was thus performed: one model examined mortality during the first 8 weeks of TB treatment, while the second model examined mortality during the period beyond the first 8 weeks of TB treatment but prior to TB treatment completion. The PH assumption was examined using the graphical method. Age was treated as a continuous variable in the multivariate analyses.
For all statistical tests, P < 0.05 (two-tailed) was considered significant. Statistical analyses were performed using Stata 9.2 (Stata Corporation, College Station, TX, USA) and SAS, version 9.1 (SAS Institute Inc, Cary, NC, USA) software packages.
RESULTS
A total of 5311 patients were reported to the North Carolina TB Control Program between 1993 and 2003; their demographic and social characteristics are shown in Table 1. Mean age was 51.5 years (standard deviation = 21.9 years). Over one third of the cohort was female and 18.1% (n = 1834) of patients were born outside the United States. Little drug resistance was seen in the study population: 1.7% (n = 90) of subjects’ isolates that underwent drug susceptibility testing showed monoresistance to isoniazid (INH), 1.4% (n = 76) showed resistance to both INH and at least one other drug, excluding rifampin (RMP), and 0.4% (n = 21) had both INH and RMP resistance. Drug resistance in our cohort was not significantly associated with mortality (data not shown). Three quarters (n = 3982) of the cohort had pulmonary TB (PTB), and an additional 4.6% (n = 243) had both PTB and extra-pulmonary disease. The use of directly observed therapy (DOT) increased markedly during the study period; 26.6% of TB cases reported in 1993 vs. 73.2% of those reported in 2003 received DOT during their entire treatment course (P < 0.0001). Cases were also less likely to be managed solely by private providers (as opposed to the county health department) over time; 12.9% of cases in 1993 were managed by private providers vs. 9.5% in 2003 (P < 0.0001).
Table 1.
Characteristics of TB patients reported in North Carolina, 1993–2003 (N = 5311)
| Characteristic | n | % | Characteristic | n | % |
|---|---|---|---|---|---|
| Age, years | Resident of long-term care facility at TB diagnosis | ||||
| 0–4 | 111 | 2.1 | Yes | 250 | 4.7 |
| 5–14 | 121 | 2.3 | No | 5061 | 95.3 |
| 15–24 | 319 | 6.0 | Homeless within past year | ||
| 25–44 | 1614 | 30.4 | Yes | 377 | 7.1 |
| 45–64 | 1522 | 28.7 | No | 4934 | 92.9 |
| ≥65 | 1624 | 30.6 | |||
| Injection drug use within past year | |||||
| Sex | Yes | 96 | 1.8 | ||
| Male | 3477 | 65.5 | No | 4420 | 83.2 |
| Female | 1834 | 34.5 | Unknown | 795 | 15.0 |
| Race | Non-injection drug use within past year | ||||
| Non-Hispanic White | 1357 | 25.6 | Yes | 483 | 9.1 |
| Non-Hispanic Black | 3043 | 57.3 | No | 4047 | 76.2 |
| Asian/Pacific Islander | 305 | 5.7 | Unknown | 781 | 14.7 |
| American Indian/Alaskan Native | 44 | 0.8 | |||
| Hispanic | 559 | 10.5 | Excess alcohol use within past year | ||
| Unknown | 3 | 0.1 | Yes | 1147 | 21.6 |
| NO | 3411 | 64.2 | |||
| Country of origin | Unknown | 753 | 14.2 | ||
| US-born | 4348 | 81.9 | |||
| Foreign-born | 963 | 18.1 | Initial organism drug susceptibility | ||
| Resistant to INH alone | 90 | 1.7 | |||
| Previous diagnosis of TB | Resistant to INH and to ≥ 1 drug other than RMP |
||||
| Yes | 296 | 5.6 | 76 | 1.4 | |
| No | 5015 | 94.4 | Resistant to RMP and INH | 21 | 0.4 |
| Site of current TB | Resistant to RMP alone | 10 | 0.2 | ||
| Pulmonary | 3982 | 75.0 | No resistance to INH or RMP documented | 5114 | 96.3 |
| Pulmonary and extra-pulmonary | 243 | 4.6 | Manner in which TB treatment was provided | ||
| Extra-pulmonary alone | All DOT | 2939 | 55.3 | ||
| Lymphatic | 278 | 5.2 | Combination of DOT and SAT | 1162 | 21.9 |
| Miliary | 119 | 2.2 | ALL SAT | 1019 | 19.2 |
| Pleural | 286 | 5.4 | Unknown | 191 | 3.6 |
| Bone/joint | 126 | 2.4 | |||
| Other | 277 | 5.2 | TB treatment provider | ||
| Health department only | 1856 | 35.0 | |||
| HIV status | Health department and private/other provider | 2660 | 50.1 | ||
| Positive | 549 | 10.3 | Private/other provider only | 690 | 13.0 |
| Negative | 2650 | 49.9 | Unknown | 105 | 2.0 |
| Unknown | 2109 | 39.7 | |||
| Resident of correctional facility at TB diagno | |||||
| Yes | 106 | 2.0 | |||
| No | 5205 | 98.0 |
TB = tuberculosis; HIV = human immunodeficiency virus; INH = isoniazid; RMP = rifampin; DOT = directly observed therapy; SAT = self-administered therapy.
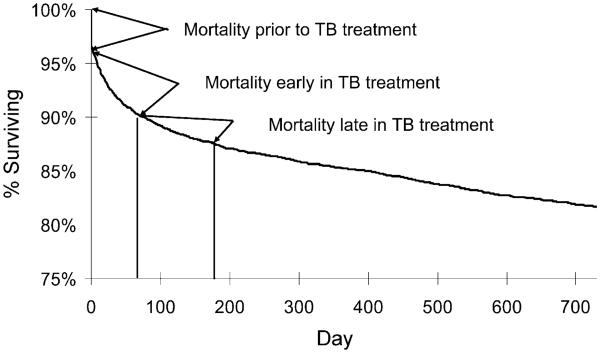
Of the total study cohort, 3.4% (n = 181) died before initiation of TB treatment (Figure). The remaining patients were started on anti-tuberculosis therapy, but 540 patients died before completion of therapy. Of these, 305 (5.7%) died within the first 8 weeks of treatment and 235 (4.4%) survived the initial 8 weeks of TB treatment but died prior to treatment completion. The overall mortality rate during treatment was 0.23 per person-year (py; 0.39/py in the first 8 weeks, 0.15/py during the rest of treatment). There were no secular trends in mortality rate in this cohort.
Mortality prior to initiation of treatment
Multivariate analyses showed that increasing age, TB-HIV co-infection, miliary/meningeal disease, and Native American race were significant predictors of death in the period prior to initiation of anti-tuberculosis therapy (Table 2). Patients with TB-HIV co-infection had a higher risk of mortality compared to TB patients whose HIV status was negative or unknown; this finding only reached statistical significance in White and Black patients due to the small patient numbers in other ethnic groups. Among patients with pulmonary TB (adjusting for all other factors in Table 2), those with cavitary disease were less likely to die prior to TB treatment than those with non-cavitary findings on chest radiograph (odds ratio 0.62, 95% confidence interval [CI] 0.40–0.98).
Table 2.
Risk factors for death prior to and during TB treatment among TB patients in North Carolina
| Characteristic | Death prior to TB treatment OR (95%CI) |
Death early (first 8 weeks) in TB treatment HR (95%CI) |
Death late (8–26 weeks) in TB treatment HR (95%CI) |
|---|---|---|---|
| Age | |||
| Per year increase | 1.04 (1.03–1.05)* | 1.06 (1.05–1.07)* | 1.05 (1.04–1.07)* |
| Per decade increase | 1.48 (1.34–1.63)* | 1.79 (1.63–1.97)* | 1.63 (1.48–1.88)* |
| Race and HIV status | |||
| Non-Hispanic White, HIV− | Referent | Referent | Referent |
| Non-Hispanic White, HIV+ | 4.11 (1.04–16.34)* | 2.77 (0.85–9.05) | 2.93 (0.47–18.50) |
| Non-Hispanic Black, HIV− | 1.16 (0.81–1.65) | 1.38 (1.06–1.78)* | 1.17 (0.82–1.67) |
| Non-Hispanic Black, HIV+ | 3.00 (1.56–5.77)* | 2.08 (1.17–3.71)* | 5.79 (3.20–10.48)* |
| Hispanic, HIV− | 1.33 (0.56–3.16) | 1.30 (0.58–2.91) | 0.56 (0.13–2.39) |
| Hispanic, HIV+ | 1.80 (0.35–9.21) | Undefined | 1.71 (0.19–15.32) |
| Asian, HIV− | 0.53 (0.16–1.73) | 0.75 (0.30–1.85) | 0.42 (0.10–1.72) |
| Native American, HIV− | 3.35 (1.12–10.03)* | 1.92 (0.70–5.27) | 0.97 (0.13–7.05) |
| Sex | |||
| Male | Referent | Referent | Referent |
| Female | 1.03 (0.75–1.43) | 0.86 (0.67–1.10) | 0.92 (0.66–1.28) |
| Excess alcohol use | 0.90 (0.54–1.50) | 1.62 (1.13–2.32)* | 0.77 (0.44–1.34) |
| Injection drug use | 2.39 (0.73–7.81) | 1.80 (0.71–4.55) | 1.60 (0.57–4.47) |
| Non-injection drug use | 0.43 (0.17–1.08) | 0.67 (0.36–1.26) | 1.13 (0.54–2.34) |
| Homeless | 1.08 (0.51–2.29) | 1.24 (0.73–2.12) | 0.83 (0.38–1.81) |
| Nursing home resident | 1.41 (0.86–2.30) | 1.65 (1.20–2.29)* | 1.41 (0.85–2.32) |
| Disease site | |||
| Pulmonary | Referent | Referent | Referent |
| Miliary/meningeal | 2.36 (1.28–4.34)* | 1.93 (1.18–3.17)* | 2.07 (1.07–3.98)* |
| Extra-pulmonary (not miliary/meningeal) | 0.91 (0.59–1.38) | 0.62 (0.43–0.89)* | 1.23 (0.83–1.81) |
Statistically significant.
TB = tuberculosis; OR = odds ratio; CI = confidence interval; HR = hazards ratio; HIV = human immunodeficiency virus; HIV− = HIV−negative; HIV+ = HIV−positive.
Mortality in the early weeks of treatment
Significant predictors of death in the initial 8 weeks of anti-tuberculosis therapy were increasing age, Black race, history of excess alcohol use within the previous 12 months, miliary/meningeal disease, and residency in a long-term care facility at the time of diagnosis (Table 2). HIV was significantly associated with increased mortality in the first 8 weeks of treatment only in persons of Black race (adjusted hazards ratio [aHR] 2.15, 95%CI 1.21–3.81), and non-significantly associated with increased mortality among white persons (aHR 2.79, 95%CI 0.86–9.11); there were no deaths among the few HIV-positive Hispanic patients. However, persons of Black race who were not known to be HIV-infected were also at higher risk of death than White persons (aHR 1.36, 95%CI 1.05–1.76). The presence of cavitation in patients with PTB was not associated with mortality in the first 8 weeks of treatment (aHR 1.20, 95%CI 0.91–1.58). In a separate univariate analysis, patients treated with DOT had a higher risk of mortality during the initial 8 weeks of treatment than those treated with self-administered therapy alone (SAT): 5/983 (0.51%) patients treated with SAT alone, 17/1159 (1.47%) patients treated with a mixture of DOT and SAT (HR 2.89, 95%CI 1.07–7.84) and 277/2924 (9.47%) of patients treated exclusively with DOT (HR 19.51, 95%CI 8.06–47.23) died during the first 8 weeks of treatment.
Mortality in the later weeks of treatment
Increasing age and miliary/meningeal disease predicted death in the later weeks of anti-tuberculosis therapy (Table 2). Furthermore, TB-HIV co-infected subjects had a higher risk of mortality in the later weeks of treatment, although this finding only reached statistical significance among Black patients. Cavitary disease was not associated with increased mortality risk among PTB patients in the later weeks of therapy (aHR 0.90, 95%CI 0.58–1.40). In a separate univariate analysis, patients treated exclusively with DOT had higher mortality risk during the later weeks of treatment than patients treated with SAT alone. Of 978 patients, 27 (2.76%) treated with SAT alone, 39/1142 (3.42%) treated with a mixture of DOT and SAT (HR 1.32, 95%CI 0.69–2.53) and 169/2639 (6.40%) treated exclusively with DOT (HR 3.18, 95%CI 1.86–5.43) died later in treatment.
DISCUSSION
Increasing age, miliary or meningeal disease, and HIV were predictors of TB mortality in North Carolina throughout treatment. In addition, we found that being a person of Native American race specifically predicts death prior to TB treatment, while being a resident of a long-term care facility or having a history of excess alcohol use specifically predict death in the first 8 weeks of treatment. Cavitary disease was actually associated with a lower risk of death prior to TB treatment initiation, possibly because the finding of a cavity may raise the suspicion for TB in clinicians’ minds and reduce diagnostic delay. DOT was associated with increased mortality during treatment, but this finding is almost certainly due to patient selection (DOT was at the discretion of the health department during this time; it has been mandatory only since 2006 in North Carolina), as use of DOT was significantly associated with minority status, HIV status, and substance abuse (data not shown).
Our findings suggest that increased mortality falls on the shoulders of social groups that have been traditionally disenfranchised from mainstream society and thus may have reduced access to health care. This is a finding noted in other studies performed in the United States,8,17,23 as well as in Mexico,9 Russia,11,24 Australia25 and South Africa.4 Such findings suggest that it is important for individual TB programs to analyze their own data so they may identify groups at especially high risk for TB-associated mortality, and develop relevant interventions for improvement.
Previous studies have reported between 3% and 37% TB-related mortality prior to receiving anti-tuberculosis treatment;16,25 in our cohort, 3.8% died before initiation of anti-tuberculosis therapy. Of those in our cohort who survived to start treatment, 5.7% of the remaining patients then died within the initial 8 weeks of anti-tuberculosis therapy. Early mortality among TB patients is multifactorial, but diagnostic delay,26,27 severe (miliary/meningeal) disease,28 and comorbidities17 are contributory. The finding that cavitary disease was associated with lower risk of death suggests that reduced diagnostic delay in patients with cavitation may reduce mortality. In low-incidence settings with an adequate public health infrastructure, mortality rates may improve with improved surveillance efforts targeted at specific vulnerable groups, as TB in these settings may be more common in highly vulnerable groups than in resource-poor countries with high transmission rates.11
Our study had some limitations. TB patient deaths were determined by the RVCT form, a match with NDI, or both. As some TB patient information may not have been recorded correctly on the RVCT form or death certificate, we may have missed a few deaths during the NDI match and may have underestimated early death among TB patients. However, we believe underestimation to be minimal, given the completeness of the RVCT report in North Carolina.
With regard to variables, with values labeled as ‘positive’, ‘negative’ or ‘unknown’, such as HIV status, those with ‘unknown’ values were assigned to the ‘negative’ category during analyses. Because some of those ‘unknowns’ may be positive, assigning them to the negative group would likely bias our relative risk estimate towards null. This does not limit our findings with respect to the risk factors (e.g., HIV) that were found to be statistically significant, but may have reduced the ability to ascertain the significance of some of the other risk factors.
CONCLUSIONS
Despite these limitations, we drew several conclusions from this study. First, even in low-incidence settings where anti-tuberculosis therapy can be administered effectively, increased mortality occurs among the most vulnerable populations, such as the elderly or those with HIV. Second, the death rate among TB patients is highest during the first 8 weeks of TB treatment, suggesting that many of these early deaths are caused by TB. Death due to TB may be attributable to factors such as delayed diagnosis or poor functional status. Resources directed at increased TB surveillance and improvement of overall health care in these specific populations may help reduce future mortality.
Figure.
Survival during TB treatment among TB patients reported in 1993–2003 in North Carolina, United States. TB = tuberculosis; vertical lines = approximate time points for early and late in treatment, respectively.
Acknowledgements
The North Carolina TB Control Program and staff contributed data and discussion around the study design and findings. This work was supported by National Institutes of Health grants K23 AI051409 to JES and K24 AI001833 to CDH.
References
- 1.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Harries AD, Hargreaves NJ, Gausi F, Kwanjana JH, Salaniponi FM. High early death rate in tuberculosis patients in Malawi. Int J Tuberc Lung Dis. 2001;5:1000–1005. [PubMed] [Google Scholar]
- 3.Oursler KK, Moore RD, Bishai WR, Harrington SM, Pope DS, Chaisson RE. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiological factors. Clin Infect Dis. 2002;34:752–759. doi: 10.1086/338784. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez GG, Thembela BL, Muller FJ, Clinch J, Singhal N, Cameron DW. Tuberculosis at Edendale Hospital in Pietermaritzburg, KwaZulu Natal, South Africa. Int J Tuberc Lung Dis. 2004;8:1472–1478. [PubMed] [Google Scholar]
- 5.Archibald LK, den Dulk MO, Pallangyo KJ, Reller LB. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis. 1998;26:290–296. doi: 10.1086/516297. [DOI] [PubMed] [Google Scholar]
- 6.Pablos-Mendez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with TB. JAMA. 1996;276:1223–1228. doi: 10.1001/jama.1996.03540150025026. [DOI] [PubMed] [Google Scholar]
- 7.Haar CH, Cobelens GJ, Kalisvaart NA, van Gerven PJH, van de Have JJ. HIV-related mortality among tuberculosis patients in the Netherlands, 1993–2001. Int J Tuberc Lung Dis. 2007;11:1038–1041. [PubMed] [Google Scholar]
- 8.Sterling TR, Zhao Z, Chaisson RE, et al. Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis. 2006;10:542–549. [PubMed] [Google Scholar]
- 9.Bustamante-Montes LP, Escobar-Mesa A, Borja-Aburto VH, Gomez-Munoz A, Becerra-Posada F. Predictors of death from pulmonary tuberculosis: the case of Veracruz, Mexico. Int J Tuberc Lung Dis. 2000;4:208–215. [PubMed] [Google Scholar]
- 10.Garcia-Garcia M de L, Ponce-De-Leon A, Garcia-Sancho MC, et al. Tuberculosis-related deaths within a well-functioning DOTS control program. Emerg Infect Dis. 2002;8:1327–1333. doi: 10.3201/eid0811.020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathew TA, Ovsyanikova TN, Shin SS, et al. Causes of death during tuberculosis treatment in Tomsk Oblast, Russia. Int J Tuberc Lung Dis. 2006;10:857–863. [PubMed] [Google Scholar]
- 12.Sacks LV, Pendle S. Factors related to in-hospital deaths in patients with tuberculosis. Arch Intern Med. 1998;158:1916–1922. doi: 10.1001/archinte.158.17.1916. [DOI] [PubMed] [Google Scholar]
- 13.Santha T, Garg R, Frieden TR, et al. Risk factors associated with default, failure and death among tuberculosis patients treated in a DOTS programme in Tiruvallur District, South India, 2000. Int J Tuberc Lung Dis. 2002;6:780–788. [PubMed] [Google Scholar]
- 14.Fielder JF, Chaulk CP, Dalvi M, Gachuhi R, Comstock GW, Sterling TR. A high tuberculosis case-fatality rate in a setting of effective tuberculosis control: implications for acceptable treatment success rates. Int J Tuberc Lung Dis. 2002;6:1114–1117. [PubMed] [Google Scholar]
- 15.Cayla JA, Caminero JA, Rey R, Lara N, Valles X, Galdos-Tanguis H. Current status of treatment completion and fatality among tuberculosis patients in Spain. Int J Tuberc Lung Dis. 2004;8:458–464. [PubMed] [Google Scholar]
- 16.Farah MG, Tverdal A, Steen TW, Heldal E, Brantsaeter AB, Bjune G. Treatment outcome of new culture positive pulmonary tuberculosis in Norway. BMC Public Health. 2005;5:14. doi: 10.1186/1471-2458-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansel NN, Merriman B, Haponik EF, Diette GB. Hospitalizations for tuberculosis in the United States in 2000: predictors of in-hospital mortality. Chest. 2004;126:1079–1086. doi: 10.1378/chest.126.4.1079. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson CR, Forouhi NG, Roglic G, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234–241. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4:20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinson NA, Karstaedt A, Venter WDF, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21:2043–2050. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 21.Santo AH. Deaths attributed to multiple causes and involving tuberculosis in the state of Rio de Janeiro Brazil between 1999-2001. J Bras Pneumol. 2006;32:544–552. doi: 10.1590/s1806-37132006000600012. [DOI] [PubMed] [Google Scholar]
- 22.Racial disparities in tuberculosis—selected southeastern states, 1991-2002. MMWR. 2004;53:556–559. [PubMed] [Google Scholar]
- 23.Friedman LN, Williams MT, Singh TP, Frieden TR. Tuberculosis, AIDS, and death among substance abusers on welfare in New York City. N Engl J Med. 1996;334:828–833. doi: 10.1056/NEJM199603283341304. [DOI] [PubMed] [Google Scholar]
- 24.Dewan PK, Arguin PM, Kiryanova H, et al. Risk factors for death during tuberculosis treatment in Orel, Russia. Int J Tuberc Lung Dis. 2004;8:598–602. [PubMed] [Google Scholar]
- 25.Walpola HC, Siskind V, Patel AM, Konstantinos A, Derhy P. Tuberculosis-related deaths in Queensland, Australia, 1989- 1998: characteristics and risk factors. Int J Tuberc Lung Dis. 2003;7:742–750. [PubMed] [Google Scholar]
- 26.Palmieri F, Girardi E, Pellicelli AM, et al. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002;30:68–74. doi: 10.1007/s15010-002-2062-9. [DOI] [PubMed] [Google Scholar]
- 27.Greenaway C, Menzies D, Fanning A, Grewal R, Yuan L, FitzGerald J M. Delay in diagnosis among hospitalized patients with active tuberculosis—predictors and outcomes. Am J Respir Crit Care Med. 2002;165:927–933. doi: 10.1164/ajrccm.165.7.2107040. [DOI] [PubMed] [Google Scholar]
- 28.Kourbatova EV, Leonard MK, Jr, Romero J, Kraft C, del Rio C, Blumberg HM. Risk factors for mortality among patients with extra-pulmonary tuberculosis at an academic inner-city hospital in the US. Eur J Epidemiol. 2006;21:715–721. doi: 10.1007/s10654-006-9060-7. [DOI] [PubMed] [Google Scholar]