Abstract
OBJECTIVE
Patients with inflammatory bowel disease (IBD) frequently receive immunosuppressive therapy. The immune response in these patients to vaccines has not been well studied. We conducted a prospective, open label study to evaluate the serologic response to influenza vaccine in children with IBD.
METHODS
Serum was obtained from 146 children and young adults with IBD (96 CD, 47 UC, 3 IC) for baseline influenza titer, immediately followed by immunization with trivalent [A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004 (B)] inactivated influenza vaccine. Subjects returned for repeat titers 3-9 weeks later. Seroprotection against each influenza strain was defined as hemagglutination inhibition (HAI) titer ≥40. Patients were categorized as non-immunosuppressed [(NIS), aminosalicylates only, antibiotics only, or no therapy] or immunosuppressed [(IS), any immunosuppressive agent]. IS patients were further subcategorized as: (1) tacrolimus; (2) TNF-alpha inhibitor; (3) immunomodulator; and (4) corticosteroids only.
RESULTS
More patients were seroprotected against strains A/H1N1 and A/H3N2 than B strain (p<0.02), regardless of immunosuppression status. The proportion seroprotected and geometric mean titers at post-vaccination were similar between NIS and IS groups for all three strains. Subanalysis of patients not seroprotected at baseline showed that those receiving anti-TNF therapy were less likely seroprotected against strain B (14%) compared to patients in the NIS group (39%, p=0.025). There were no serious vaccine-associated adverse events.
CONCLUSION
Influenza vaccination produces a high prevalence of seroprotection in IBD patients, particularly against A strains. The vaccine is well tolerated. Routine influenza vaccination in IBD patients is recommended, irrespective of whether patients receive immunosuppressive medications.
INTRODUCTION
Patients with Crohn’s disease (CD) and ulcerative colitis (UC) are presumed to have chronic intestinal inflammation as a result of an altered immune response to an environmental or infectious trigger. [1] A number of genetic mutations associated with CD and/or UC, suggest that affected patients have subtle defects in innate immunity. [2-5] Inflammatory bowel disease (IBD) patients are often placed on long-term anti-inflammatory and immunosuppressive therapies, including aminosalicylates, corticosteroids, 6-mercaptopurine, azathioprine, methotrexate, and infliximab. [6] While a majority of IBD patients do not develop serious infections, reports of life-threatening infections (e.g., varicella, tuberculosis) have been published, primarily in patients receiving immunosuppressive therapy. [7-10]
In 2004, a consensus report of a committee organized by the Crohn’s and Colitis Foundation of America (CCFA) recommended the administration of routine inactivated vaccines to IBD patients. [11] However, there is a paucity of formal vaccine studies that examine the serologic response to routine immunizations in this population. One prior study by Mamula et al [12] suggested that children with IBD may mount an inadequate immune response to one strain of the inactivated influenza vaccine compared to healthy controls.
Influenza is a contagious acute respiratory infection that may cause serious complications. [13] In the United States, influenza was responsible for more than 226,000 hospitalizations annually from 1979-2001 and 36,000 deaths annually from 1990-1999. [14, 15] People with certain medical conditions (e.g., chronic lung disease, malignancy) may be at a higher risk of having complications if they are infected with influenza. [16, 17] Other groups of patients (e.g., those with pulmonary or cardiac disease) may have exacerbations of their underlying illness. [13] In one study of children younger than 16 years old, the influenza vaccine had an efficacy rate between 77% (Influenza A/H1N1) and 91% (A/H3N2) in protecting against culture proven influenza infections. [18] The vaccine may be less effective in people with certain medical conditions (e.g., systemic lupus erythematosus) [19, 20] or in patients receiving immunosuppressive medications. [12, 21]
The serologic response to vaccines (including influenza vaccine) in children with IBD is not well studied. To further evaluate this question, we conducted a prospective, open label study to evaluate the safety and immunogenicity of inactivated influenza vaccine in a cohort of pediatric IBD patients. We hypothesized that children receiving immunosuppressive therapy would be less likely to develop a serologic response to the vaccine.
METHODS
Subjects
We recruited patients ages 5 years or older from the IBD outpatient program or during hospital admissions at Children’s Hospital Boston who had documented CD, UC, or indeterminate colitis (IC). The lower age limit was set at 5 years to capture the vast majority of the pediatric IBD population. The diagnosis of CD, UC, and IC [22, 23] was established by standard clinical, radiographic, endoscopic, and histologic criteria.
Exclusion criteria included: (1) contraindication to receiving influenza vaccine (e.g., allergic reaction to prior influenza vaccine, egg allergy); (2) previous influenza vaccination during the current influenza season; (3) fever within 48 hours prior to influenza vaccination; and (4) receipt of another vaccine within 48 hours prior to influenza vaccination. We asked that patients not receive another immunization within 48 hours after influenza vaccination to minimize confounding of acute adverse event reporting.
Groups
Patients were categorized into two main groups depending on whether their IBD treatments were thought to cause immunosuppression: (1) non-immunosuppressed (NIS) or (2) immunosuppressed (IS). Subjects were classified as NIS if they received no therapy, antibiotics only, or aminosalicylates only at the time of vaccination (Table 1). Subjects were classified as IS if they received tacrolimus, infliximab, adalimumab, thalidomide, 6-mercaptopurine, azathioprine, methotrexate, or corticosteroids at the time of vaccination. Standard concomitant medications (e.g., antibiotics, antihistamines, acetaminophen) were allowed.
Table 1.
Therapeutic agents used to treat patients with IBD in the study
| IBD Group | Type of Therapy | Individual Medications |
|---|---|---|
|
| ||
| Non-immunosuppressed | No therapy | none |
|
| ||
| Antibiotic | metronidazole | |
| ciprofloxacin | ||
|
| ||
| Aminosalicylate | mesalamine | |
| sulfasalazine | ||
| balsalazide | ||
| olsalazine | ||
|
| ||
| Immunosuppressed | Calcineurin inhibitor | tacrolimus |
|
| ||
| Tumor necrosis factor (TNF) alpha inhibitor | infliximab | |
| adalimumab | ||
| thalidomide | ||
|
| ||
| Immunomodulator | azathioprine | |
| 6-mercaptopurine | ||
| methotrexate | ||
|
| ||
| Corticosteroids | prednisone | |
| prednisolone | ||
| methylprednisolone | ||
A priori, we assumed there were varying degrees of immune response to influenza vaccination depending on the type of immunosuppressive therapy patients received. Therefore, subjects within the IS group were further subcategorized by the specific medications they received at the time of vaccination: (1) tacrolimus; (2) TNF-alpha inhibitor (infliximab, adalimumab, thalidomide); (3) immunomodulator (6-mercaptopurine, azathioprine, methotrexate); and (4) corticosteroids only (prednisone, prednisolone, methylprednisolone) (Table 1). Patients receiving both an immunosuppressive medication and prednisone were categorized by the immunosuppressive therapy (e.g., a patient receiving both 6-mercaptopurine and prednisone was placed in the 6-mercaptopurine group). Patients who received at least two immunosuppressive therapies were classified by the medication that had the greatest immunosuppressive effect. Tacrolimus and TNF-alpha inhibitors were thought to have the most immunosuppressive potency, and immunomodulators the least immunosuppressive potency of these 3 classes of medications. For example, a patient on infliximab and 6-mercaptopurine was placed in the TNF-alpha inhibitor group. None of our patients received both tacrolimus and TNF-alpha inhibitor.
Study Design
Patients were recruited between October 2007 and December 2007. Each patient had two study visits (entry and post-vaccination), as outlined in Figure 1.
Figure 1.
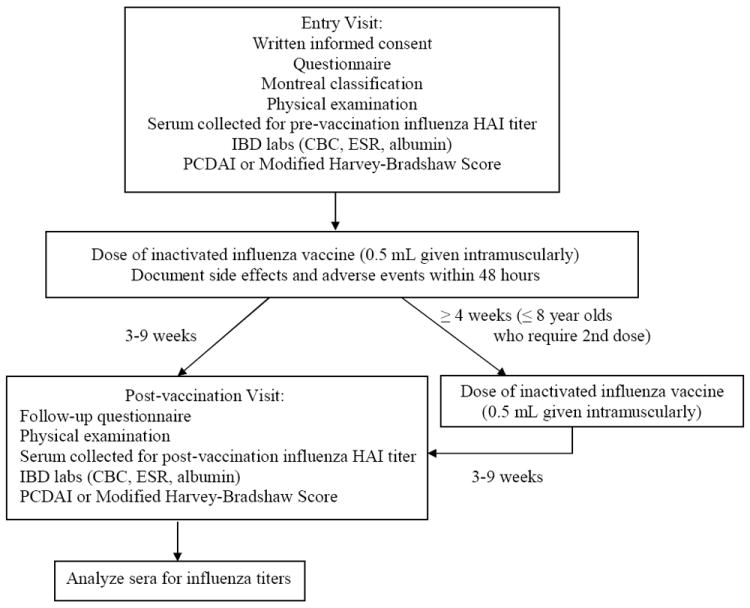
Study schedule
Entry visit
Written informed consent was obtained from the parent/guardian of patients <18 years old, and from patients if they were ≥ 18 years old. Each subject answered a questionnaire focused on demographics, medical history related to IBD, medications, and possible influenza-like symptoms. Based on the clinical information obtained, the Montreal classification [24] (a widely used disease phenotyping system in IBD research) was used to define disease location and behavior. In each patient, a physical examination was performed and clinical IBD laboratory studies were obtained including: complete blood count (CBC), erythrocyte sedimentation rate (ESR), and albumin. Disease activity was assessed by the Pediatric Crohn’s Disease Activity Index (PCDAI) [25] for patients with Crohn’s disease, and the Modified Harvey Bradshaw Score [26] for patients with UC or indeterminate colitis.
Serum was obtained for determination of baseline influenza titer (pre-vaccination), immediately followed by immunization with a 0.5 mL dose of trivalent [A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004 (B)] inactivated influenza vaccine administered intramuscularly (Fluzone; Sanofi Pasteur Inc., Swiftwater, PA). In accordance with recommendations of the Advisory Committee on Immunization Practices (ACIP) from the Centers for Disease Control and Prevention (CDC), children ≤ 8 years old received two doses ≥ 4 weeks apart if they were receiving the influenza vaccine for the first time, or if they had received only one dose of influenza vaccine during the previous influenza season as their first dose. [13]
A standard 0.5 mL dose of vaccine contained 15 micrograms hemagglutinin of each influenza strain, residual egg protein, and gelatin. There were no antibiotics or thimerosal in the manufacture of the vaccines used. [27] Participants were instructed to contact the study staff immediately if they experienced adverse events (e.g., life-threatening event or hospitalization related to the vaccine) after immunization. If subjects did not contact the study staff, they were called approximately 48 hours after vaccination to inquire about side effects and adverse events.
Post-vaccination visit
Patients returned for the post-vaccination visit 3-9 weeks after the single dose (children ≥ 8 yrs old) or second of two doses of vaccination (children ≤ 8 yrs old). Subjects answered another questionnaire regarding new events related to IBD, changes in medications, and influenza-like symptoms since the entry visit. A physical examination was performed. A serum sample was again obtained from each subject to measure influenza titer (post-vaccination) and clinical IBD laboratory studies (CBC, ESR, and albumin). Severity of disease was reassessed using the PCDAI or Modified Harvey-Bradshaw Score.
The study was approved by the Institutional Review Board at Children’s Hospital Boston. An independent Data Safety Monitor Board monitored the study for adverse events.
Specimen handling and laboratory analysis
Sera to be used for measurement of influenza titers were stored at -80 degrees Celsius. Samples were sent on dry ice in a batch to the Laboratory for Specialized Clinical Studies at Cincinnati Children’s Hospital Medical Center (Cincinnati, OH) for analysis. The laboratory was blinded to subject identifiers, diagnosis, and medication group. Sera were assessed for antibody to each of the three components of the vaccine by hemagglutination inhibition (HAI) assay using standard methods. [28] In brief, sera were treated with Receptor-Destroying Enzyme (RDE; Denka-Seiken, Japan) to remove non-specific inhibitors of hemagglutination prior to testing. Following RDE treatment, the samples were further diluted to 1:10 in Phosphate Buffered Saline (PBS). The sera were then treated with packed red blood cells (RBCs) to remove non-specific inhibitors. The RBCs were spun out of the sera and the samples were ready for testing. Starting at 1:10 dilution, the sera were diluted two-fold through 1:2560 in V-bottom microtiter plates.
Egg-derived, inactivated viral antigens (A/H1N1, A/H3N2 and B/Malaysia) representative of the 2007-2008 vaccine were obtained from the CDC, added to serially diluted sera, and incubated at room temperature for 30 minutes. RBCs from turkey blood (Viromed Laboratories, Minnetonka, MN) were suspended at a concentration of 0.5% in PBS, added to the serum/viral antigen mixture, and incubated at room temperature for 30 minutes. Plates were tilted and read. The antibody titers were reported as the reciprocal of the last serum dilution to completely inhibit RBC agglutination. Sera without reactivity were assigned a value of < 10. Sera with initial titers of ≥ 2560 were retested at a higher starting dilution in order to obtain a reportable titer.
Study Outcomes
The primary outcome was seroprotection, defined as a hemagglutination inhibition (HAI) titer ≥ 40 [27] to each influenza strain. The secondary outcome was the antibody titer to each influenza strain. The tertiary outcome was the number and type of acute vaccine-associated side effects and adverse events.
Statistical Analysis
Characteristics of Patients Groups
Demographic characteristics, medical history, medications, disease activity, laboratory data, and Montreal classification were described for NIS and IS groups. For continuous variables, differences between groups were compared using t tests. If there was evidence for non-normality, non-parametric statistics were used. For dichotomous variables, differences between groups were compared using chi-square or Fisher’s exact tests.
Seroprotection
At each time point (pre- and post-vaccination), we calculated the proportion of subjects in the NIS and IS groups with a seroprotective titer (HAI ≥ 40) to each influenza strain (proportion seroprotected). We examined the difference in proportions between the two groups using a Fisher’s exact test. We performed the above analysis in three ways: (1) among patients who completed the study regardless of their titer at entry; (2) among patients who were not seroprotected at entry (HAI < 40); and (3) among patients who were seronegative at entry (HAI < 10).
Geometric Mean Titer (GMT)
We compared the post-vaccination geometric mean titers (GMTs) between the NIS and IS groups using two-sample t tests for each influenza strain. If there was evidence for non-normality, the Wilcoxon test was used.
Medication subgroup analyses
We stratified the IS group by medication class (tacrolimus, TNF-alpha inhibitor, immunomodulator, and corticosteroids only). We used logistic regression analysis to compare the proportion seroprotected in each of the four subgroups to the NIS group. For example, four dummy variables were created, with NIS as the reference group. We used ANOVA to compare differences in post-vaccination titers across the medication and NIS groups. We used Tukey’s HSD to adjust for multiple comparisons.
Acute side effects and adverse events
We evaluated the difference in frequency of types of acute side effects and adverse events between the two IBD groups using a chi-square or Fisher’s exact test. In addition, we compared pre-and post-vaccination disease activity scores using the Sign test. All statistical tests were performed using SPSS 15.0 for Windows. [29]
Power calculations
Before conducting the study, we calculated the differences in proportion seroprotected between the NIS and IS groups. We used the proportions seroprotected in a prior influenza immunization study of healthy children by Chui et al [30] to estimate the proportion seroprotected in the NIS group. We assumed an alpha of 0.05 with continuity correction and 80% power. Given 65 patients per group (IS, NIS), we determined that we would be able to detect a difference of 16% for H1N1, 14% for H3N2, and 20% for B strain between the NIS and IS groups. We enrolled additional subjects in the IS group, so that we could conduct subanalyses based on the class of immunosuppressive agent. All power calculations were performed using the program PASS. [31]
RESULTS
Study Demographics
We enrolled 146 subjects (96 CD, 47 UC, 3 IC) into the study: 20 were receiving no therapy, antibiotics only, or aminosalicylates only [non-immunosuppressed (NIS) group], and 126 were receiving immunosuppressive therapy [immunosuppressed (IS) group]. The number of patients who completed the study was 137 (Figure 2). The baseline characteristics for enrolled patients are shown in Table 2. The median disease activity scores for both CD and UC groups were compatible with disease in remission.
Figure 2.
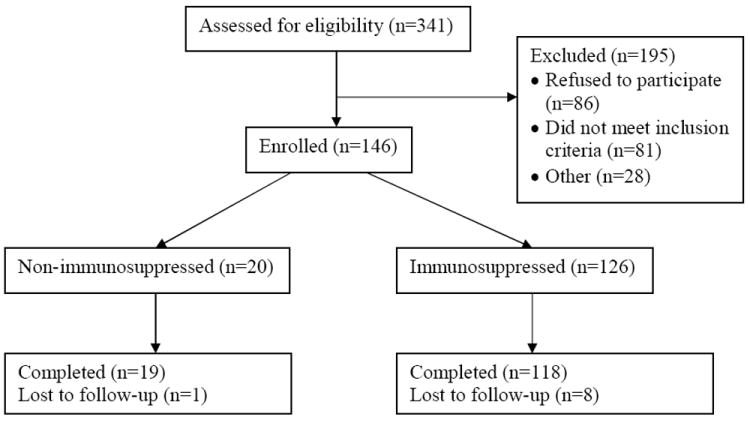
Conduct of study
Table 2.
Baseline characteristics for the study patients (n=146)
| Characteristics | Non-immunosuppressed (n=20) | Immunosuppressed (n=126) | p* |
|---|---|---|---|
| Gender | 1.00 | ||
| Male | 12 (60%) | 76 (60%) | |
| Female | 8 (40%) | 50 (40%) | |
| Age (years, median) | |||
| At enrollment (range 5-26) | 14.5 | 16.0 | 0.20 |
| At diagnosis (range 3-21) | 11.0 | 12.0 | 0.93 |
| Race/Ethnicity | 1.00 | ||
| Caucasian | 18 (90%) | 111 (88%) | |
| Non-Caucasian | 2 (10%) | 15 (12%) | |
| Diagnosis | 0.03† | ||
| Crohn’s disease | 8 (40%) | 88 (70%) | |
| Ulcerative colitis | 11 (55%) | 36 (28.5%) | |
| Indeterminate colitis | 1 (5%) | 2 (1.5%) | |
| Montreal classification | |||
| Crohn’s disease (n=96) | |||
| Disease location | 0.72 | ||
| L1 (terminal ileum) | 1 (12.5%) | 8 (9%) | |
| L2 (colon) | 0 (0%) | 6 (7%) | |
| L3 (ileocolon) | 7 (87.5%) | 74 (84%) | |
| Upper GI disease | 0.02† | ||
| L4 | 2 (10%) | 48 (38%) | |
| Disease behavior | 0.89 | ||
| B1 (non-stricturing, non-penetrating) | 5 (62.5%) | 61 (69%) | |
| B2 (stricturing) | 2 (25%) | 16 (18%) | |
| B3 (penetrating) | 1 (12.5%) | 11 (13%) | |
| Perianal disease (p) | 2 (10%) | 36 (29%) | 0.10 |
| UC or IC (n=50) | 0.04† | ||
| E1 (proctitis) | 2 (16.5%) | 0 (0%) | |
| E2 (left-sided colitis) | 2 (16.5%) | 7 (18%) | |
| E3 (pancolitis) | 8 (67%) | 31 (82%) | |
| Disease activity at time of study entry (median) | |||
| PCDAI (range 0-40, n=89)‡ | 0.0 | 5.0 | 0.51 |
| Modified Harvey Bradshaw Score (range 0-16, n=50) | 1.0 | 0.5 | 0.97 |
| Medications | |||
| No therapy | 3 (15%) | 0 (0%) | |
| Antibiotic only | 1 (5%) | 0 (0%) | |
| Aminosalicylate only | 16 (80%) | 0 (0%) | |
| Tacrolimus | 0 (0%) | 10 (8%) | |
| TNF-alpha inhibitor | 0 (0%) | 45 (36%) | |
| Immunomodulator | 0 (0%) | 59 (47%) | |
| Corticosteroids only§ | 0 (0%) | 12 (9%) | |
| Received prior influenza vaccine | 16 (80%) | 90 (72%) | 0.59 |
| Days between visits (median, range 21-65) | 39.0 | 38.0 | 0.33 |
The reported p values are the results of a chi-square or Fisher’s exact test comparing the characteristic (e.g., male or female) between the non-immunosuppressed and immunosuppressed groups.
Statistically significant
7 missing values
All patients in the “corticosteroids only” group were receiving a dose of < 2 mg/kg/day, with a mean dose of 0.73 ± 0.50 mg/kg/day, at the time of vaccination.
The proportion seroprotected at post-vaccination is similar between non-immunosuppressed (NIS) and immunosuppressed (IS) IBD patients
The proportion seroprotected against each influenza vaccine strain pre- and post-vaccination are shown by medication group in Table 3. The proportion seroprotected at post-vaccination for each influenza A strain (A/H1N1 and A/H3N2) was compared to the B strain for each IBD group. There was a statistically significant higher proportion seroprotected in both A strains than the B strain, irrespective of whether patients were receiving immunosuppressive therapy (p<0.02 for all comparisons).
Table 3.
Proportion seroprotected [hemagglutination inhibition (HAI) ≥ 40, n=137] pre- and post-vaccination
| Group | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Pre/Post-vaccination | Pre/Post | Pre/Post | |
| Non-immunosuppressed (n=19) | 58% / 100%* | 68% / 84%* | 5% / 42% |
| Immunosuppressed (n=118) | 63% / 95%* | 59% / 89%* | 15% / 39% |
| Tacrolimus (n=10) | 70% / 90% | 30% / 100% | 20% / 60% |
| TNF-alpha inhibitor (n=42) | 67% / 93% | 60% / 79% | 12% / 21% |
| Immunomodulator (n=56) | 66% / 98% | 64% / 93% | 16% / 41% |
| Corticosteroids only (n=10) | 20% / 90% | 50% / 100% | 20% / 80% |
| Healthy historical controls† (n=76) | 20% / 85% | 18% / 84% | 5% / 57% |
A higher proportion of patients were seroprotected at post-vaccination against strains A/H1N1 and A/H3N2 compared to B strain (p<0.02), regardless of immunosuppression status.
Pediatric historical healthy controls were immunized with the same influenza strains during the same influenza season (2007-2008) as IBD patients in our study. Data for these historical controls were obtained from studies reported to the Vaccines and Related Biological Products Advisory Committee (VRBPAC) from the Food and Drug Administration (FDA). [32]
At post-vaccination, the proportion seroprotected was similar between the NIS and IS groups for all three influenza strains (p>0.46 for all strains): A/H1N1 (NIS 100%, IS 95%), A/H3N2 (NIS 84%, IS 89%), and B (NIS 42%, IS 39%).
Overall, the proportion seroprotected at post-vaccination was similar between the NIS and IS groups for all three influenza strains.
We also compared the proportion seroprotected in each subclass within the IS group (tacrolimus, TNF-alpha inhibitor, immunomodulator, and corticosteroids only) to the NIS group. We found no significant differences in the proportion seroprotected between any medication subgroup and the NIS group. Of patients receiving TNF-alpha inhibitors, a lower percentage (21%) responded to the B strain. However, the difference was not statistically significant.
In Table 3, we compared the proportion of patients who were seroprotected at post-vaccination in our study to a cohort of pediatric historical healthy controls who were immunized with the same influenza strains during the same influenza season (2007-2008) as the IBD patients in our study. Data for these historical controls were obtained from studies reported to the Vaccines and Related Biological Products Advisory Committee (VRBPAC) from the Food and Drug Administration (FDA). [32] In these studies (N=76), the proportion seroprotected against the A/H1N1 and A/H3N2 were similar between the historical healthy controls and the NIS or IS group in our study. The data in healthy controls suggested that strain B was less immunogenic overall; the proportion seroprotected was 57% for controls, compared to 42% for our NIS group and 39% for our IS group. [32]
The proportion seroprotected at post-vaccination is similar between NIS and IS groups among patients who did not have seroprotective levels (HAI < 40) at entry
We conducted a subanalysis to determine the proportion seroprotected at post-vaccination among patients who did not have a seroprotective titer (HAI < 40) at entry (Table 4). Overall, we did not find a difference in the proportion seroprotected between the NIS and IS groups for any of the three influenza strains.
Table 4.
Proportion seroprotected at post-vaccination among patients who were not seroprotected [hemagglutination inhibition (HAI) < 40] at entry
| Group | A/H1N1 #/total (%) |
A/H3N2 #/total (%) |
B #/total (%) |
|---|---|---|---|
| Non-immunosuppressed | 8/8 (100%) | 3/6 (50%) | 7/18 (39%) |
| Immunosuppressed | 38/44 (86%) | 36/49 (74%) | 30/100 (30%) |
| Tacrolimus | 2/3 (67%) | 7/7 (100%) | 4/8 (50%) |
| TNF-alpha inhibitor | 11/14 (79%) | 8/17 (47%) | 5/37 (14%)* |
| Immunomodulator | 18/19 (95%) | 16/20 (80%) | 15/47 (32%) |
| Corticosteroids only | 7/8 (88%) | 5/5 (100%) | 6/8 (75%) |
At post-vaccination, the proportion seroprotected was similar between the NIS and IS groups for all three influenza strains (p>0.34 for all strains): A/H1N1 (NIS 100%, IS 86%), A/H3N2 (NIS 50%, IS 74%), and B (NIS 39%, IS 30%).
In an analysis of IS subgroups, only patients receiving TNF-alpha inhibitors had a significantly lower proportion seroprotected against strain B (14%) compared to patients in the NIS group (39%, p=0.025).
However, in an analysis of IS subgroups, patients receiving TNF-alpha inhibitors had a significantly lower proportion seroprotected against strain B (14%) compared to patients in the NIS group (39%, p=0.025). There were no significant differences in the proportion seroprotected between each of the other subclass medication groups compared to the NIS group for any vaccine strain. Additionally, there was no difference in the proportion seroprotected between patients on both TNF-alpha inhibitor and an immunomodulator compared to patients on TNF-alpha inhibitor without an immunomodulator (data not shown).
The proportion seroprotected at post-vaccination is similar between NIS and IS groups among patients who were seronegative levels (HAI < 10) at entry
We conducted another subanalysis of the proportion seroprotected at post-vaccination among patients who were seronegative (HAI < 10) at entry (Table 5). Overall, we did not identify a difference in the proportion seroprotected between the NIS and IS groups for any of the three influenza strains. There were also no differences in the proportion seroprotected between each medication within the IS group compared to the NIS group for any vaccine strain.
Table 5.
Proportion seroprotected at post-vaccination among patients who were seronegative [hemagglutination inhibition (HAI) < 10] at entry
| Group | A/H1N1 #/total (%) |
A/H3N2 #/total (%) |
B #/total (%) |
|---|---|---|---|
| Non-immunosuppressed | 3/3 (100%) | 0/1 (0%) | 3/11 (27%) |
| Immunosuppressed | 14/19 (74%) | 10/17 (59%) | 13/50 (26%) |
| Tacrolimus | All seropositive at baseline | 1/1 (100%) | 0/2 (0%) |
| TNF-alpha inhibitor | 2/5 (40%) | 3/8 (38%) | 2/21 (10%) |
| Immunomodulator | 10/11 (91%) | 4/6 (67%) | 9/24 (38%) |
| Corticosteroids only | 2/3 (67%) | 2/2 (100%) | 2/3 (67%) |
At post-vaccination, the proportion seroprotected was similar between the NIS and IS groups for all three influenza strains (p>0.44 for all strains): A/H1N1 (NIS 100%, IS 74%), A/H3N2 (NIS 0%, IS 59%), and B (NIS 27%, IS 26%).
The post-vaccination geometric mean titer (GMT) is similar between NIS and IS IBD patients
Geometric mean titers (GMTs) are a quantitative measure of antibody response to immunization. The pre- and post-vaccination GMTs for each group in our study population and for the pediatric historical healthy controls are presented in Table 6. Overall, there was no difference in post-vaccination GMTs between the NIS and IS groups for any of the 3 influenza strains.
Table 6.
Pre- and post-vaccination geometric mean titers (GMTs, n=137)
| Group | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Pre/Post-vaccination | Pre/Post | Pre/Post | |
| Non-immunosuppressed (n=19) | 39 / 230 | 59 / 153 | 12 / 22 |
| Immunosuppressed (n=118) | 48 / 212 | 47 / 132 | 14 / 25 |
| Tacrolimus (n=10) | 70 / 394 | 24 / 160 | 18 / 37 |
| TNF-alpha inhibitor (n=42) | 54 / 154 | 47 / 85 | 13 / 19 |
| Immunomodulator (n=56) | 48 / 215 | 57 / 154 | 14 / 24 |
| Corticosteroids only (n=10) | 18 / 390 | 34 / 299 | 15 / 80* |
| Healthy historical controls† (n=76) | 5/ 183 | 9 / 147 | 6 / 45 |
At post-vaccination, the GMTs were similar between the NIS and IS groups for all three influenza vaccine strains (p>0.52 for all strains): A/H1N1 (NIS 230, IS 212), A/H3N2 (NIS 153, IS 132), and B (NIS 22, IS 25).
The “corticosteroids only” group had a significantly higher post-vaccination GMT for strain B (80) compared to the NIS group (22, p=0.005) and other immunosuppressive medication groups (p≤0.003) except tacrolimus (p=0.33).
Pediatric historical healthy controls were immunized with the same influenza strains during the same influenza season (2007-2008) as IBD patients in our study. Data for these historical controls were obtained from studies reported to the Vaccines and Related Biological Products Advisory Committee (VRBPAC) from the Food and Drug Administration (FDA). [32]
The GMTs for each subclass of immunosuppressive medication were compared with those for the NIS group. The “corticosteroids only” group had a significantly higher post-vaccination GMT for strain B (80) compared to the NIS group (22, p=0.005) and other immunosuppressive medication groups (p≤0.003) except tacrolimus (p=0.33). There were no differences between these two groups for post-immunization GMTs to the two A strains. There was no difference in any of the other post-vaccination GMTs for the IS subclasses compared to the NIS group.
Adverse events
There were no serious adverse events related to the influenza vaccine during the study. Reported side effects from vaccination are listed in Table 7. The most common side effect was soreness at the injection site (38%, n=146). Local reactions to vaccination, upper respiratory symptoms, headache, and systemic symptoms occurred in ≤ 5% of all subjects. There was no difference in side effects from vaccination between the NIS and IS groups.
Table 7.
Side effects from influenza vaccination
| Side effect | Non-immunosuppressed (n=20) |
Immunosuppressed (n=126) |
p |
|---|---|---|---|
| Sore arm | 50% | 36% | 0.23 |
| Erythema around site | 0% | 1% | 0.86 |
| Nasal symptoms | 5% | 3% | 0.53 |
| Sore throat | 5% | 2% | 0.45 |
| Cough | 5% | 0% | 0.14 |
| Headache | 0% | 5% | 0.41 |
| Fever | 5% | 0% | 0.14 |
| Fatigue | 0% | 1% | 0.86 |
| Myalgia | 0% | 1% | 0.86 |
| Malaise | 0% | 1% | 0.86 |
During the period of patient follow-up for this study (i.e. between the patient’s first and second study visits), 6 patients were hospitalized for IBD-related issues: 4 for electively scheduled colectomies, and 2 for flares of inflammatory bowel disease. None of these hospitalizations were thought to be related to influenza immunization.
In addition, there was no statistically significant difference between pre-and post-vaccination disease activity scores (p=0.69 for PCDAI, p=0.85 for Modified Harvey Bradshaw Score).
DISCUSSION
Our prospective study of IBD patients who received influenza vaccine indicates that the vaccine is both safe and immunogenic in this population. The influenza vaccine was well tolerated with a low percentage of side effects and without serious adverse events related to the vaccine. Severity of disease activity was not affected by immunization. A high proportion of patients with CD and UC immunized with the standard influenza vaccine were seroprotected irrespective of whether they were receiving immunosuppressive therapy. However, on subanalysis of patients who were not seroprotected prior to immunization, a lower proportion of patients receiving TNF-alpha inhibitors were seroprotected against influenza strain B/Malaysia than other patient groups. This has important implications because these patients are at risk for acquiring influenza strain B and its complications. Concomitant use of an immunomodulator with TNF-alpha inhibitor did not affect the proportion seroprotected and post-vaccination GMT for any strains (data not shown).
We were surprised to learn that patients on tacrolimus did not have a lower proportion seroprotected, since both TNF-alpha inhibitors and tacrolimus are immunosuppressive therapies that affect T-cells. Even though corticosteroids may also affect T-cells, the patients in the “corticosteroids only” group were receiving only a relatively low dose of the medication.
Vaccine trials conducted in patients with chronic illness have demonstrated varying degrees of seroprotection. Adults with systemic lupus erythematosus (SLE) have a lower antibody response to pneumococcus, [33, 34] tetanus [35] and influenza [19, 20] compared to controls. In contrast, response to the influenza vaccine appears adequate in adults with asthma receiving corticosteroids [36] and in rheumatoid arthritis. [37] A study of pediatric oncology patients who received two doses of influenza vaccine demonstrated a 40-65% seroconversion rate and 38-72% seroprotection rate to the three influenza strains in unprimed patients. However, patients receiving chemotherapy had a lower immune response to influenza A strains compared to patients who completed chemotherapy 1-6 months prior to the study. This latter group of children who no longer received chemotherapy exhibited a similar immune response to healthy children. [21]
Only one other study has prospectively evaluated vaccine serologic responses in children with IBD. Mamula et al [12] compared the immune response to the 2002-2004 inactivated influenza vaccines in 51 pediatric IBD patients and 29 healthy children. In their study, the authors noted an 86% seroprotection rate to the B/Hong Kong strain in IBD patients on non-immunosuppressive therapy, 70% seroprotection rate in patients on immunomodulators, and 38% in patients on infliximab combined with immunomodulators. Our study differed from the prior study in two major ways: we had a larger sample size, and we conducted the entire study over one influenza season. However, our findings are similar to the study by Mamula and colleagues. We demonstrate that both NIS and IS patients mount a similar serologic response to inactivated influenza vaccine, and we suggest that the subset of patients receiving TNF-alpha inhibitors may have a poorer response.
Our study had some limitations. We initially planned to enroll 65 patients in the NIS group, but we only enrolled 20. Most of our eligible patients were receiving immunosuppression. In addition, parents whose children were not immunosuppressed were less willing to participate and preferred to get vaccinated by their primary care physician and not have blood drawn to measure HAI titers. To address this limitation, we obtained control data from studies reported to the VRBPAC. [32] Another limitation is that we measured HAI titers, but did not document culture positive cases of influenza. However, other studies clearly show that the development of antibody protects against disease. In addition, while we classified patients according to their type of immunosuppressive therapy, it is unclear precisely how potent various immunosuppressive treatments truly are. To compare subgroups, we utilized multiple statistical comparisons, which potentially could result in a false positive. However, we are reassured that our finding of a lower response in patients receiving TNF-alpha inhibitors was also identified in the study by Mamula et al. [12] Lastly, some of the sample sizes in our subanalyses were small.
In summary, our study confirms the current recommendations that patients with IBD should receive influenza vaccine irrespective of their degree of immunosuppression. However, we and others have identified a group of patients (those receiving TNF-alpha inhibitor therapy) who are at risk of developing an inadequate serologic response. Future studies should attempt to validate this finding and to determine if booster immunizations are needed in this patient group, and to potentially evaluate the immunogenicity of other vaccines.
STUDY HIGHLIGHTS.
-
Current knowledge
Immunosuppressed people are at higher risk for complications from influenza.
Guidelines recommend inactivated influenza vaccine for IBD patients.
Paucity of data on immune response in IBD patients to influenza vaccine.
-
New knowledge
Influenza vaccine is safe and immunogenic in IBD patients.
Influenza vaccine was well tolerated (few side effects and no serious vaccine-associated adverse events).
Severity of disease activity was unaffected by immunization.
Most IBD patients have good immune response irrespective of immunosuppression status.
Patients receiving anti-TNF therapy had decreased response rate to strain B.
Acknowledgments
We thank the Thrasher Research Fund for their generous research grant in supporting this study. Part of this research was also supported by the Wolfman, Wolpow, Stefanis and MacInnes Families, and by the General Clinical Research Center (GCRC) grant # MO1-RR02172. This study could not be conducted without the help of the dedicated staff at the GCRC and Center for Ambulatory Treatment and Clinical Research (CATCR) at Children’s Hospital Boston. We also appreciate the help received from Dr. Zhiping Ye at the FDA/Center for Biologics Evaluation and Research (CBER) regarding data on the immune response to the influenza vaccine used during the 2007-2008 season in healthy children. We also thank Dr. Samuel Katz for reviewing the manuscript.
This research was funded by the Thrasher Research Fund and General Clinical Research Center (GCRC) grant # MO1-RR02172. We also received financial support from the Wolfman, Wolpow, Stefanis and MacInnes Families.
Footnotes
Ying Lu is the guarantor of this manuscript.
The following list describes the contributions from each author:
- Ying Lu was involved in the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and obtaining funding
- Denise L. Jacobson was involved in the conception and design of the study, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and supervision.
- Lori A. Ashworth was involved in the acquisition of data, critical revision of the manuscript for important intellectual content, and administrative support.
- Richard J. Grand was involved in the conception and design of the study, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, administrative support, and supervision.
- Anthony L. Meyer was involved in the acquisition of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and administrative support.
- Monica M. McNeal was involved in the acquisition of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and administrative support.
- Matt C. Gregas was involved in the analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and statistical analysis.
- Sandra K. Burchett was involved in the analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and supervision.
- Athos Bousvaros was involved in the conception and design of the study, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, administrative support, and supervision.
Please note that the Laboratory for Specialized Clinical Studies located at Cincinnati Children’s Hospital Medical Center was contracted to run the HAI assays on the sera. The lab was blinded to the treatment and vaccine given to study subjects while completing the HAI assays. This lab does contract work for a number of sponsors including Sanofi Pasteur, Inc., but has no conflict of interest and received no financial gain due to the results of this study. Dr. Bousvaros is on the Speaker’s Bureau and a sub-investigator on a clinical trial supported by Abbott. Drs. Bousvaros and Jacobson are the recipients of a research grant from Merck & Co., Inc.
CONFLICT OF INTEREST:
There are no competing interests.
References
- 1.Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12(30):4807–12. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 3.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411(6837):603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 4.Baldassano RN, Bradfield JP, Monos DS, Kim CE, Glessner JT, Casalunovo T, et al. Association of variants of the interleukin-23 receptor gene with susceptibility to pediatric Crohn’s disease. Clin Gastroenterol Hepatol. 2007;5(8):972–6. doi: 10.1016/j.cgh.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rufo PA, Bousvaros A. Current therapy of inflammatory bowel disease in children. Paediatr Drugs. 2006;8(5):279–302. doi: 10.2165/00148581-200608050-00002. [DOI] [PubMed] [Google Scholar]
- 7.Rau R, Fitzhugh CD, Baird K, Cortez KJ, Li L, Fischer SH, et al. Triad of severe abdominal pain, inappropriate antidiuretic hormone secretion, and disseminated varicella-zoster virus infection preceding cutaneous manifestations after hematopoietic stem cell transplantation: utility of PCR for early recognition and therapy. Pediatr Infect Dis J. 2008;27(3):265–8. doi: 10.1097/INF.0b013e31815cb239. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch DE, Olson AD, Kraker S, Dickinson CJ. Overwhelming varicella pneumonia in a patient with Crohn’s disease treated with 6-mercaptopurine. J Pediatr Gastroenterol Nutr. 1995;20(3):351–3. doi: 10.1097/00005176-199504000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Korelitz BI, Fuller SR, Warman JI, Goldberg MD. Shingles during the course of treatment with 6-mercaptopurine for inflammatory bowel disease. Am J Gastroenterol. 1999;94(2):424–6. doi: 10.1111/j.1572-0241.1999.871_w.x. [DOI] [PubMed] [Google Scholar]
- 10.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 11.Sands BE, Cuffari C, Katz J, Kugathasan S, Onken J, Vitek C, et al. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10(5):677–92. doi: 10.1097/00054725-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Mamula P, Markowitz JE, Piccoli DA, Klimov A, Cohen L, Baldassano RN. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5(7):851–6. doi: 10.1016/j.cgh.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56(RR-6):1–54. [PubMed] [Google Scholar]
- 14.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. Jama. 2004;292(11):1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 15.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Griffin MR, Coffey CS, Neuzil KM, Mitchel EF, Jr, Wright PF, Edwards KM. Winter viruses: influenza- and respiratory syncytial virus-related morbidity in chronic lung disease. Arch Intern Med. 2002;162(11):1229–36. doi: 10.1001/archinte.162.11.1229. [DOI] [PubMed] [Google Scholar]
- 17.Kempe A, Hall CB, MacDonald NE, Foye HR, Woodin KA, Cohen HJ, et al. Influenza in children with cancer. J Pediatr. 1989;115(1):33–9. doi: 10.1016/s0022-3476(89)80325-7. [DOI] [PubMed] [Google Scholar]
- 18.Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J. 2001;20(8):733–40. doi: 10.1097/00006454-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Shakra M, Press J, Varsano N, Levy V, Mendelson E, Sukenik S, et al. Specific antibody response after influenza immunization in systemic lupus erythematosus. J Rheumatol. 2002;29(12):2555–7. [PubMed] [Google Scholar]
- 20.Williams GW, Steinberg AD, Reinertsen JL, Klassen LW, Decker JL, Dolin R. Influenza immunization in systemic lupus eruthematosus. A double-blind trial. Ann Intern Med. 1978;88(6):729–34. doi: 10.7326/0003-4819-88-6-729. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzaki A, Suminoe A, Koga Y, Kinukawa N, Kusuhara K, Hara T. Immune response after influenza vaccination in children with cancer. Pediatr Blood Cancer. 2005;45(6):831–7. doi: 10.1002/pbc.20470. [DOI] [PubMed] [Google Scholar]
- 22.Escher JC. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis--the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41(1):1–7. doi: 10.1097/01.mpg.0000163736.30261.82. [DOI] [PubMed] [Google Scholar]
- 23.Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44(5):653–74. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 25.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12(4):439–47. [PubMed] [Google Scholar]
- 26.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1(8167):514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 27.Package insert. Influenza Virus Vaccine, Fluzone, No Preservative, 2007-2008 Formula. Sanofi Pasteur Inc.; Swiftwater, PA: 2007. [Google Scholar]
- 28.Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, et al. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. Jama. 2007;297(14):1577–82. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- 29.SPSS version 15.0. SPSS Inc.; Chicago, IL: [Google Scholar]
- 30.Chiu SS, Peiris JS, Chan KH, Wong WH, Lau YL. Immunogenicity and safety of intradermal influenza immunization at a reduced dose in healthy children. Pediatrics. 2007;119(6):1076–82. doi: 10.1542/peds.2006-3176. [DOI] [PubMed] [Google Scholar]
- 31.Hintze J. NCSS 2001 and PASS 2002: Number cruncher statistical systems. Kaysville, UT: 2004. [Google Scholar]
- 32.Ye Z. Influenza vaccine responses. 2008 http://www.fda.gov/ohrms/dockets/ac/08/slides/2008-4348S2-5_files/frame.htm.
- 33.Jarrett MP, Schiffman G, Barland P, Grayzel AI. Impaired response to pneumococcal vaccine in systemic lupus erythematosus. Arthritis Rheum. 1980;23(11):1287–93. doi: 10.1002/art.1780231110. [DOI] [PubMed] [Google Scholar]
- 34.Elkayam O, Ablin J, Caspi D. Safety and efficacy of vaccination against streptococcus pneumonia in patients with rheumatic diseases. Autoimmun Rev. 2007;6(5):312–4. doi: 10.1016/j.autrev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Nies K, Boyer R, Stevens R, Louie J. Anti-tetanus toxoid antibody synthesis after booster immunization in systemic lupus erythematosus. Comparison of the in vitro and in vivo responses. Arthritis Rheum. 1980;23(12):1343–50. doi: 10.1002/art.1780231203. [DOI] [PubMed] [Google Scholar]
- 36.Kubiet MA, Gonzalez-Rothi RJ, Cottey R, Bender BS. Serum antibody response to influenza vaccine in pulmonary patients receiving corticosteroids. Chest. 1996;110(2):367–70. doi: 10.1378/chest.110.2.367. [DOI] [PubMed] [Google Scholar]
- 37.Chalmers A, Scheifele D, Patterson C, Williams D, Weber J, Shuckett R, et al. Immunization of patients with rheumatoid arthritis against influenza: a study of vaccine safety and immunogenicity. J Rheumatol. 1994;21(7):1203–6. [PubMed] [Google Scholar]


