Abstract
We assessed the combination of sirolimus, tacrolimus and low-dose methotrexate as acute graft versus host disease prophylaxis after reduced intensity conditioning allogeneic peripheral blood stem cell transplantation from matched related (MRD, n=46) and unrelated (URD, n=45) donors. All patients received fludarabine and intravenous busulfan conditioning followed by transplantation of mobilized peripheral blood stem cells. The median time to neutrophil engraftment was 13 days. The cumulative incidence of grade II–IV and III–IV acute GVHD were 16% and 7%, respectively. There was no difference in the incidence of acute GVHD between MRD and URD cohorts. Two year cumulative incidence of extensive chronic GVHD was 40%. Relapse-free survival at two years was 34%: 21% in MRD and 45% in URD. Overall survival at two years was 59%: 47% in MRD and 67% in URD. High levels (>90%) of donor derived hematopoiesis were achieved in 59% of patients early after transplantation. The addition of sirolimus to tacrolimus and low-dose methotrexate as GVHD prophylaxis following reduced intensity conditioning with fludarabine and low dose intravenous busulfan is associated with rapid engraftment, low rates of acute GVHD, and achievement of high levels of donor chimerism.
Introduction
The development of acute graft-versus-host disease (GVHD) remains a barrier to successful allogeneic reduced intensity conditioning peripheral blood stem cell transplantation (PBSC). A variety of regimens to prevent the development of acute GVHD have been explored and an optimal regimen has yet to be defined. A calcineurin-inhibitor combined with either methotrexate or mycophenolate mofetil is commonly used.1,2 Other prophylaxis regimens have included the use of anti T-cell specific antibodies such as anti-thymocyte globulin (ATG) or alemtuzumab.3,4 Given the need to balance the prevention of acute GVHD with maintaining the graft versus malignancy effect, which is the curative aspect of reduced intensity conditioning transplantation, it is important that regimens prevent GVHD while not eliminating or reducing the graft versus malignancy effect.
Sirolimus is an inhibitor of the mammalian target of Rapamycin (mTOR). Sirolimus binds to FK binding protein 12 (FKBP12) forming a complex with mTOR and the raptor/rictor proteins5,6 This sirolimus-FKBP12-mTOR complex inhibits several pathways, resulting in a reduction in DNA transcription, DNA translation, protein synthesis and cell cycling, resulting in T cell immunosuppression and impairment of dendritic cell function. Moreover, in experimental models, sirolimus results in an expansion of CD4+CD25+FoxP3 regulatory T cells and does not impair GVL. 7,8,9
Sirolimus and calcineurin inhibitors agents work synergistically10 and have been used to prevent the development of acute GVHD after myeloablative allogeneic stem cell transplantation. The combination of sirolimus, tacrolimus and low-dose methotrexate has been demonstrated to be effective GVHD prophylaxis in HLA-matched and partially mismatched URD transplantation.11 Sirolimus and tacrolimus without methotrexate also have been used successfully to prevent acute GVHD in both matched related and unrelated donor myeloablative allogeneic PBSC transplantation.12,13 In this report, we demonstrate the effectiveness of combining sirolimus, tacrolimus and low-dose methotrexate as prophylaxis against acute GVHD for patients receiving reduced intensity conditioning allogeneic transplantation from matched related or unrelated donors for the treatment of hematologic malignancies.
Methods
Study Design
Patients with hematologic malignancies receiving unmanipulated HLA matched allogeneic transplantation from related or unrelated donors were eligible. Transplantation eligibility requirements included ECOG performance status 0–2, absence of uncontrolled infection at the time of study entry, left ventricular ejection fraction >30%, and normal or near normal parameters of kidney and liver function. All patients receiving reduced intensity conditioning were evaluated for myeloablative transplantation and considered to have contraindications to that approach. Relative contraindications to myeloablative transplantation included prior myeloablative transplantation, age >50 years, or significant organ dysfunction. The Human Subjects Protection Committee of Dana-Farber Cancer Institute approved investigational protocols. Written informed consent was obtained in all cases.
All donors included in this analysis were HLA matched at A, B, and DR loci. Unrelated donors were required to match recipients at HLA-DR loci by molecular analysis. Class II typing was performed with sequence specific oligonucleotide probes (SSOP). All patients received filgrastim mobilized peripheral blood stem cells (PBSC). Donors were mobilized with filgrastim at 10 μg /kg/day for 5 days. Stem cell collection was initiated on the fifth day of filgrastim and continued until a sufficient number of CD34+ cells were obtained. Target cell dose was 1 × 107 CD34+ cells/kg. PBSCs were mobilized from unrelated donors according to local donor center practices. The first day of stem cell infusion corresponded to Day 0.
Study Therapy
Patients received fludarabine (30 mg/m2/day on days –6, -5, -4, -3) and intravenous busulfan (0.8 mg/kg/d days –6, -5, -4, -3). Tacrolimus was administered by mouth at 0.05 mg/kg/day starting on day –3 with a target serum concentration of 5–10 ng/mL. Sirolimus was administered as a 12 mg oral loading dose on day –3, followed by a 4 mg/day single dose, with a target serum concentration of 3–12 ng/mL by HPLC. Methotrexate was administered at 5 mg/m2 intravenously days 1, 3 and 6 after transplantation. Filgrastim was administered at 5 μg/kg/day beginning on day +1 and was continued until neutrophil engraftment. Patients received prophylactic anti-viral therapy against herpes virus infections and prophylaxis against Pneumocystis carinii. No prophylactic antifungal therapy was administered. All patients were monitored for CMV reactivation, without prophylactic therapy. Acute GVHD was graded according to the consensus grading scale14. If a patient developed evidence of recurrent disease, taper of the immune suppression was at the discretion of the treating physician.
Chimerism Analysis
Unfractionated donor chimerism was assessed from bone marrow aspirates at approximately day +30–45 post transplant. Genotype of donor and recipient were determined using DNA extracted from pre transplant samples. Nine short tandem repeat (STR) loci were typed using the ABI Profiler Plus Kit (Applied Biosystems Inc) and the ABI 310 Genetic Analyzer to resolve alleles. “Informative” alleles that were present only in the donor or recipient were used in the chimerism calculations. In cases of gender mismatched donor-recipient pairs where molecular chimerism analysis was not available, assessment of donor chimerism was based on fluorescent in-situ hybridization (FISH) for X and Y chromosomes, or cytogenetic analysis.
Statistical Analysis
Descriptive statistics was provided for patient baseline characteristics. Two-sided Fisher’s exact test was used to compare incidence of GVHD, and two-sided Wilcoxon-Rank-Sum test was used for two-sample comparison of number of stem cells infused and time to engraftment.
Cumulative incidence curves for aGVHD, cGVHD and relapse were constructed reflecting early death, death and relapse, and non-relapse treatment-related death as competing risks, respectively15. Time to relapse and time to treatment-related death were measured from the date of stem cell infusion. Patients who were alive without relapse were censored at the time last seen alive. Overall survival (OS) and relapse-free survival (RFS) were calculated using the Kaplan-Meier method. Overall survival was defined as the time from stem cell infusion to death from any cause. Relapse-free survival was defined as the time from stem cell infusion to relapse or death from any cause. The Log-rank test was used for group comparisons of OS or RFS. Prognostic factors for OS and RFS were examined in the Cox proportional hazards model.
Results
Patient Characteristics
The characteristics of all 91 patients are detailed in Table 1. Forty-five patients (49%) had an unrelated donor and 46 patients (51%) had a related donor. Thirty-seven patients (41%) had AML or MDS and these diagnoses were the most common indication for transplantation. Low risk disease was defined as acute leukemia in CR1, de-novo early stage myelodysplastic syndrome (RA or RARS), and first chronic phase CML. Seventeen patients (19%) had low risk disease and 74 patients (81%) had advanced stage disease at the time of transplantation. The median age was 57 years (range 20 to 69 years). Thirty-two patients (35%) had undergone prior myeloablative transplantation. The median follow-up for those still alive is 24 months (range 3.4 to 44 months).
Table 1.
Baseline Characteristics
| Sample Size | 91 |
|---|---|
| Median Age (Range) | 57 (20, 69) |
| Sex | |
| Male | 62 (68%) |
| Female | 29 (32%) |
| Gender mismatch (donor/recipient) | |
| M/M, F/F | 46 (50%) |
| F/M, M/F | 45 (50%) |
| Disease | |
| AML | 24 (26%) |
| CR1 | 10 |
| >CR1 | 14 |
| CML | 7 (8%) |
| CP | 5 |
| AP/BC | 2 |
| CLL | 17 (19%) |
| NHL | 18 (20%) |
| HD | 6 (7%) |
| MDS | 13 (15%) |
| RA/RARS | 5 |
| RAEB/RAEBT | 8 |
| Myeloproliferative Diseases | 3 (3%) |
| ALL/>CR 1 | 1 (1%) |
| Plasma Cell Dyscrasia | 2 (2%) |
| Prior Transplantation | 32 (35%) |
| Remission Status at Time of Transplant | |
| Complete Remission/First Chronic Phase | 27 (30%) |
| Active Disease | 64 (70%) |
| Risk Status | |
| High Risk | 74 (81%) |
| Low Risk | 17 (19%) |
Stem Cell Product and Engraftment
All patients received unmanipulated PBSC. The median number of CD34+ cells infused was 8.6 × 106 CD34+ cells/kg (range 2.4 × 106 to 2.9 × 106). Unrelated donors received a median of 9.5 × 106 CD34+ cells/kg (range 2.4 × 106 to 29 × 106), which was statistically higher than MRD recipients with a median of 7.8 × 106 CD34+ cell/kg (range 2.5 × 106 to 19 × 106)(p=0.02).
All patients engrafted. The majority of patients (52%) did not develop neutropenia (ANC<500). Of the 44 patients who did develop neutropenia or who were neutropenic prior to receiving the conditioning regimen, the median time to neutrophil recovery (defined as day to absolute neutrophil count >500) was 13 days (range 3 to 32). There were no significant differences in the time to engraftment of MRD and URD donors. Donor derived hematopoiesis was assessed at day 30 to 45 after transplantation. By day 45, 89% of patients had achieved >50% donor derived hematopoiesis with 59% of all patients achieving >90% donor derived hematopoiesis.
Acute and Chronic Graft-versus-Host Disease
In a competing risk model, the cumulative incidence of grade II–IV acute GVHD was 10% by day 100 and 16% by day 200 post transplantation (Figure 1). Of the 14 patients developing grade 2–4 acute GVHD, 8 (9%) developed grade 2 acute GVHD and 6 patients (7%) developed grade 3–4 acute GVHD. There was a difference in incidence of acute GVHD between MRD (11%) and URD (20%) cohorts, however, this difference was not statistically significant (p=0.22). Eight patients were mismatched at HLA-C (7 URD and 1 MRD). One of these eight patients developed grade II acute GVHD.
Figure 1.
Cumulative incidence of grade 2–4 acute graft versus host disease after transplantation. MRD: Matched Related Donor, URD: Unrelated Donor
Two-year cumulative incidence of extensive chronic GVHD was 40%. There was a trend for a higher incidence of extensive chronic GVHD in recipients of URD transplantation (49%) compared with recipients of MRD transplantation (30%)(p=0.06). The median time to onset was 6.5 months (range 3–12 months). The majority of patients developed de novo chronic GVHD (81%) while a small number of patients progressed from acute to chronic GVHD (19%). There was no correlation between the development of acute GVHD and the development of chronic GVHD.
Survival and Outcome
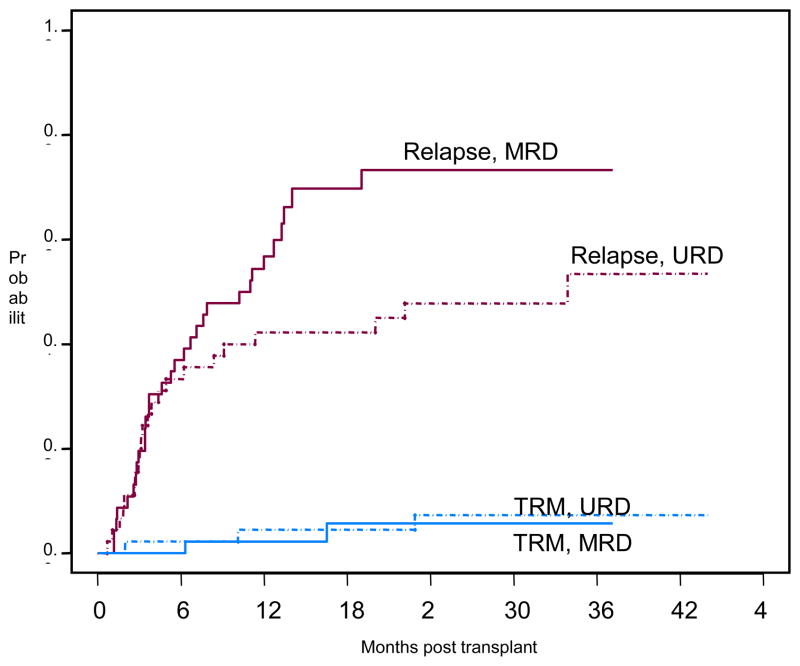
Non-relapse mortality at 100 days was 1%. Two-year cumulative incidence of treatment related mortality (TRM) was 6%. Non-relapse causes of treatment failure included GVHD (1), infection (2) and pulmonary toxicity (3).(Figure 2) No patients developed VOD or evidence of TTP/HUS. The primary cause for treatment failure was relapse of disease. Overall survival (OS) estimates at 1 and 2 years are 74% and 59%, respectively (Figure 3a). Relapse free survival (RFS) after transplantation at 1 and 2 years are 47% and 34%, respectively. (Figure 3b). Although the URD cohort showed superior OS and RFS, neither was statistically significantly different (p=0.37, p=0.1, respectively). The cumulative incidence of relapse at 2 years is 60%. The cumulative risk of relapse for patient with unrelated donors was 42% compared with 73% for patients with matched related donors but this difference does not reach statistical significance (p=0.09).
Figure 2.
Cumulative incidence of relapse with treatment related mortality as a competing risk. MRD: Matched Related Donor; URD: Unrelated Donor; TRM: treatment related mortality
Figure 3.
Figure 3a-Overall Survival
Figure 3b-Progression Free Survival
Factors Associated with Outcome and Toxicity
Cox regression analysis was performed to identify factors associated with overall survival, relapse free survival and treatment related mortality. Factors analyzed included age, patient-donor sex mismatch, donor type (related versus unrelated), history of a prior transplant, risk status at time of transplantation, recipient CMV status, number of CD34 cells infused, and day 30–45 chimerism. Patients with positive CMV status had a shorter overall survival (HR=2.1, p=0.06). Patients receiving a higher dose of CD34+ cells (>5× 106 CD34+ cells/kg) had an improved RFS (HR=0.43, p=0.02) but not overall survival. No other factors including chimerism were found to be significant.
Discussion
The development of acute GVHD after reduced intensity conditioning (RIC) transplantation contributes to transplant related morbidity and mortality, as well as prevents the administration of post transplant immune therapies for patients with persistent disease. While initially hypothesized that the incidence of acute GVHD would be lower after non-myeloablative regimens because of less tissue damage resulting in lack of pro-inflammatory cytokines, the incidence of acute GVHD after RIC transplantation remains significant. Two strategies have been pursued to prevent the development of acute GVHD; pharmacologic prophylaxis often using calcineurin inhibitors, or T cell depletion either through the use of anti-T cell antibodies such as ATG or alemtuzumab or cell separation technologies. In RIC transplantation defining the correct balance between controlling GVHD by suppression of T cell function and preserving graft versus leukemia activity is critical since the success of RIC transplantation is based on the preservation of the GVL effect. In the current report, we used a potent immunosuppressive agent, sirolimus, combined with tacrolimus and low-dose methotrexate and demonstrate low rates of acute GVHD for both related and unrelated donors after receiving RIC allogeneic PBCS transplantation.
Sirolimus has been effective in reducing the incidence of acute GVHD after myeloablative stem cell transplantation. When combined with low-dose methotrexate, sirolimus improved GVHD control in patients receiving transplantation from mismatched related and unrelated donors.11 Sirolimus combined with tacrolimus without the use of methotrexate also results in excellent prevention of acute GVHD in both matched related and unrelated donor myeloablative stem cell transplantation and is associated with a low treatment related mortality.13 A randomized trial comparing tacrolimus and sirolimus as GVHD prophylaxis with tacrolimus and methotrexate in patients undergoing matched related myeloablative transplantation is currently underway to confirm these findings. An increased risk of VOD has been noted in patients receiving sirolimus as GVHD after myeloablative transplantation.16 In the current study, no patients developed VOD or TTP/HUS. This suggests that the endothelial or other tissue damage mediated by myeloablative conditioning in the setting of sirolimus administration is needed to predispose to VOD.
The incidence of acute GVHD noted in this study of RIC compares favorably with other pharmacologic prophylactic strategies of GVHD after RIC transplantation. Rates for developing acute GVHD for patients receiving cyclosporine and MMF range from 16%–47% for related donors17,18 and in reports of URD transplantation up to52%.19 Reports have suggested that cyclosporine and MMF may not be sufficient to prevent GVHD in unrelated donors.20 Rates for developing acute GVHD after RIC transplantation using tacrolimus or cyclosporine and methotrexate range from 12%–36% for patients with related and unrelated donors21,22 Incorporation of alemtuzumab as GVHD prophylaxis is associated with a low incidence of acute and chronic GVHD4, however, many patients have persistent or progressive disease after transplantation suggesting that some element of the GVL effect has been compromised.
Sirolimus, in addition to its immune suppressive properties which prevent acute GVHD, has several potential advantages including possible anti-tumor activity and may facilitate engraftment of donor cells. Sirolimus has been identified as having direct anti-tumor activity in several hematologic malignancies23. Anti-tumor activity has been demonstrated in vitro in AML, CLL, multiple myeloma and lymphoma.24–29 The anti-leukemic activity of rapamycin appears to be mediated through inhibition of the mTOR pathway and inhibits immature cells by blockade in G0/G1 phase of the cell cycle. Demonstrating in vivo activity, 4 of 9 patients with refractory/relapsed AML demonstrated significant clinical responses following treatment with sirolimus30. Preliminary results incorporating sirolimus into a RIC regimen in a group of patients with high risk leukemia suggested improved disease control after transplant.31 In the current study, despite the use of sirolimus relapse of disease remains the principle reason for treatment failure. It is unclear if the doses used to prevent acute GVHD are sufficient to mediate anti-tumor activity. Larger series will be needed to assess the impact of sirolimus on disease control in specific diseases.
High levels of donor derived hematopoeisis early after RIC transplantation were noted in patients in this trial. Several studies have demonstrated that high levels of donor derived hematopoiesis predict improved outcome for certain patient populations after RIC transplantation.32–34 Preclinical data suggests that sirolimus combined with cyclosporine facilitates stable mixed chimerism in animals given sublethal total body irradiation35,36 and facilitates the development of tolerance in a mismatched rodent stem cell transplant model37. Comparison of sirolimus containing GVHD prophylactic regimens with other regimens is needed to see if the addition of sirolimus augments donor chimerism and if this can be translated into improved outcome.
Patients receiving transplantation from a URD had a marginally improved relapse-free survival (p=0.1) when compared with patients receiving transplantation from a MRD. This observation is consistent with other publications of RIC transplantation.38 Presumably the increased genetic disparity between donor and host may also facilitate greater donor-versus-malignancy effect. With improved control of acute GVHD and resulting reduction in treatment related mortality associated with URD transplantation, the impact of donor source on disease control is now recognizable.
Sirolimus when combined with tacrolimus and low-dose methotrexate provide adequate prophylaxis against the development of acute GVHD in patients undergoing RIC PBSC transplantation from related and unrelated donors. This combination was safe and well tolerated in a patient population that was at high risk for complications after transplantation. Given that sirolimus has several properties that may be advantageous when used after RIC transplantation, such as anti-tumor activity and facilitation of chimerism, this agent when used in combination with other agents should be compared with other GVHD prophylactic regimens.
Table 2.
Cause of Treatment Failure
| Cause of Treatment Failure | N |
|---|---|
| Relapse | 34 (77%) |
| GVHD | 1 (2%) |
| Infection | 2 (5%) |
| Idiopathic Pneumonia Syndrome | 3 (7%) |
Acknowledgments
Supported in part by P01 HL070149 from the National Heart, Lung, and Blood Institute, an unrestricted educational grant from (Protein Design Labs), the Ted and Eileen Pasquarello Research Fund, and the Jock and Bunny Adams Education and Research Fund
Footnotes
Scientific Heading: Transplantation
Authorship Statement
Study Design: Alyea, Antin, Kim
Patient Accrual and Care: Alyea, Ho, Cutler, Soiffer, Antin
Data Collection and Analysis: Alyea, Li, Kim
Manuscript Preparation: Alyea
Manuscript Review: Kim, Cutler, Li, Ho, Soiffer, Antin
The Authors have no financial conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 2.McSweeney P, Wagner J, Maloney D, et al. Outpatient PBSC allografts using immunosuppression with low-dose TBI before and cyclosporine (CSP) and mycophenolate mofetil (MMF) after transplant. Blood. 1998;92 (Suppl 1):519a. [Google Scholar]
- 3.Slavin S, Ackerstein A, Weiss L, Nagler A, Or R, Naparstek E. Immunotherapy of minimal residual disease by immunocompetant lymphocytes and their activation by cytokines. Cancer Investigation. 1992;10:221–227. doi: 10.3109/07357909209032764. [DOI] [PubMed] [Google Scholar]
- 4.Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96:2419–2425. [PubMed] [Google Scholar]
- 5.Adawi A, Zhang Y, Baggs R, et al. Blockade of CD40-CD40 ligand interactions protects against radiation- induced pulmonary inflammation and fibrosis. Clin Immunol Immunopathol. 1998;89:222–230. doi: 10.1006/clin.1998.4606. [DOI] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 7.Baan CC, van der Mast BJ, Klepper M, et al. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation. 2005;80:110–117. doi: 10.1097/01.tp.0000164142.98167.4b. [DOI] [PubMed] [Google Scholar]
- 8.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 10.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 11.Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102:1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 12.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:328–336. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 13.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2006 doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1994;15:825–828. [PubMed] [Google Scholar]
- 15.Gray R. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 16.Cutler C, Stevenson K, Kim H, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative transplantation. BMT. 2008;41(S1):s7. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieto Y, Patton N, Hawkins T, et al. Tacrolimus and mycophenolate mofetil after nonmyeloablative matched-sibling donor allogeneic stem-cell transplantations conditioned with fludarabine and low-dose total body irradiation. Biol Blood Marrow Transplant. 2006;12:217–225. doi: 10.1016/j.bbmt.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 19.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 20.Loren AW, Luger SM, Stadtmauer EA, et al. Intensive graft-versus-host disease prophylaxis is required after unrelated-donor nonmyeloablative stem cell transplantation. Bone Marrow Transplant. 2005;35:921–926. doi: 10.1038/sj.bmt.1704887. [DOI] [PubMed] [Google Scholar]
- 21.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10:178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 22.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 23.Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Curr Treat Options Oncol. 2006;7:285–294. doi: 10.1007/s11864-006-0038-1. [DOI] [PubMed] [Google Scholar]
- 24.Yan H, Frost P, Shi Y, et al. Mechanism by which mammalian target of rapamycin inhibitors sensitize multiple myeloma cells to dexamethasone-induced apoptosis. Cancer Res. 2006;66:2305–2313. doi: 10.1158/0008-5472.CAN-05-2447. [DOI] [PubMed] [Google Scholar]
- 25.Mirshahi P, Toprak SK, Faussat AM, et al. Malignant hematopoietic cells induce an increased expression of VEGFR-1 and VEGFR-3 on bone marrow endothelial cells via AKT and mTOR signalling pathways. Biochem Biophys Res Commun. 2006;349:1003–1010. doi: 10.1016/j.bbrc.2006.08.132. [DOI] [PubMed] [Google Scholar]
- 26.Leseux L, Hamdi SM, Al Saati T, et al. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006;108:4156–4162. doi: 10.1182/blood-2006-05-026203. [DOI] [PubMed] [Google Scholar]
- 27.Vega F, Medeiros LJ, Leventaki V, et al. Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res. 2006;66:6589–6597. doi: 10.1158/0008-5472.CAN-05-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ringshausen I, Peschel C, Decker T. Mammalian target of rapamycin (mTOR) inhibition in chronic lymphocytic B-cell leukemia: a new therapeutic option. Leuk Lymphoma. 2005;46:11–19. doi: 10.1080/10428190400005353. [DOI] [PubMed] [Google Scholar]
- 29.Hipp S, Ringshausen I, Oelsner M, Bogner C, Peschel C, Decker T. Inhibition of the mammalian target of rapamycin and the induction of cell cycle arrest in mantle cell lymphoma cells. Haematologica. 2005;90:1433–1434. [PubMed] [Google Scholar]
- 30.Recher C, Beyne-Rauzy O, Demur C, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105:2527–2534. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 31.Claxton DF, Ehmann C, Rybka W. Control of advanced and refractory acute myelogenous leukaemia with sirolimus-based non-myeloablative allogeneic stem cell transplantation. Br J Haematol. 2005;130:256–264. doi: 10.1111/j.1365-2141.2005.05600.x. [DOI] [PubMed] [Google Scholar]
- 32.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 33.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Brown J, Kim H, Li S, et al. Predictors of Improved Progression-Free Survival Following Nonmyeloablative Allogeneic Stem Cell Transplantation for Advanced CLL. Biology of Blood and Marrow Transplantation. 2006 doi: 10.1016/j.bbmt.2006.06.004. in press. [DOI] [PubMed] [Google Scholar]
- 35.Hogan WJ, Little MT, Zellmer E, et al. Postgrafting immunosuppression with sirolimus and cyclosporine facilitates stable mixed hematopoietic chimerism in dogs given sublethal total body irradiation before marrow transplantation from DLA-identical littermates. Biol Blood Marrow Transplant. 2003;9:489–495. doi: 10.1016/s1083-8791(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 36.Powell JD, Fitzhugh C, Kang EM, Hsieh M, Schwartz RH, Tisdale JF. Low-dose radiation plus rapamycin promotes long-term bone marrow chimerism. Transplantation. 2005;80:1541–1545. doi: 10.1097/01.tp.0000185299.72295.90. [DOI] [PubMed] [Google Scholar]
- 37.Jager MD, Liu JY, Timrott KF, et al. Sirolimus promotes tolerance for donor and recipient antigens after MHC class II disparate bone marrow transplantation in rats. Exp Hematol. 2007;35:164–170. doi: 10.1016/j.exphem.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Sorror ML, Maris MB, Sandmaier BM, et al. Hematopoietic Cell Transplantation After Nonmyeloablative Conditioning for Advanced Chronic Lymphocytic Leukemia. J Clin Oncol. 2005 doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]