Abstract
An increased incidence of cardiovascular complications has been documented in recipients of allogeneic hematopoietic stem cell transplantation (HSCT). Despite this, little is known about the risk factors for hyperlipidemia or the role of lipid-lowering therapy early after transplantation. We performed a retrospective analysis of all patients who underwent allogeneic HSCT at the Dana-Farber Cancer Institute from 1998 to 2008 and who survived more than 100 days. The incidence of hypercholesterolemia and hypertriglyceridemia in the first two years after transplantation was 73.4% and 72.5%, respectively. In multivariable analysis, the development of acute graft-versus-host disease was independently associated with both hypercholesterolemia (O.R. 1.62) and hypertriglyceridemia (O.R. 1.54) after transplantation. Statin use was instituted in 29% of patients and was associated with a significant net reduction in total cholesterol (65 mg/dL, P<0.0001), triglyceride (118 mg/dL P<0.0001), and LDL levels (59 mg/dL P<0.0001) without any significant adverse effects. These data suggest that hyperlipidemia is common in the first two years after allogeneic transplantation when most patients remain under the care of the transplantation physician and lipid-lowering therapy may be under-utilized. Given the cardiovascular risk associated with hyperlipidemia and the tolerability of statins, further prospective evaluation of lipid abnormalities and their treatment seems well warranted.
Introduction
There is a growing appreciation that coronary artery disease and myocardial infarction are relatively common late complications of allogeneic hematopoietic stem cell transplantation (HSCT)[1–3]. Hyperlipidemia is a well-known risk factor for atherosclerotic heart disease [4]; several reports have documented the incidence of hyperlipidemia and cardiovascular events late after HSCT [1, 3, 5–8]. However, little is known about the incidence of hyperlipidemia within the first two years. If there is a characteristic pattern of changes in lipid profiles early after transplantation, more aggressive lipid modification therapy at that time may help ameliorate late cardiovascular complications.
HMG-CoA reductase inhibitors (statins) are the mainstay of treatment for hyperlipidemia, have salutary effects on LDL, and are widely used in the general population [9]. However because of the concern of potential liver toxicity, drug interactions, and monitoring for liver graft-versus-host disease (GVHD), they are often discontinued at the time of HSCT and may not be started after transplantation. The reported rate of statin use in HSCT patients ranges from 5% to 15% in previously reported series, though it is unclear whether their use is safe and/or effective in this setting [10–12].
We performed a retrospective chart review on all patients who underwent allogeneic HSCT at the Dana-Farber Cancer Institute/Brigham and Women’s Hospital from 1998 to 2008, survived more than 100 days post-transplantation, and had lipid measurements after transplantation. We describe the incidence and time course of hyperlipidemia after allogeneic HSCT, identify risk factors for hyperlipidemia, and examine the use and effect of statin therapy for hyperlipidemia.
Methods
Patient population
The subjects of this retrospective study were 1493 consecutive patients who underwent allogeneic HSCT at the Dana-Farber Cancer Institute from 1998–2008 and who survived more than 100 days following transplantation. A total of 732 subjects had no total cholesterol measurements from day 30 to two years after transplantation and were excluded from the analysis. Patients were treated according to investigational protocol, if applicable, or institutional standard of care. Myeloablative conditioning regimens included cyclophosphamide (1800 mg/m2 × 2 days) plus total body irradiation (14 Gy total in 7 fractions over 4 days) or busulfan (16 mg/kg orally or 12.8 mg/kg intravenously total) in 16 divided doses. Reduced intensity conditioning (RIC) regimens consisted of fludarabine (30 mg/m2 intravenously) and busulfan (0.8 or 1.6 mg/kg intravenously) for 4 days. GVHD prophylaxis consisted predominantly of a calcineurin inhibitor and methotrexate, tacrolimus plus sirolimus with or without low dose methotrexate, or ex vivo T cell depletion with or without additional immune suppression. Lipid profiles were analyzed by standard methods either at the discretion of the primary transplantation physician or according to protocol. Routine practice was for lipid profiles to be drawn after a minimum 9-hour fast. This retrospective analysiswas approved by the Office for the Protection of Research Subjectsat the Dana-Farber CancerInstitute.
Data extraction
Patient and donor characteristics, stem cell source, conditioning and prophylactic regimens, and incidence and severity of acute and chronic GVHD were retrieved from the BMT data repository at the Dana-Farber Cancer Institute. Lipid values and outpatient medication history were accessed via an electronic medical record system covering more than ten hospitals and associated clinics in the greater Boston area. Hyperlipidemia was defined as any outpatient total cholesterol value ≥ 200 mg/dL or triglyceride value ≥ 200 mg/dL. These cutoffs were derived from current treatment guidelines[4]. Lipid values obtained within 30 days of transplantation were excluded from the statistical analysis. Patients with any 30-day prescription for a statin listed among their current or discontinued medications were considered to have been treated with statins. Only prescriptions starting from day 0 to two years after transplantation were considered.
Statistical analysis
Patient baseline characteristics were reported descriptively and the χ2 test or the Wilcoxon-Rank-Sum test was used for group comparison. Differences in lipid levels between pre- and post-transplantation measurements were tested using the Wilcoxon-signed rank test. Whether the status of hyperlipidemia was in agreement before and after transplantation was tested using McNemar’s test. Cumulative incidence of acute and chronic GVHD was calculated reflecting time to relapse or death without developing GVHD as a competing risk. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method. Potential prognostic factors for hyperlipidemia after HSCT were examined in the univariable and multivariable logistic regression analysis. The functional form of year of transplantation was examined using restricted cubic spline function[13]. All tests were two-sided. All calculations were performed using SAS 9.2 (SAS Institute, Cary, NC) and R 2.10.1 (The CRAN project).
Results
Patient characteristics
The characteristics of the patients included in the study are detailed in Table 1. Among the 761 patients included in the study, median age at transplantation was 49 years (range 17–73), 60% had myeloid neoplasms, 42% received grafts from matched related donors, 46% from matched unrelated donors, and 55% received myeloablative conditioning. Greater than 95% of patients received tacrolimus-based GVHD prophylaxis. Sirolimus was included as GVHD prophylaxis for 50%. The cumulative incidence of grade II-IV acute GVHD was 26% at 200 days after transplantation; the cumulative incidence of chronic GVHD at 2 years after transplantation was 60% (Table 2). Also listed in Tables 1 and 2 are the baseline characteristics and cumulative incidence of GVHD for 732 excluded patients who underwent HSCT within the same time period, were alive at day 100, but did not have lipid values checked within two years after transplantation. The patients who had lipid values checked were older (P=0.001), more likely to be male (P=0.016), more likely to have received peripheral blood stem cell grafts (P<0.001), more likely to have received sirolimus-based GVHD prophylaxis (P<0.001), more likely to have received RIC regimens (P=0.047), and were more likely to be overweight (P=0.02) compared with patients who did not have their lipid values checked (Table 1). Lipid values were checked more frequently in the year 2002 and later than before 2002 (P<0.001, Table 1). There was no difference in the cumulative incidence of acute GVHD at 200 days after transplantation in either group, though chronic GVHD was more common in those who had their lipids checked (P<0.001, Table 2).
Table 1. Patient and Transplant Characteristics.
Table of baseline and transplant characteristics for patients included and excluded from the study.
| Included (n=761) | Excluded (n=732) | ||||
|---|---|---|---|---|---|
| N | % | N | % | P | |
| Age at HSCT | 0.001 | ||||
| < 50 | 397 | 52 | 444 | 61 | |
| ≥ 50 | 364 | 48 | 288 | 39 | |
| median age (range) | 49 (17*-73) | 46 (18–73) | 0.001 | ||
| Patient Gender | 0.016 | ||||
| M | 462 | 61 | 399 | 55 | |
| F | 299 | 39 | 333 | 45 | |
| Patient-Donor Gender | 0.046 | ||||
| M<-F | 201 | 26 | 160 | 22 | |
| other | 560 | 74 | 572 | 78 | |
| Donor Type | 0.21 | ||||
| Matched unrelated | 350 | 46 | 299 | 41 | |
| Matched related | 322 | 42 | 343 | 47 | |
| Mismatched unrelated | 80 | 11 | 78 | 11 | |
| Mismatched related | 9 | 1 | 12 | 2 | |
| Cell Source | <0.001 | ||||
| Bone Marrow | 146 | 19 | 258 | 35 | |
| PBSC | 575 | 76 | 444 | 61 | |
| Cord Blood | 36 | 5 | 30 | 4 | |
| Acute GVHD prophylaxis | <0.001 | ||||
| sirolimus-containing | 384 | 50 | 248 | 34 | |
| no sirolimus | 377 | 50 | 484 | 66 | |
| Conditioning Regimen | 0.047 | ||||
| Myeloablative | 419 | 55 | 441 | 60 | |
| RIC | 342 | 45 | 291 | 40 | |
| Diagnosis | 0.38 | ||||
| Myeloid Malignancy | 455 | 60 | 412 | 56 | |
| Lymphoid Malignancy | 272 | 36 | 298 | 41 | |
| Other | 34 | 4 | 22 | 3 | |
| Body mass index (BMI) at HSCT | 0.048 | ||||
| median (range) | 27 (16–65) | 26 (17–69) | |||
| Overweight at HSCT (BMI ≥ 25) | 0.02 | ||||
| No | 254 | 33 | 287 | 39 | |
| Yes | 507 | 67 | 445 | 61 | |
| Obese at HSCT (BMI ≥ 30) | 0.77 | ||||
| No | 552 | 73 | 536 | 73 | |
| Yes | 209 | 27 | 196 | 27 | |
one patient was 17y 9mo at the time of HSCT
Table 2. Cumulative Incidence of Acute and Chronic GVHD.
Cumulative incidence of acute and chronic GVHD for patients included and excluded from the study.
| Included | Excluded | P | ||
|---|---|---|---|---|
| Acute GVHD Gr II-IV | 100 day CI | 23% | 26% | 0.43 |
| 200 day CI | 26% | 28% | ||
| Chronic GVHD | 1 year CI | 53% | 42% | <0.001 |
| 2 year CI | 60% | 47% |
Frequency of hyperlipidemia
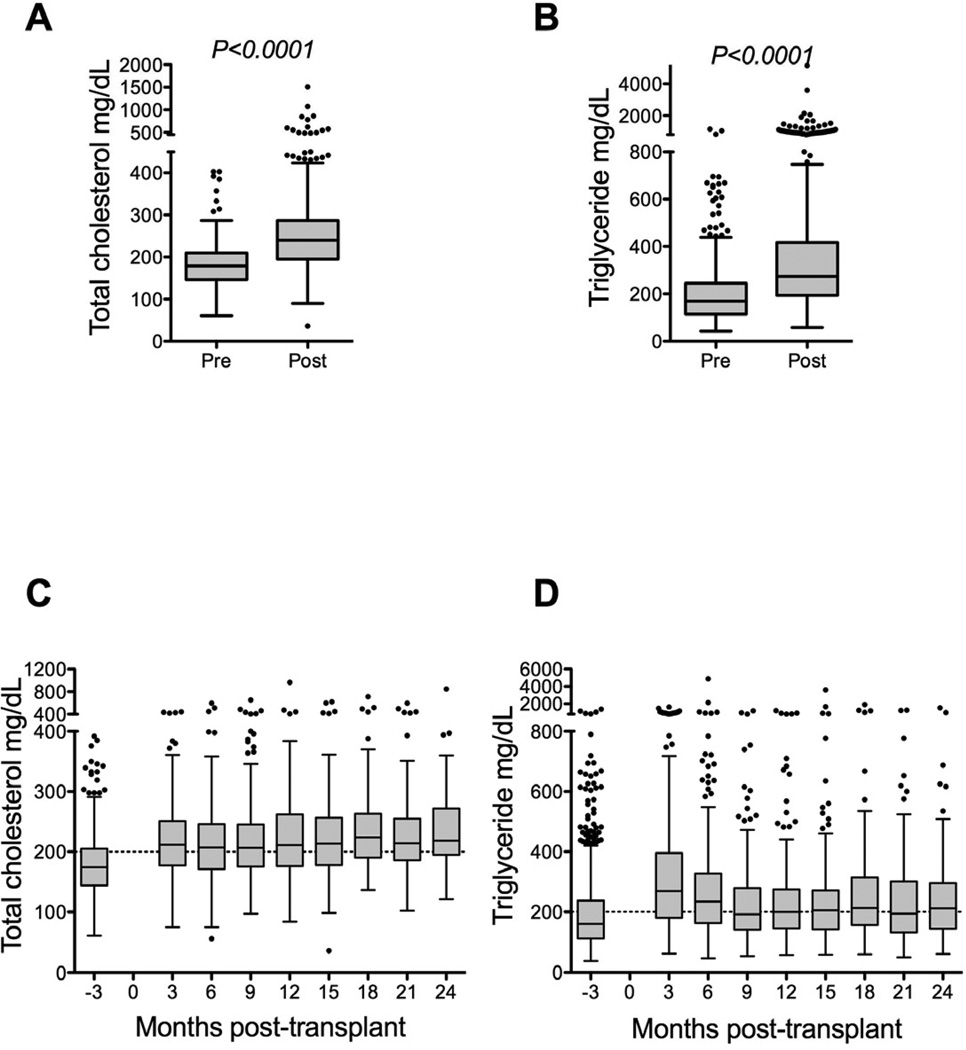
Of the 761 patients included in the study, 556 (73.4%) had at least one post-transplantation total cholesterol value greater than or equal to 200 mg/dL and were considered hypercholesterolemic according to the ATP-III treatment guidelines (Table 3). Five hundred sixty patients also had at least one total cholesterol value recorded in the 90 days prior to transplantation. Of these, 179 (32.0%) were hypercholesterolemic prior to transplantation (Table 3). Among the 560 patients with both pre- and post-transplantation values, the median peak total cholesterol levels before and after transplantation were 178 and 241 mg/dL, respectively (pre-post change: 62 mg/dL, P<0.0001, Figure 1A). Post-transplantation hypertriglyceridemia (defined as any triglyceride measurement greater than or equal to 200 mg/dL) was present in 531 patients (72.5%, Table 3). Five hundred seventy three patients had pre-transplantation triglyceride levels measured. Of these, 225 (39.3%) were hypertriglyceridemic (Table 3). The number of patients with both pre- and post-transplantation triglyceride values was 554; among these, the median peak triglyceride levels before and after transplantation were 171 and 275 mg/dL, respectively (pre-post change: 109 mg/dL, P<0.0001, Figure 1B). Compiled total cholesterol and triglyceride values for the first two years after transplantation are presented in Figure 1C and 1D.
Table 3. Overall Frequency of Hyperlipidemia.
Frequency of hyperlipidemia in allogeneic HSCT recipients. Cutoffs were derived from current ATP-III treatment guidelines.
| Pre-Transplantation | Post-Transplantation | |||||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Total cholesterol | 0 -199 mg/dL | 381 | 68.0 | 201 | 26.6 | |
| ≥ 200 mg/dL | 179 | 32.0 | 556 | 73.4 | ||
| Triglyceride | 0 -199 mg/dL | 348 | 60.7 | 201 | 27.5 | |
| ≥ 200 mg/dL | 225 | 39.3 | 531 | 72.5 | ||
Figure 1.
(A, B) Box and whisker plots demonstrating peak total cholesterol (A, N=560) and triglyceride (B, N=554) values within the first 90 days prior to transplantation (pre) and 30 days to two years after transplantation (post). Only patients with both pre- and post-transplantation values are included in Figures 1A and 1B. (C,D) Box and whisker plots demonstrating compiled total cholesterol and triglyceride levels from all patients included in the study.
Comparison of hyperlipidemia before and after transplantation
To clarify the changes in lipid levels occurring with transplantation, we identified patients who had pre-existing hyperlipidemia and those who had de novo hyperlipidemia after transplantation. Among 179 patients with pre-existing hypercholesterolemia, 164 patients (92%) remained hypercholesterolemic after transplantation. Of the 381 patients with pre-transplant total cholesterol <200 mg/dL, 249 patients (65%) became newly hypercholesterolemic after transplantation. Among patients who experienced a change in their status (hypercholesterolemic or not) before and after transplantation, 94% developed de novo hypercholesterolemia (P<0.001, McNemar’s test). For patients with pre-existing hypercholesterolemia, total cholesterol was higher after transplantation compared with those who had de novo hypercholesterolemia after transplantation (275 mg/dL vs. 259 mg/dL, P=0.004).
Among 216 patients with pre-existing hypertriglyceridemia, 193 patients (89%) remained hypertriglyceridemic after transplantation. Of the 338 patients with pre-transplant triglyceride <200 mg/dL, 215 patients (64%) became newly hypertriglyceridemic after transplantation. Among patients who experienced a change in their status (hypertriglyceridemic or not) before and after transplantation, 90% developed de novo hypertriglyceridemia (P<0.001, McNemar’s test). For patients with pre-existing hypertriglyceridemia, triglyceride values were higher after transplantation compared with those who had de novo hypertriglyceridemia after transplantation (398 mg/dL vs. 305 mg/dL P<0.001).
To confirm that pre-transplantation lipid levels were drawn on a random subset of patients, the rate of post-transplantation hypercholesterolemia and hypertriglyceridemia for those with and without pre-transplantation lipids were compared. There was no difference in the rate of post-transplantation hypercholesterolemia for patients with and without pre-transplantation values (74% vs. 73%, P=0.75). Similarly, there was no difference in the rate of post-transplantation hypertriglyceridemia for patients with and without pre-transplantation values (74% vs. 69%, P=0.24).
Risk factors for hyperlipidemia
To identify risk factors for post-transplantation hyperlipidemia, patients with at least one total cholesterol ≥ 200 mg/dL or triglyceride ≥ 200 mg/dL from day 30 to two years after transplantation were identified. Logistic regression analysis was performed using baseline patient characteristics (age ≥ 50, male gender, type of malignancy, overweight (BMI ≥ 25)), transplantation variables (unrelated or mismatched donor, stem cell source, conditioning regimen intensity, sirolimus-based GVHD prophylaxis) and post-transplantation variables (presence of grade II-IV acute GVHD). In univariable analysis, being overweight at the time of transplantation and the development of grade II-IV acute GVHD were associated with both post-transplantation hypercholesterolemia and hypertriglyceridemia (data not shown). Male gender and sirolimus use were associated only with post-transplantation hypertriglyceridemia (data not shown).
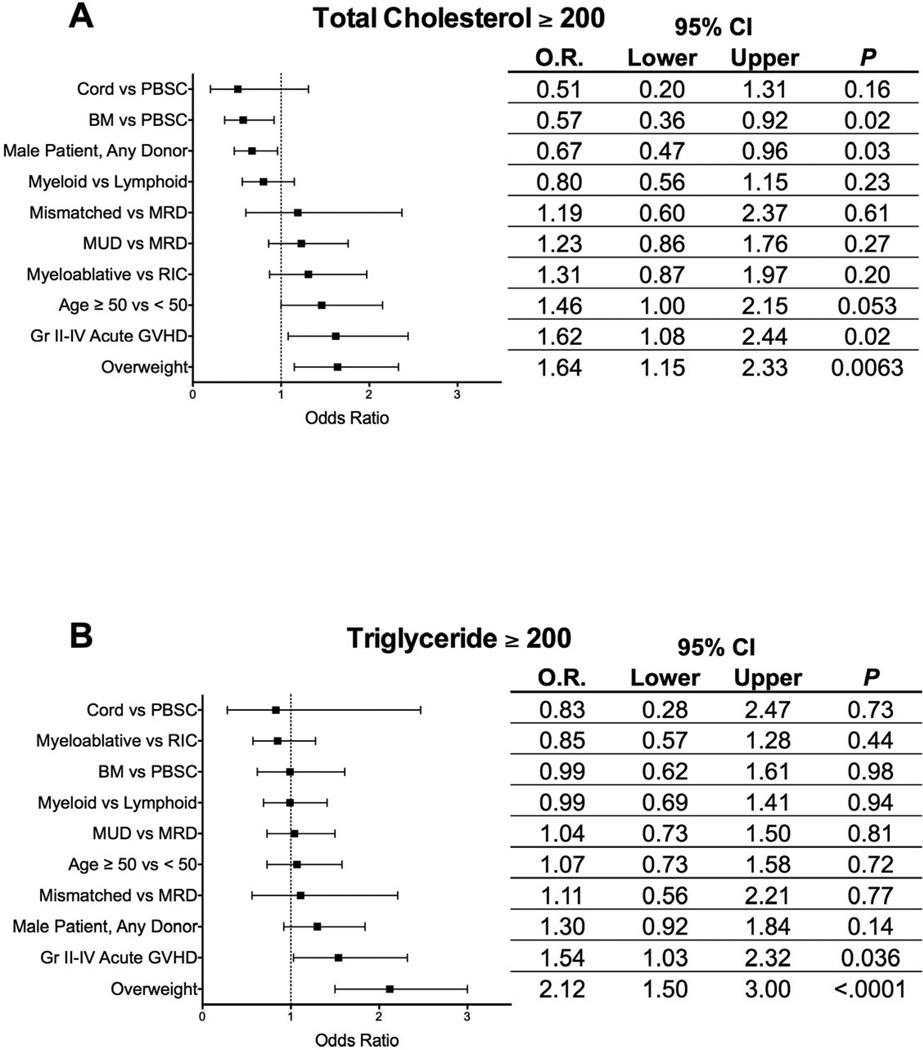
Multivariable logistic regression analysis was also performed using similar variables in the model (Figure 2A-B). There was a strong inverse correlation between sirolimus use and the presence of acute GVHD, so sirolimus use was not included in the model. The development of grade II-IV acute GVHD was an independent risk factor for both post-transplantation hypercholesterolemia and hypertriglyceridemia (O.R. 1.62, P=0.02 and O.R. 1.54, P=0.036, respectively) as was being overweight at the time of transplantation (O.R. 1.64, P=0.0063 and O.R. 2.12, P<0.0001 respectively). Male recipients (O.R. 0.67, P=0.03) and those receiving bone marrow allografts (O.R. 0.57, P=0.02) were less likely to have post-transplantation hypercholesterolemia compared with female recipients or those receiving peripheral blood stem cell allografts.
Figure 2.
Multivariable analysis for odds ratio identifying risk factors for peak post-transplantation total cholesterol ≥ 200 mg/dL (A) or triglyceride ≥ 200 mg/dL (B). Error bars represent 95% confidence intervals.
Hyperlipidemia in patients with acute GVHD
Among the patients with grade 0-I acute GVHD, the frequency of post-transplantation hypercholesterolemia was 71% while among patients with grade II-IV acute GVHD the frequency was 81% (P=0.007). In patients with grade 0-I acute GVHD, the frequency of post-transplantation hypertriglyceridemia was 70%, while among patients with grade II-IV acute GVHD the frequency was 79% (P=0.01). The median peak total cholesterol level was 235 mg/dL for patients with grade 0-I acute GVHD and 262 mg/dL for patients with grade II-IV acute GVHD (P<0.001). The median peak triglyceride level was 264 mg/dL for patients with grade 0-I acute GVHD and 300 mg/dL for patients with grade II-IV acute GVHD (P=0.002).
Corticosteroid therapy is known to elevate serum lipids. Of the hypercholesterolemic patients with grade II-IV acute GVHD, 81% were being treated with steroids at the time of their peak total cholesterol measurement. Of the hypertriglyceridemic patients with grade II-IV acute GVHD, 73% were being treated with steroids at the time of their peak triglyceride measurement.
Statin usage after transplantation
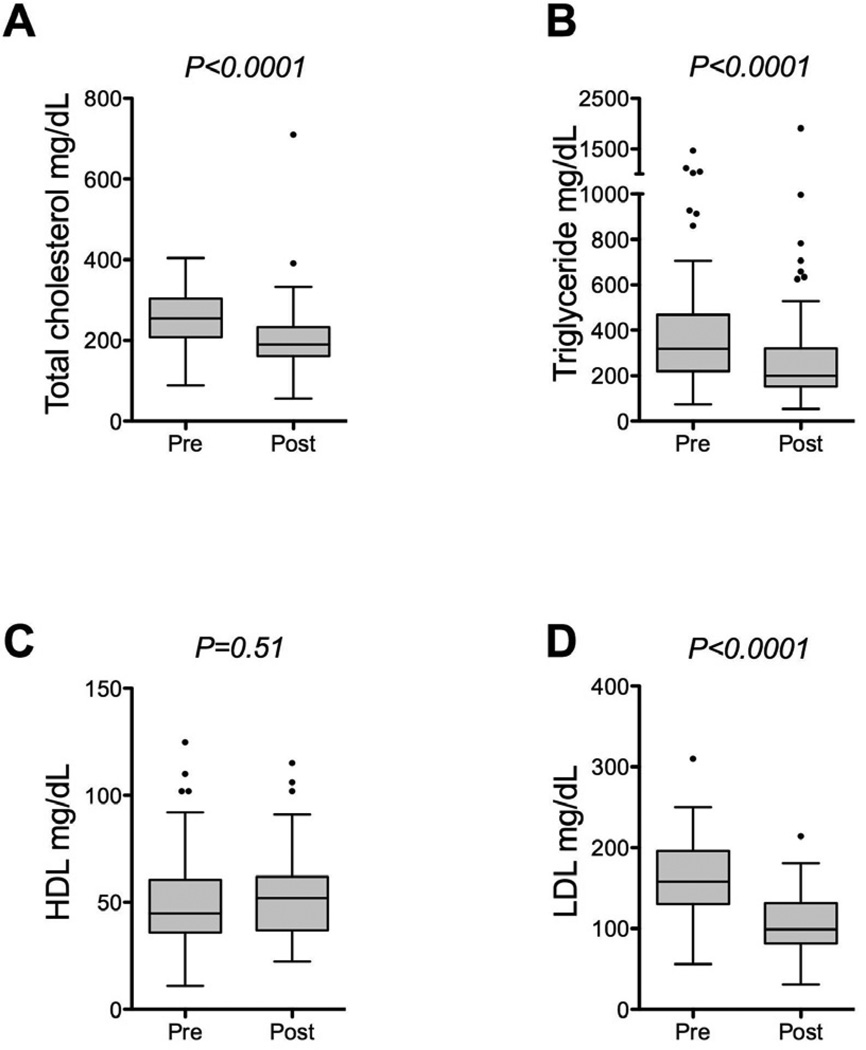
Outpatient prescription records of the 761 patients in the study were searched for the presence of any statin medication. Sixty-two patients (8%) had been prescribed any statin medication prior to transplantation. Among these, statin therapy was resumed in 53 patients after transplantation (85%). Overall, 220 patients (29%) were prescribed statin therapy within the first two years after transplantation. Atorvastatin was the most frequently prescribed statin (54%), followed by simvastatin (29%), pravastatin (8%), rosuvastatin (7%), lovastatin (2%), and fluvastatin (< 1%). The median day of initiation of statin therapy was 400 days after transplantation (range: 1 day to 723 days). Changes in lipid values were calculated for patients who had values recorded in the 90 days prior to initiation of statin therapy and who had values recorded from 180 to 270 days following initiation of statin therapy. Median values obtained immediately before and after statin initiation are plotted in Figure 3 (total cholesterol pre-statin: 255 mg/dL, post-statin: 190 mg/dL, N=94, P<0.0001; triglyceride pre-statin: 318 mg/dL, post-statin: 200 mg/dL, N=91, P<0.0001; HDL pre-statin: 45 mg/dL, post-statin: 52 mg/dL, N=76, P=0.51; LDL pre-statin 158 mg/dL, post-statin 99 mg/dL, N=49, P<0.0001).
Figure 3.
(A-D) Box and whisker plots demonstrating paired lipid values drawn up to 90 days before (pre) and 270 days after (post) starting statin therapy. Total cholesterol (A, N=94 patients), triglyceride (B, N=91 patients), HDL (C, N=76 patients) and LDL (D, N=49 patients) values are plotted.
The medical records of the 220 statin-treated patients were reviewed for the presence of adverse drug reactions leading to discontinuation of the statins. In 76 patients (35%), statin therapy had been discontinued. The reasons for discontinuation included reaching target LDL (N=11, 5% of all statin treated patients), relapse of disease with reinduction chemotherapy or second transplantation (N=13, 6%), and evidence of possible statin toxicity (N=7, 3%). Of the 7 patients with possible statin toxicity, two had biopsy-proven acute GVHD of the liver, one had suspected acute GVHD of the liver and one had veno-occlusive disease of the liver documented within the same time period as the discontinuation of statin therapy. Only one patient had elevation of liver enzymes attributed to statin therapy, though the measurements did not meet accepted criteria for statin toxicity[14]. One patient had muscle cramps without an elevation in creatine phosphokinase (CPK) and one patient had rhabdomyolysis with CPK=3186 U/L. Four patients (2%) discontinued statin therapy because of personal preference. In 41 patients (19% of all statin treated patients, 54% of all discontinuations), the reason for discontinuation of statin therapy was not documented in the medical record. There was no clear evidence of statin liver toxicity or rhabdomyolysis in any of these 41 cases.
Discussion
Hyperlipidemia and atherosclerotic heart disease are increasingly recognized as late complications of allogeneic HSCT[8, 15]. Several small studies have reported the incidence of post-transplantation hyperlipidemia to range from 11% to 58% [1, 5–7, 16, 17]. The incidence of adverse cardiovascular events including myocardial infarction and stroke may also be more common in allogeneic HSCT recipients, particularly in those with acute GVHD, when compared with siblings or recipients of autologous HSCT[1, 3, 18]. The present study examines the early post-transplantation changes in serum lipids in a large cohort of allogeneic HSCT recipients. We have described a pattern of elevated total cholesterol and triglyceride levels in the patients who had their lipids measured within the first two years after transplantation. In the largest series of patients reported to date, we have demonstrated that acute GVHD is an independent risk factor for both post-transplantation hypercholesterolemia and hypertriglyceridemia. Statins appeared to be effective in improving the lipid profiles in those in whom they were used and the incidence of toxicity appeared to be low. Whether early initiation of statin therapy after allogeneic HSCT would be effective in lowering the risk of cardiovascular events attributable to transplantation is currently unknown.
As a retrospective study, our analysis has several inherent limitations. Since lipid panels were collected at the discretion of outpatient providers, our results may not accurately estimate the true incidence of hyperlipidemia in all HSCT recipients. In particular, the group of patients who had their lipids checked was older, more likely to be overweight and more likely to be male than the group of patients who did not have their lipids checked. Total cholesterol and triglyceride levels were chosen for the primary analyses because these data were available in the majority of patients while LDL levels were not. While total cholesterol and triglyceride levels are a part of the recommended cardiovascular risk assessment, therapeutic goals are based on LDL levels [4]. Further, the medical record did not allow verification that lipid panels were drawn in the fasting state, although our routine practice has been for these labs to be drawn after a 9–12 hour fast. As our institution draws from a large referral base, some patients living further away from our center may have had their lipids checked and managed locally. Their lipid measurements, prescription history and potential adverse reactions to statins may not have been available for this study. In addition, since more than 95% of our patient population received tacrolimus for GVHD prophylaxis, we were unable to assess the contribution of this or cyclosporine to hyperlipidemia. Finally, the association of acute GVHD with hyperlipidemia may be confounded by concomitant steroid use in many cases.
In addition to their beneficial lipid lowering effects, statins are known to possess anti-inflammatory properties which likely contribute to their clinical benefit. A large randomized, placebo-controlled trial of rosuvastatin in patients with normal lipid profiles showed a reduction in C-reactive protein levels and improved survival [19]. Several randomized studies of cardiac transplantation recipients have demonstrated a mortality benefit for statin use and two have shown a decrease in major cardiac allograft rejection by statins[20–24]. Similar findings have been made in lung and renal allograft recipients, though not all studies have shown benefit in the latter[25–28]. Further, a number of trials in patients with rheumatoid arthritis and multiple sclerosis have demonstrated improved outcomes in patients treated with statins[29–33]. Thus, statins have a proven clinical benefit in multiple settings as adjunctive immune suppressive therapy.
Recent reports have begun to investigate the effect of immunomodulation with statins in allogeneic HSCT. A study in 2008 reported decreased incidence of grade II-IV acute GVHD in recipients who had been on statins one month prior to transplantation and who continued on them for three months after transplantation[10]. A subsequent larger study showed decreased incidence of acute GVHD in recipients of grafts from statin-treated donors[11]. Acute GVHD was not prevented when statin treatment was limited to the recipient alone. Recipient treatment was associated with decreased rates of chronic GVHD and an increased rate of relapse or disease progression[12]. Interpretation of these studies is confounded by the low rate of statin use in recipients and the potential biased use of statin therapy in patients free from complications of transplantation for other reasons. The mechanism underlying these observation is unknown but in vitro and animal studies suggest Th2 polarization of the donor T cell pool may play a role[34, 35].
With the growing body of evidence that statins may be important as adjuvant immune suppressive therapy and for controlling hyperlipidemia after transplantation, it is necessary to consider the frequency and efficacy of their use after HSCT. The frequency of statin use in our population of patients was higher when compared with prior studies (29% vs. 5% in Rotta et al. and 15% in Hamadani et al.)[10,11]. NHANES study data shows a steady rise in statin use in the general population from 6.5% of all US adults in 1999–2000 to 11.7% in 2003–2004, the latest available published data[9]. Since we required only one month of statin use to qualify, our study may overestimate the rate of statin use compared to these other works. Despite this, the efficacy of statin use in our study does not appear to be inferior to that seen in the general population. According to NHANES data from 2003 to 2004, the average LDL of hyperlipidemic US adults in the absence or presence of statin therapy was 158.4 and 100.7 mg/dL, respectively, which is consistent with our findings in Figure 3[9].
There are a number of obvious barriers to statin use after HSCT including interaction with other hepatotoxic or hepatically metabolized medications, the potential for confounding the diagnosis of or worsening active GVHD or veno-occlusive disease of the liver, and the risk of inhibiting graft-versus-leukemia effects[12, 36, 37]. Statins are routinely used in cardiac transplantation suggesting that their co-administration with calcineurin inhibitors is safe, though the use of azoles in such patients is much less common than in the allogeneic HSCT population[21–23]. Further, multiple studies in patients with chronic hepatitis C and primary biliary cirrhosis have shown that statin therapy is well tolerated in patients with chronic liver disease[38–42]. Our data suggest that significant adverse reactions to statin therapy are relatively infrequent the post-transplantation setting. Clearly, close monitoring should accompany the use of these medications in allogeneic HSCT patients.
These data provide the first detailed look at the patterns of hyperlipidemia that occur early after HSCT. They provide the strongest evidence to date that acute GVHD is an independent risk factor for hyperlipidemia. Current guidelines recommend screening for hyperlipidemia in HSCT recipients at the same frequency as in the general population [43]. These guidelines were published before the cardiovascular risks of HSCT were well-appreciated and they do not account for the rising age of the population undergoing HSCT [1, 3, 8, 44, 45]. Our data lend further support to the proposal of Griffith et al. that lipid profiles be assessed four weeks after transplantation and then every three months while on immune suppressive therapy [15]. Clinicians should be particularly attuned to lipid abnormalities in patients receiving sirolimus or in those who develop GVHD. Nevertheless, the risks and benefits of aggressive lipid-lowering therapy have not yet been established in this population. Several important questions remain: Is it safe to treat to established LDL goals? What are the risks of statin therapy? What role do statins play as adjuvant immune suppression? How can non-LDL lipid goals be safely achieved? Answering such questions will require a prospective trial of the use of statins in allogeneic HSCT.
Acknowledgements
This work was supported by the Jock and Bunny Adams Research and Education Endowment and by the National Institutes of Health (grant CA142106).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement
The authors have no financial disclosures relevant to this study.
References
- 1.Tichelli A, Bucher C, Rovó A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 2.Armenian SH, Bhatia S. Cardiovascular disease after hematopoietic cell transplantation--lessons learned. Haematologica. 2008;93:1132–1136. doi: 10.3324/haematol.13514. [DOI] [PubMed] [Google Scholar]
- 3.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program NCEP Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults Adult Treatment Panel III. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 5.Socié G, Mary JY, Esperou H, et al. Health and functional status of adult recipients 1 year after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2001;113:194–201. doi: 10.1046/j.1365-2141.2001.02678.x. [DOI] [PubMed] [Google Scholar]
- 6.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43:49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Seaborn T, Mao FJ, et al. Frequency of abnormal findings detected by comprehensive clinical evaluation at 1 year after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:416–420. doi: 10.1016/j.bbmt.2008.12.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 9.Mann D, Reynolds K, Smith D, Muntner P. Trends in Statin Use and Low-Density Lipoprotein Cholesterol Levels Among US Adults: Impact of the 2001 National Cholesterol Education Program Guidelines. The Annals of Pharmacotherapy. 2008;42:1208. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 10.Hamadani M, Awan FT, Devine SM. The impact of HMG-CoA reductase inhibition on the incidence and severity of graft-versus-host disease in patients with acute leukemia undergoing allogeneic transplantation. Blood. 2008;111:3901–3902. doi: 10.1182/blood-2008-01-132050. [DOI] [PubMed] [Google Scholar]
- 11.Rotta M, Storer BE, Storb RF, et al. Donor statin treatment protects against severe acute graft-versus-host disease after related allogeneic hematopoietic cell transplantation. Blood. 2010;115:1288–1295. doi: 10.1182/blood-2009-08-240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotta M, Storer BE, Storb R, et al. Impact of recipient statin treatment on graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1463–1466. doi: 10.1016/j.bbmt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrell F. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York City, NY: Springer-Verlag; 2001. [Google Scholar]
- 14.Pasternak RC, Smith SC, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40:567–572. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 15.Griffith ML, Savani BN, Boord JB. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: evaluation and management. Blood. 2010;116:1197–1204. doi: 10.1182/blood-2010-03-276576. [DOI] [PubMed] [Google Scholar]
- 16.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 17.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 18.Armenian SH, Sun C-L, Mills G, et al. Predictors of Late Cardiovascular Complications in Survivors of Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 20.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 21.Kobashigawa JA, Moriguchi JD, Laks H, et al. Ten-year follow-up of a randomized trial of pravastatin in heart transplant patients. J Heart Lung Transplant. 2005;24:1736–1740. doi: 10.1016/j.healun.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Wenke K, Meiser B, Thiery J, et al. Simvastatin reduces graft vessel disease and mortality after heart transplantation: a four-year randomized trial. Circulation. 1997;96:1398–1402. doi: 10.1161/01.cir.96.5.1398. [DOI] [PubMed] [Google Scholar]
- 23.Wenke K, Meiser B, Thiery J, et al. Simvastatin initiated early after heart transplantation: 8-year prospective experience. Circulation. 2003;107:93–97. doi: 10.1161/01.cir.0000043241.32523.ee. [DOI] [PubMed] [Google Scholar]
- 24.Mehra MR, Raval NY. Metaanalysis of statins and survival in de novo cardiac transplantation. Transplant Proc. 2004;36:1539–1541. doi: 10.1016/j.transproceed.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BA, Iacono AT, Zeevi A, McCurry KR, Duncan SR. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med. 2003;167:1271–1278. doi: 10.1164/rccm.200205-410OC. [DOI] [PubMed] [Google Scholar]
- 26.Katznelson S, Wilkinson AH, Kobashigawa JA, et al. The effect of pravastatin on acute rejection after kidney transplantation--a pilot study. Transplantation. 1996;61:1469–1474. doi: 10.1097/00007890-199605270-00010. [DOI] [PubMed] [Google Scholar]
- 27.Navaneethan SD, Perkovic V, Johnson DW, Nigwekar SU, Craig JC, Strippoli GFM. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database Syst Rev. 2009;(2):CD005019. doi: 10.1002/14651858.CD005019.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Tuncer M, Süleymanlar G, Ersoy FF, Yakupoğlu G. Comparison of the effects of simvastatin and pravastatin on acute rejection episodes in renal transplant patients. Transplant Proc. 2000;32:622–625. doi: 10.1016/s0041-1345(00)00921-0. [DOI] [PubMed] [Google Scholar]
- 29.Shirinsky IV, Zheltova OI, Solovyova NY, Kozlov VA, Shirinsky VS. Changes in disease activity, cytokine production, and proliferation of peripheral blood mononuclear cells in patients with rheumatoid arthritis after simvastatin treatment. Scand J Rheumatol. 2009;38:23–27. doi: 10.1080/03009740802363776. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto H, Koizumi K, Kamitsuji S, et al. Beneficial action of statins in patients with rheumatoid arthritis in a large observational cohort. J Rheumatol. 2007;34:964–968. [PubMed] [Google Scholar]
- 31.Togha M, Karvigh SA, Nabavi M, et al. Simvastatin treatment in patients with relapsing-remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Mult Scler. 2010;16:848–854. doi: 10.1177/1352458510369147. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer T, Key L, Durkalski V, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 33.Lanzillo R, Orefice G, Quarantelli M, et al. Atorvastatin combined to interferon to verify the efficacy (ACTIVE) in relapsing-remitting active multiple sclerosis patients: a longitudinal controlled trial of combination therapy. Mult Scler. 2010;16:450–454. doi: 10.1177/1352458509358909. [DOI] [PubMed] [Google Scholar]
- 34.Dunn SE, Youssef S, Goldstein MJ, et al. Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med. 2006;203:401–412. doi: 10.1084/jem.20051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeiser R, Youssef S, Baker J, Kambham N, Steinman L, Negrin RS. Preemptive HMG-CoA reductase inhibition provides graft-versus-host disease protection by Th-2 polarization while sparing graft-versusleukemia activity. Blood. 2007;110:4588–4598. doi: 10.1182/blood-2007-08-106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong J, Laport G, Lowsky R. Rhabdomyolysis after concomitant use of cyclosporine and simvastatin in a patient transplanted for multiple myeloma. Bone Marrow Transplant. 2005;36:739–740. doi: 10.1038/sj.bmt.1705128. [DOI] [PubMed] [Google Scholar]
- 37.Hong R, Sequeira W. Rhabdomyolysis in a patient taking simvastatin after addition of cyclosporine therapy. J Clin Rheumatol. 2000;6:324–327. doi: 10.1097/00124743-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Ritzel U, Leonhardt U, Näther M, Schäfer G, Armstrong VW, Ramadori G. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol. 2002;36:454–458. doi: 10.1016/s0168-8278(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 39.Stojakovic T, Putz-Bankuti C, Fauler G, et al. Atorvastatin in patients with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid. Hepatology. 2007;46:776–784. doi: 10.1002/hep.21741. [DOI] [PubMed] [Google Scholar]
- 40.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–1292. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Abu Rajab M, Kaplan MM. Statins in primary biliary cirrhosis: are they safe? Dig Dis Sci. 2010;55:2086–2088. doi: 10.1007/s10620-009-0988-9. [DOI] [PubMed] [Google Scholar]
- 42.Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroenterol Hepatol. 2006;4:902–907. doi: 10.1016/j.cgh.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Tichelli A, Passweg J, Wójcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 45.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]