Abstract
Hypertensive heart disease causes significant mortality in older patients, yet there is an incomplete understanding of molecular mechanisms that regulate age-dependent hypertensive left ventricular hypertrophy (LVH). Therefore, we tested the hypothesis that the cGMP-dependent protein kinase G I alpha (PKGIα) attenuates hypertensive LVH by evaluating the cardiac phenotype in mice with selective mutations of the PKGIα leucine zipper domain. These leucine zipper mutant (LZM) mice develop basal hypertension. Compared with wild-type controls, 8-month-old adult LZM mice developed increased left ventricular end-diastolic pressure but without frank LVH. In advanced age (15 months), the LZM mice developed overt pathological LVH. These findings reveal a role of PKGIα in normally attenuating hypertensive LVH. Therefore, mutation of the PKGIα LZ domain produces a clinically relevant model for hypertensive heart disease of aging.
Key Words: Left ventricular hypertrophy, Hypertension, Protein kinase G.
In patients aged more than 65, congestive heart failure (CHF) comprises the most common cause of death in the United States (1) and is predicted to cause 33% of U.S. annual deaths by 2050 (2). Elderly patients represent more than 75% of the heart failure patients in the United States (1). In this population, the syndrome of CHF usually results from a process that begins with left ventricular hypertrophy (LVH) (3). Chronic hypertension is a leading cause of LVH in elderly patients (4), comprising the first and second most common etiologies of heart failure in elderly women and men, respectively (1). This combination of hypertension-induced LVH and resultant CHF is termed hypertensive hypertrophic cardiomyopathy (1). There is a lack of animal models of chronic hypertensive hypertrophic cardiomyopathy of elderly patients, with the majority of published models instead relying on acute surgically induced pressure overload by transaortic constriction (TAC) (5). TAC is almost exclusively performed on young mice, making its relevance to chronic hypertension in older patients questionable. Furthermore, experimental induction of hypertrophy has different effects when applied to older animals, rather than young adult animals (6,7). Therefore, there is a need for models of age-dependent hypertensive LVH that might ultimately aid in the identification of novel therapeutic targets for this highly prevalent disease in older adults.
Cyclic GMP (cGMP) is one of the few signaling molecules identified to date which likely attenuates chronic hypertension and resultant LVH (8,9). cGMP is synthesized by guanylate cyclases, activated by NO or natriuretic peptides (10). There are several cGMP effectors in the cardiovascular system, including cGMP-dependent protein kinase G I alpha (PKGIα). Intrinsic PKGI activators, such as ANP, increase in the serum and in cardiac tissue during aging (11). However, whether PKGI, or its specific isoforms, normally attenuates age-dependent hypertensive LVH has not been tested. The PKGIα N-terminal leucine zipper domain mediates a number of important protein interactions with PKGIα substrates (12). Therefore, to investigate the cardiovascular role of PKGIα LZ-mediated interactions, we developed a mouse model, termed the PKGIα leucine zipper mutant (LZM) mouse, which harbors discrete mutations in the LZ domain. In this model, PKGIα kinase activity is maintained, but LZ-dependent protein–protein interactions are disrupted (12). We previously investigated the vascular effects of the LZ mutation and observed disorders of vasomotor tone and chronic hypertension in LZM mice (12). Young prehypertensive LZM mice develop accelerated TAC-induced cardiac hypertrophy and remodeling (13).
However, older adults with hypertension-induced LVH and CHF more frequently have experienced chronic, moderate elevations in afterload, rather than acute, severe afterload induced by TAC. Additionally, sustained vascular abnormalities, such as those observed in the LZM model, are thought to contribute to the pathogenesis of hypertension in elderly patients (4). We therefore hypothesized that PKGIα normally inhibits hypertensive hypertrophic cardiomyopathy of aging. This study tested this by investigating the basal LZM cardiovascular phenotype during aging.
Methods
Animal Models
PKGIα LZM mice were generated on the C57/BL6 background as described (12). Animal care was in accordance with and approved by the Institutional Animal Care and Use Committee of Tufts University School of Medicine and Tufts Medical Center.
Left Ventricular Hemodynamic Measurements
After anesthetizing mice with 2.5% isoflurane, hemodynamic analyses were performed from the right carotid artery using a fully calibrated 1.0 Fr catheter (PVR-1045; Millar Instruments, Houston, TX). Hemodynamics were recorded with IOX version 1.8.11 software (EMKA Instruments, Falls Church, VA). Mice were maintained at 37°C. Pressure volume analysis was performed as described (14).
Organ Weights and Histology
Hearts were prepared as described (13), and images at the midpapillary level were acquired at ×40 using SPOT Basic 4.7. Myocytes with visible central nuclei were traced using Image-Pro version 6.2. Statistical analysis was performed by one-way ANOVA.
Transthoracic Echocardiography
Echocardiography was performed under isoflurane anesthesia (2.5% induction and 1.5% maintenance). Left ventricular end-diastolic and end-systolic diameters (EDD and ESD, respectively), posterior wall thickness, and heart rate were measured by averaging values from five cardiac cycles. Fractional shortening (FS) was calculated using the following equation: FS = [(EDD − ESD)/EDD] × 100.
Western Blots
Left ventricles from 12-month-old mice were snap frozen in liquid nitrogen, and protein lysates were made by homogenation as described (13). Lysate of 15 μg per heart was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by immunoblotting with antibodies to phospho-AKT (pAKT) and total AKT (Cell Signaling, 1:800 dilution). AKT and pAKT positive controls were obtained from Cell Signaling.
Statistics
All data are reported as mean ± SEM. Comparisons between two groups were made using Student’s t test. For experiments with greater than two groups, comparisons were made by one- or two-way ANOVA, and p values shown indicate the effect of genotype on the age-related response. Correction for multiple comparisons was performed with the Student–Newman Keul test. A p value of <.05 was considered statistically significant.
Results
Generation and Vascular Phenotype of LZM Mice
The generation and vascular phenotype of the PKGIα LZM mouse were extensively characterized and reported previously (12). These mice harbor mutations replacing the first four hydrophobic leucine/isoleucine residues of the PKGIα LZ domain with alanines, to disrupt the tertiary structure of this region, yielding a protein with retained PKGI kinase activity but abolished binding with proteins whose binding is dependent on the LZ. These knockin mice display significant systemic hypertension after 6 months of age (12).
Age-Dependent Left Ventricular Functional Changes in LZM Mice
We explored the cardiac phenotype of male LZM mice at ages of 6 weeks, 8 months, and 15 months, representing pre-adult, adult, and elderly ages, respectively. At 6 weeks, there were no significant differences in systolic, diastolic, or mean blood pressure between wild-type (WT) and LZM male mice, as assessed by invasive hemodynamics and by tailcuff blood pressures, consistent with our previous observations (12). Ventricular masses and function did not differ between genotypes at this age (Supplementary Table).
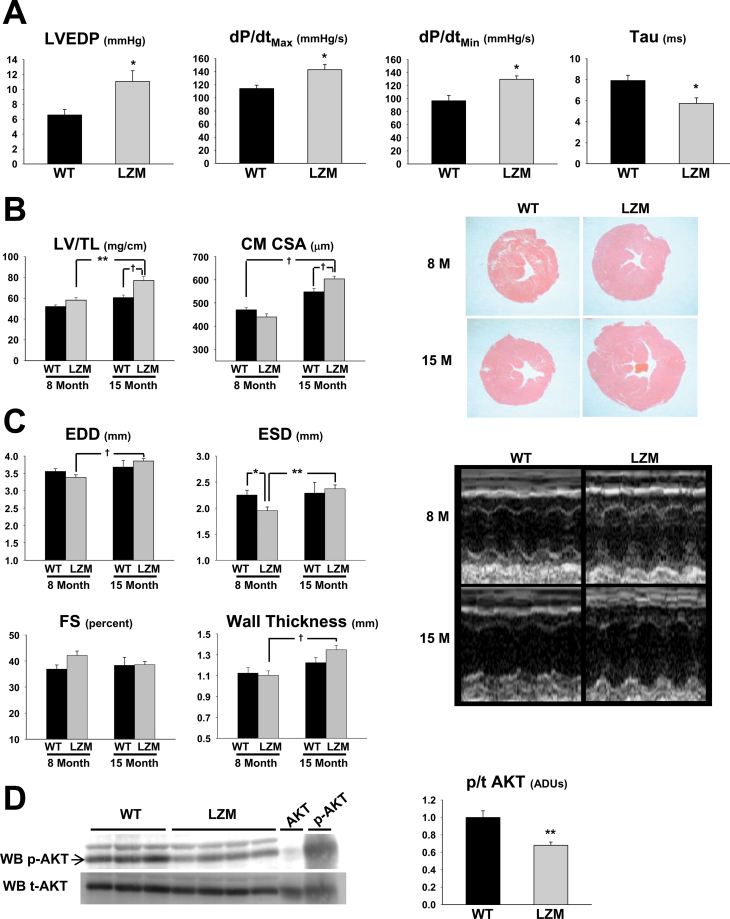
At 8 months, arterial blood pressure increased significantly in LZM, compared with WT male mice (WT systolic 106.6±0.8 mmHg, LZM systolic 118.8±6.1 mmHg, p < .05; WT diastolic 72.9±1.1 mmHg, LZM diastolic 82.1±4.6 mmHg, p < .05), consistent with prior observations (12). Invasive indices of afterload-dependent systolic (dP/dt Max) and diastolic (tau, dP/dt Min) functions were accelerated in LZM left ventricles, consistent with increased left ventricular workload (Figure 1A). However, the left ventricular end-diastolic pressure (LVEDP) was elevated in LZM compared with WT left ventricles, consistent with decreased diastolic compensation in LZM left ventricles (Figure 1A).
Figure 1.
Age-dependent pathological left ventricular hypertrophy in protein kinase G I alpha LZM mice. (A) Summary data of left ventricular hemodynamic measurements obtained from 8-month WT and LZM mice. n = 4 per group. (B) Summary data of left ventricular mass normalized to tibia length; representative left ventricular mass cut in cross-section at the midpapillary level and stained with Hematoxylin and Eosin; and left ventricular cardiac myocyte cross-sectional area. Summary data of WT and LZM mice at 8 and 15 months of age. n = 11 WT, 9 LZM 8 month, 5 WT, and 13 LZM 14 month; n > 120 myocytes in each experimental group. (C) Summary data and representative images of left ventricle echocardiography from WT and LZM mice at 8 and 15 months of age. (D) Immunoblot of phospho- and total AKT from left ventricles of WT and LZM 12-month-old mice. AKT and p-AKT, positive controls for total and phosphorylated AKT, respectively. n = 3 WT, 4 LZM. LVEDP = left ventricular end-diastolic pressure; LV dP/dt Max = peak rate of LV pressure rise; LV dP/dt Min = peak rate of LV pressure decline; Tau = time constant of LV relaxation; LV/TL = left ventricular mass normalized to tibia length; CSA = cross-sectional area; EDD = left ventricular end-diastolic dimension; ESD = left ventricular end-systolic dimension; FS = left ventricular percent fractional shortening; WB = Western blot; ADU = arbitrary densitometric unit; LSM = leucine zipper mutant; WT = wild type. *p < .05; **p < .01; † p < .001.
Age-Dependent Pathological LVH in LZM Mice
Cardiac chamber weights are shown in Table 1. As in the 6-week-old mice, no significant differences in left ventricle mass were detected between the 8-month WT and LZM mice (Figure 1B). However, by 15 months, the LZM mice developed robust LVH compared with both 15-month WT littermates and the 8-month LZM mice. Cardiac myocyte cross-sectional area also increased in 15-month LZM compared with 8- and 15-month WT hearts (Figure 1B). Echocardiography (Figure 1C) revealed increased left ventricular EDD in 15-month LZM compared with 8-month LZM left ventricles. At 8 months of age, the left ventricular ESD was decreased in LZM, compared with WT left ventricles, consistent with increased cardiac workload. Unlike the WT mice, the wall thickness of the LZM left ventricles was significantly elevated at 15 months of age, compared with 8 months.
Table 1.
Baseline Organ Masses and Echocardiographic Parameters of 8- and 15-Month-Old Male Mice
| Genotype | 8 Months | 15 Months | ||
|---|---|---|---|---|
| WT | LZM | WT | LZM | |
| n | 9 | 11 | 5 | 13 |
| Body weight (g) | 38.7±1.4 | 38.7±2.0 | 39.3±3.7 | 41.2±7.3 |
| LV (mg) | 94.7±3.0 | 106.4±5.0 | 112.6±6.8 | 140.2±7.1*,† |
| RV (mg) | 22.8±1.0 | 5.0±1.6 | 29.0±1.9‡ | 31.9±1.5† |
| RV/tibia length (mg/cm) | 12.5±0.5 | 13.7±0.9 | 15.7±1.0‡ | 17.5±7.5† |
| Tibia length (cm) | 1.8±0.01 | 1.8±0.01 | 1.9±0.05‡ | 1.8±0.02§ |
| LV/body weight (mg/g) | 2.5±0.1 | 2.8±0.11 | 2.9±0.2 | 3.4±0.1 |
| RV/body weight (mg/g) | 0.6±0.03 | 0.7±0.05 | 0.8±0.1 | 0.8±0.04 |
| Heart rate (s−1) | 468.3±19.0 | 481.1±14.1 | 510.0±16.4 | 450.0±17.7 |
Note. WT = wild type; LZM = leucine zipper mutant; LV = left ventricle; RV = right ventricle.
*p < .01 vs 15-month WT.
† p < .01 vs 8-month LZM.
‡ p < .05 vs 8-month WT.
§ p < .05 vs 8-month LZM.
Decreased Cardioprotective Signaling in Elderly LZM Hearts
We next performed signaling studies on WT and LZM left ventricles from 12-month-old mice. The ratio of phosphorylated AKT to total AKT was significantly decreased in LZM, compared with WT left ventricles (Figure 1E), consistent with increased pathological, rather than physiological, hypertrophy in LZM hearts (15).
Discussion
This study tested the hypothesis that PKGIα attenuates hypertensive LVH in vivo, using the PKGIα LZM model. Young (6 weeks) LZM mice are normotensive and display no cardiac abnormalities. Adult (8 months) mice have chronic systemic hypertension and develop changes in systolic and diastolic function, as well as increased LVEDP, but they do not yet have significant LVH. Finally, in advanced age (15 months), in the setting of long-standing chronic hypertension, the LZM mice develop overt and significant LVH. Elderly LZM left ventricles also display a signaling hallmark of pathological LVH, with attenuated AKT phosphorylation. These findings identify a novel and clinically relevant mouse model of hypertensive heart disease of older patients, in which hypertension precedes the development of progressive LVH.
Despite the established importance of hypertension as a contributor to hypertensive hypertrophic cardiomyopathy and cardiovascular death in elderly patients (1,4), remarkably few animal models of this condition exist. In fact, a number of mouse models of chronic hypertension report no cardiac abnormalities (14,16). Our finding that the LZM mice develop progressive LVH preceded by the onset of hypertension therefore provides an important model for human hypertensive heart disease and supports for the first time that PKGIα attenuates this process in vivo. This is important because a number of PKGI-activating drugs have been studied primarily for their acute vasorelaxant effects, or in the setting of decompensated heart failure (17,18), but not as chronic treatments to prevent or reverse LVH in older hypertensive patients. We therefore interpret our findings to support chronic activation of PKGIα, or downstream LZ-dependent effectors, in the treatment and prevention of hypertensive LVH of aging.
We interpret the accelerated systolic and diastolic measures in LZM left ventricles to reflect the increased workload of their ventricles in response to chronic afterload, similar to that observed in hypertensive humans (19). However, LVEDP was elevated in adult LZM hearts compared with WT hearts, supporting maladaptive diastolic function in LZM hearts. This finding supports a causal role of PKGIα disruption in the development of heart failure with preserved ejection fraction (HFPEF). HFPEF is prevalent in elderly patients and is characterized by increased LVEDP leading to pulmonary edema despite normal left ventricle ejection fraction (reviewed in reference (20)). Therefore, our findings in the LZM mice support a clinically relevant role of PKGIα in maintaining diastolic function.
We and others previously identified the LZ domain of PKGIα as mediating important interactions with its vascular substrates (21). Importantly, in the LZM model, PKGI is not deleted but rather harbors discrete mutations that disrupt LZ-mediated protein interactions (12). Therefore, our findings reveal a critical role for these PKGIα LZ-mediated interactions in regulating the left ventricle response to hypertension in vivo. This study admittedly did not test the specific cell type(s) required for the PKGIα effect. However, basic studies support that intrinsic vascular and myocardial intrinsic changes of aging lead to impaired cardiac function (22,23). We speculate that both vascular and myocardial PKGIα kinase targets mediate its antihypertrophic effects, and current studies in our lab seek to identify these effectors.
In summary, we have identified the LZM mouse as a novel model of age-dependent, clinically relevant, hypertensive LVH. Our study suggests that derangements in PKGIα signaling may contribute to hypertensive heart disease in elderly patients. Therefore, our findings support further study of PKGIα, and its downstream leucine zipper-dependent targets in the cardiovascular system, as a potential therapeutic target in this condition.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Funding
This work was supported by the National Institutes of Health (1R03AG042367 to R.M.B., HL93432 to E.T., HL89297, and HL59480 to D.A.K., and HL077378, HL069770, and HL056069 to M.E.M.); the American Heart Association (10SDG2630161 to R.M.B.); and by the Fondation Leducq and Belfer Laboratory Endowment (to D.A.K.). R.M.B. was also supported by a T. Franklin Williams Scholarship Award. Funding for the Williams Award was provided by Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American College of Cardiology.
Acknowledgments
M.E.M. is currently employed by Merck but retains an appointment at Tufts University School of Medicine.
References
- 1. Rich M. Heart failure in the 21st century: A cardiogeriatric syndrome. J Gerontol A Med Sci. 2001;56A:88–96 [DOI] [PubMed] [Google Scholar]
- 2. Sonnenshein E, Brady J. Effect of population aging on proportionate mortality from heart disease and cancer, U.S. 2000–2050. J Gerontol B Social Sci. 2005;60B:110–112 [DOI] [PubMed] [Google Scholar]
- 3. Rodríguez-Calvo R, Serrano L, Barroso E, et al. Peroxisome proliferator-activated receptor alpha down-regulation is associated with enhanced ceramide levels in age-associated cardiac hypertrophy. J Gerontol A Biol Sci Med Sci. 2007;62:1326–1336 [DOI] [PubMed] [Google Scholar]
- 4. Rigaud AS, Forette B. Hypertension in older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M217–M225 [DOI] [PubMed] [Google Scholar]
- 5. Rockman HA, Ross RS, Harris AN, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Florini JR, Saito Y, Manowitz EJ. Effect of age on thyroxine-induced cardiac hypertrophy in mice. J Gerontol. 1973;28:293–297 [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Reddy AK, Ochoa LN, et al. Effect of age on peripheral vascular response to transverse aortic banding in mice. J Gerontology A Biol Sci. 2008;58A:895–899 [DOI] [PubMed] [Google Scholar]
- 8. Flaherty MP, Brown M, Grupp IL, Schultz JE, Murphree SS, Jones WK. eNOS deficient mice develop progressive cardiac hypertrophy with altered cytokine and calcium handling protein expression. Cardiovasc Toxicol. 2007;7:165–177 [DOI] [PubMed] [Google Scholar]
- 9. Oliver PM, Fox JE, Kim R, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–14735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hofmann F. The biology of cyclic GMP-dependent protein kinases. J Biol Chem. 2005;280:1–4 [DOI] [PubMed] [Google Scholar]
- 11. Wu SQ, Kwan CY, Tang F. The effect of aging on ANP levels in the plasma, heart, and brain of rats. J Gerontol A Biol Sci Med Sci. 1997;52:B250–B254 [DOI] [PubMed] [Google Scholar]
- 12. Michael SK, Surks HK, Wang Y, et al. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci U S A. 2008;105:6702–6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blanton RM, Takimoto E, Lane AM, et al. Protein kinase g iα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc. 2012;1:e003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takimoto E, Koitabashi N, Hsu S, et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119:408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeBosch B, Treskov I, Lupu TS, et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104 [DOI] [PubMed] [Google Scholar]
- 16. Johns C, Gavras I, Handy DE, Salomao A, Gavras H. Models of experimental hypertension in mice. Hypertension. 1996;28:1064–1069 [DOI] [PubMed] [Google Scholar]
- 17. Lapp H, Mitrovic V, Franz N, et al. Cinaciguat (BAY 58-2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation. 2009;119:2781–2788 [DOI] [PubMed] [Google Scholar]
- 18. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43 [DOI] [PubMed] [Google Scholar]
- 19. de Simone G, Di Lorenzo L, Costantino G, Moccia D, Buonissimo S, de Divitiis O. Supernormal contractility in primary hypertension without left ventricular hypertrophy. Hypertension. 1988;11:457–463 [DOI] [PubMed] [Google Scholar]
- 20. Ahmed A. Association of diastolic dysfunction and outcomes in ambulatory older adults with chronic heart failure. J Gerontol A Biol Sci Med Sci. 2005;60:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Surks HK, Mochizuki N, Kasai Y, et al. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase I alpha. Science. 1999;286:1583–1587 [DOI] [PubMed] [Google Scholar]
- 22. Sloboda N, Fève B, Thornton SN, et al. Fatty acids impair endothelium-dependent vasorelaxation: a link between obesity and arterial stiffness in very old Zucker rats. J Gerontol A Biol Sci Med Sci. 2012;67:927–938 [DOI] [PubMed] [Google Scholar]
- 22. Diffee GM, Chung E. Effect of aging on power output properties in rat skinned cardiac myocytes. J Gerontol A Biol Sci. 2012;66A:1267–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]