Abstract
Background.
Weakness contributes to the decline of physical function that occurs with aging. Contradictory findings have been reported as to whether neuromuscular activation is impaired with aging, and the extent to which it contributes to weakness. The present study uses a longitudinal design to assess how potential age-related change of neuromuscular activation affects strength, power, and mobility function.
Methods.
Participants included 16 healthy older adults who were healthy and high functioning at baseline. Strength was measured by leg press one repetition maximum. Power production was measured during a maximal effort rapid leg press movement with resistance set to 70% of the one repetition maximum. During the same movement, neuromuscular activation was quantified as the rate of rise of the quadriceps surface electromyogram (rate of electromyogram rise). Thigh muscle cross-sectional area was measured by computed tomography. Mobility function was assessed by the Short Physical Performance Battery.
Results.
The time between baseline and follow-up testing was almost 3 years. Between these time points, rate of electromyogram rise decreased 28% (p = .004) and power decreased 16.5% (p = .01). There was a trend for reduced anterior thigh muscle cross-sectional area (3%, p = .05), but no change in posterior thigh muscle cross-sectional area (p = .84), one repetition maximum strength (p = .72), or Short Physical Performance Battery score (p = .17). Loss of power was strongly associated with reduction in the rate of electromyogram rise (R 2 = .61, p < .001), but not with reduction of anterior thigh muscle cross-sectional area (p = .83).
Conclusions.
The present findings suggest that voluntary neuromuscular activation declines with advancing age, contributes to a reduction in power production, and precedes the decline of mobility function.
Key Words: Physical performance, Physical function, Muscle, Motor control.
Minimizing the decline of physical function in older adults is an important public health priority in our rapidly aging society. Weakness is a major determinant of functional decline (1). Given that loss of muscle size contributes to weakness, much effort has been dedicated to preventing age-related loss of muscle tissue (2,3). Yet, longitudinal studies indicate that weakness and muscle size are less tightly coupled than previously thought, with progression of weakness considerably outpacing the loss of muscle (4,5). Accordingly, other mechanisms such as impaired voluntary neuromuscular activation may also need to be addressed to prevent weakness and functional decline (6,7).
Numerous studies have examined the relationship between weakness and voluntary activation with aging. These studies primarily include cross-sectional comparisons of older and younger adults and the use of resistance training interventions to enhance activation and force production capability. A number of recent review articles provide an excellent summary on this topic, but reveal a lack of consensus on whether impaired voluntary neuromuscular activation occurs with aging and accounts for weakness (6,8,9). Various factors likely contribute to the discrepancies among previous studies, such as the age range of elderly participants (10,11), the functional status of the participants (12,13), the muscle group assessed (6,9), the measurement technique used (6), and the contraction mode and speed assessed (6,9,12,13).
Longitudinal studies are needed to better understand whether voluntary neuromuscular activation capability declines with aging and contributes to weakness and loss of physical function in older adults. Accordingly, the objective of the present study is to determine the extent to which potential changes in voluntary neuromuscular activation (quantified as the rate of electromyogram [EMG] rise) (13,14) and muscle size account for potential changes in leg press strength, leg press power, and mobility function during an approximately 2.5 year longitudinal assessment of healthy older adults. We hypothesize that reductions in both voluntary neuromuscular activation and muscle size will be found, and that these reductions will be associated with loss of strength, power, and mobility function.
Methods
Participants
The participants of this study are a subset of the high functioning older cohort from an earlier cross-sectional investigation of rate of EMG rise in older adults (13). Volunteers were screened by telephone using the following exclusion criteria: age younger than 70 or older than 85, presence of unstable chronic disease, acute or terminal illness, myocardial infarction within 6 months (or other symptomatic coronary artery disease), uncontrolled hypertension (>150/90mm Hg), fracture in the previous 6 months, disease affecting neuromuscular function, use of prescription medications, hormone replacement therapy, body mass index less than 19 or greater than 33kg/m2, significant weight loss or gain within the previous 6 months, participation in a strength or endurance training program within the previous 6 months, and presence of joint pain. Additional screening took place at the research facility, and included confirmation of blood pressure and body mass index as well as assessment on the Short Physical Performance Battery (SPPB). Volunteers with SPPB score less than 10 were excluded from participation. Participants who completed baseline testing were contacted again by telephone or mail after 2.5 years and invited to return to the lab for follow-up testing. Prior to enrollment all volunteers provided written informed consent. This study was approved by the Tufts University Health Sciences Institutional Review Board.
Protocol
All data collection procedures were identical for baseline and follow-up testing. Participants were seated on a bilateral leg press apparatus, and the seat position was adjusted such that the range of motion started with knees flexed to 90° and hips flexed to approximately 110° and ended with the knees almost fully extended and hips flexed to approximately 80°. One repetition maximum (1RM) strength was measured using previously described procedures (15). Maximal voluntary EMG magnitude was measured during an isometric maximal voluntary contraction, with the participants’ legs constrained to the starting position. Participants then performed five maximal speed leg press trials at a resistance setting equal to 70% of the 1RM. The instructions were to perform the leg press movement as rapidly as possible through the concentric phase, and then to slowly return the footplate to the starting position. Each trial was separated by at least 30 seconds of rest. A high level of resistance (ie, 70% 1RM) is desirable for this study because it requires that the leg press movement is preceded by a period of isometric force development during which the rate of EMG rise can be obtained (see Data Analysis section).
Instrumentation
Leg press performance was assessed using a commercially available device (Leg Press A420, Keiser Corporation, Fresno, CA) that we have described previously (13). Force, position, and velocity data were sampled at 400 Hz and saved to disk for off-line analysis. Muscle activation was assessed by surface EMG using a commercially available data acquisition system (Delsys Bagnoli-8, Delsys, Boston, MA). Single differential surface electrodes (Delsys 2.1, Delsys) with 1-cm interelectrode distance were placed over the muscle bellies of the rectus femoris, vastus medialis, and vastus lateralis. A custom-built trigger device marked the instant of movement initiation by changing output voltage when the footplate was lifted from the supporting base. EMG and trigger signals were recorded at a sampling rate of 1kHz using a Powerlab/16SP A/D system and Chart software (ADInstruments, Colorado Springs, CO).
Computed tomography scans of the nondominant thigh were obtained at the midpoint of the femur using a Siemens Somotom Scanner (Erlangen, Germany) operating at 120kV and 100 mA, a slice width of 10mm and a scanning time of 1 second.
Data Analysis
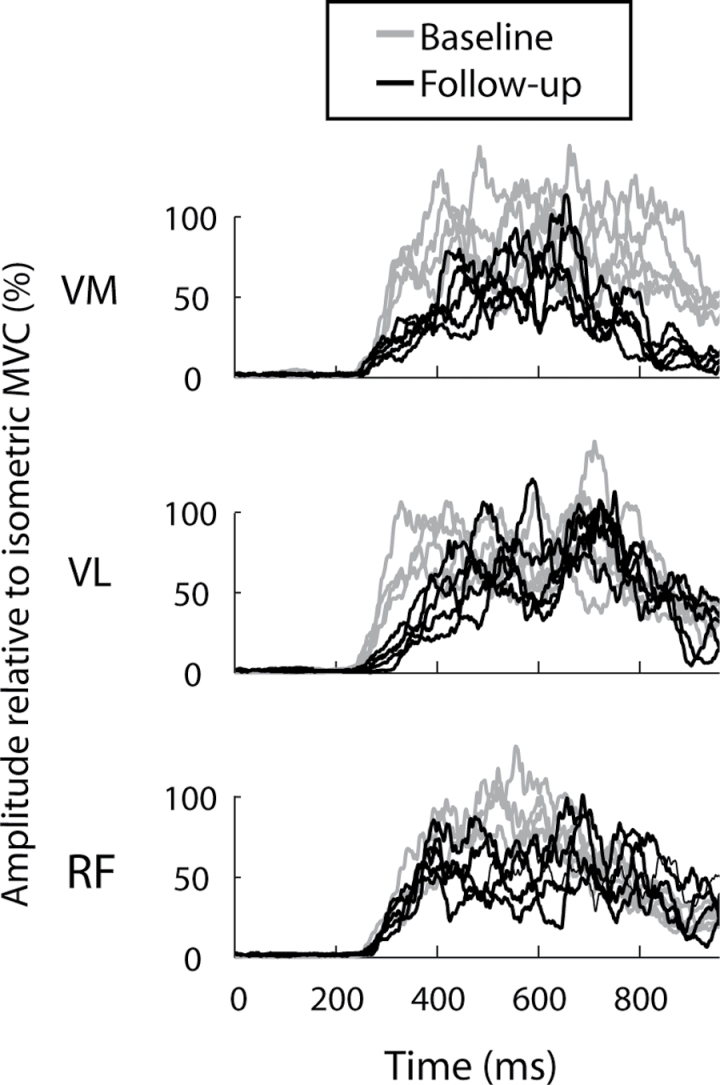
Leg press data were analyzed using a custom analysis program created in MATLAB (version 7.0, The Mathworks, Natick, MA). Power production for each rapid leg press trial was calculated as the mean value during the concentric phase of the movement. All raw EMG signals were debiased (ie, signal mean was set to zero), filtered with a zero phase lag first-order Butterworth band-pass filter (10–200 Hz), rectified, and then smoothed using a 100-ms sliding window average. The EMG recorded from each muscle during the rapid dynamic leg press trial was normalized to its own peak magnitude determined from the maximal voluntary contraction trial. Rate of EMG rise was calculated for each muscle as the mean derivative of the normalized EMG signal between activation onset and movement onset. Activation onset was defined as the first detection of activation in any muscle of the quadriceps group (EMG amplitude exceeding the resting mean plus 3 SDs in either vastus medialis, vastus lateralis, or rectus femoris). Rate of EMG rise from each muscle was averaged to produce a single, composite value for the quadriceps muscle group for each trial. Representative EMG data are presented in Figure 1.
Figure 1.
Representative processed electromyogram (EMG) data from one participant during repeated trials of the leg press movement at baseline (gray) and follow-up (black). EMG signals were filtered and rectified, and are displayed for the vastus medialis (VM), vastus lateralis (VL), and rectus femoris (RF) muscles. EMG magnitude is expressed relative to the magnitude recorded during an isometric maximal voluntary effort contraction.
Computed tomography scans were analyzed by a single, blinded assessor using SliceOmatic v4.2 software (Tomovision, Montreal, Canada). Images were reconstructed on a 512 × 512 matrix with a 25-cm field of view, and muscle cross-sectional area (CSA) was measured by manual tracing using, when applicable, intermuscular adipose tissue as a guide. Midthigh total muscle CSA was measured in the range of 0–100 Hounsfield units and calculated as the sum of low and normal density area. Midthigh subcutaneous adipose tissue CSA was also measured because changes in the thickness of the adipose tissue layer may influence surface EMG signal characteristics.
Statistics
Statistical analysis was performed with JMP statistical software (v. 9.0.2, SAS Institute Inc., Cary, NC). For the maximal effort leg press task at 70% 1RM, power and rate of EMG rise data were averaged across trials (within each participant). Longitudinal changes for rate of EMG rise, power, 1RM strength, muscle CSA, and SPPB were each analyzed separately using a one-tailed repeated measures t test, with the hypothesis that follow-up values would be less than baseline values. Change of subcutaneous adipose tissue was analyzed using a two-tailed repeated measures t test. Pearson’s correlation analysis was used to assess the associations between variables. Correction for multiple comparisons was conducted with the False Discovery Rate Procedure (16).
Results
Participant Characteristics
Participant characteristics at baseline and follow-up are shown in Table 1. Data were acquired from 25 participants at baseline and 16 participants at follow-up. Those individuals lost to follow-up either did not return for the second assessment or we were unable to acquire the EMG measurements for various pragmatic or technical reasons. The individuals lost to follow-up tended to include more female participants (six women and three men, p = .08). The mean age of the individuals lost to follow-up was less than those who completed the study (72.0 vs 75.6 years, p = .02). Baseline SPPB score did not differ (p = .86).
Table 1.
Participant Demographics
| Baseline | Follow-up | |
|---|---|---|
| Age (y) | 75.0±4.0 | 77.8±4.0 |
| Sex (male/female) | 11/5 | Same |
| Time to follow up (y) | n/a | 2.84±0.30 |
| Weight (kg) | 76.3±18.4 | 76.0±18.9 |
| Body mass index (kg/m2) | 25.7±4.2 | 25.4±4.4 |
| Prescription medications (number per person) | 0±0 | 1.19±1.28 |
| Diagnosed health conditions (number per person) | 0±0 | 1.31±1.62 |
The average time between baseline and follow-up testing was 2.84±0.30 years. At baseline, no participants were taking prescription medications and no participants had been diagnosed with health conditions. At follow-up, a general decline in health status was evident for the group. Ten of the 16 participants were taking at least one prescription medication and 9 of the 16 reported having at least one diagnosed medical condition at follow-up. Some individuals had more than one medical condition. Medical conditions included arthritis (1 participant), basal cell carcinoma (2), carpal tunnel syndrome (1), cataracts (4), colorectal polyps (2), eczema (1), enlarged prostate (1), glaucoma (1), high cholesterol (2), inguinal hernia (1), and sarcoidosis (1).
Neuromuscular and Functional Measurements
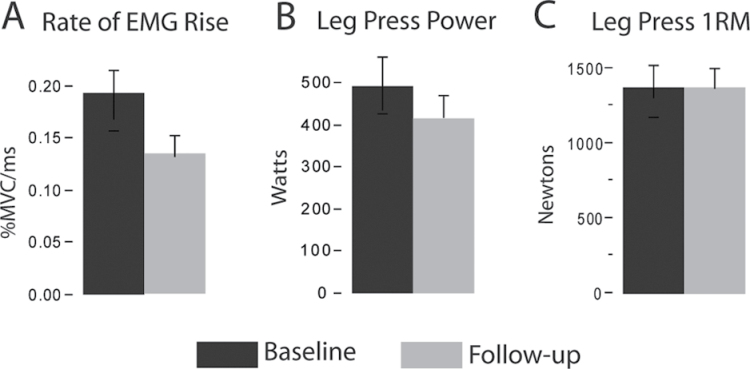
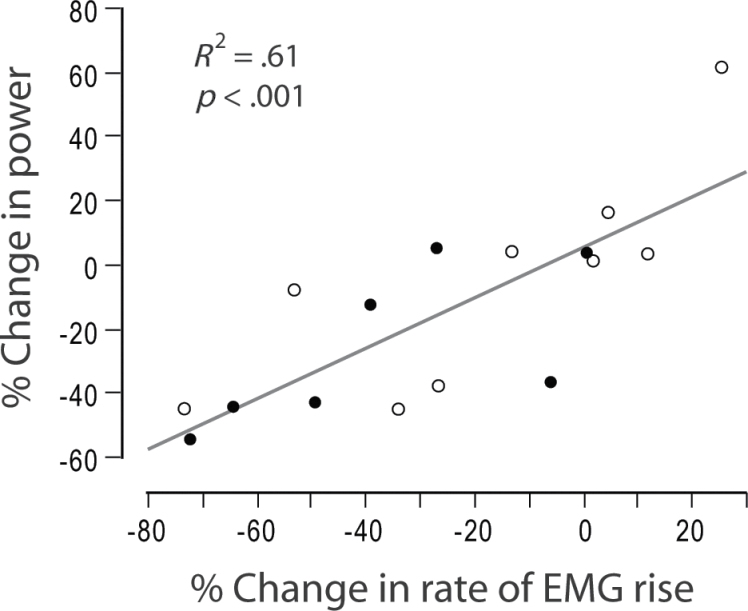
Results for each major study variable are shown in Table 2. Between baseline and follow-up testing, rate of EMG rise decreased 28% (p = .004, Figure 2A). Leg press power decreased 16.5% (p = .01, Figure 2B), but there was no change in leg press 1RM strength (p = .72, Figure 2C). Reduced rate of EMG rise was strongly associated with loss of power, accounting for 61% of the variability among participants (Figure 3, p < .001). There was a trend toward reduced anterior thigh muscle CSA (3%, p = .05), but posterior thigh muscle CSA did not change (p = .84). Reduced anterior thigh muscle CSA was not associated with loss of power (p = .83). SPPB score did not differ between baseline and follow-up testing (p = .17), nor did its subcomponents of 4-m walking speed (p = .35), repeated chair rise time (p = .65), or standing balance (p = .53).
Table 2.
Study Results
| Baseline | Follow-up | p Value | Critical p Value | |
|---|---|---|---|---|
| Rate of EMG rise (%MVC/s) | 0.19 ± .09 | 0.14 ± .08 | .004* | .008 |
| Power (W) | 494.5±225.0 | 413.0±223.7 | .012* | .014 |
| Anterior thigh CSA (cm2) | 56.4±14.7 | 54.6±14.1 | .046 | .021 |
| SPPB score (score out of 12) | 11.1±0.9 | 10.6±1.5 | .17 | .029 |
| One repetition maximum (N) | 1332.8±403.5 | 1336.6±421.4 | .723 | .036 |
| Subcutaneous adipose CSA (cm2) | 52.0±18.9 | 51.7±19.0 | .833 | .043 |
| Posterior thigh CSA (cm2) | 59.4±14.3 | 60.4±14.8 | .846 | .05 |
Notes: CSA = cross-sectional area; EMG = electromyogram; MVC = maximal voluntary contraction; SPPB = Short Physical Performance Battery. Variables are organized by p value. Critical p values indicate the level required for statistical significance after correcting for multiple comparisons with the False Discovery Rate Procedure.
*Statistically significant difference between baseline and follow-up.
Figure 2.
Longitudinal change in neuromuscular performance. Bar graphs show the mean ± standard error. Significant reductions in the rate of electromyogram (EMG) rise (A; −28%, p = .004) and leg press power (B; −16.5%, p = .01) were observed between baseline and follow-up testing. Leg press one repetition maximum (1RM) strength (C) was unchanged.
Figure 3.
Change in rate of electromyogram (EMG) rise explains change in power production. Across all participants, the change in rate of EMG rise explained 61% of the variance of change in power (p < .001). Participants diagnosed with a medical condition during the course of the study are shown as open circles, and participants without a diagnosed medical condition are shown as closed circles.
To determine if there was a link between declining health status and declining neuromuscular function, we compared rate of EMG rise and power between those participants who were diagnosed with a health condition during the study versus those who were not. Participants with a diagnosed health condition actually tended to have less of a reduction in rate of EMG rise (−17% vs −37%) and power (−5% vs −25%), but these differences were not statistically significant (p = .22 and .21, respectively).
Discussion
To our knowledge, the present findings constitute the first longitudinal evidence of impaired neuromuscular activation capacity with aging. Neuromuscular activation was quantified according to the maximal voluntary rate of EMG rise, and declined at a rate of approximately 9% per year. Rate of EMG rise has previously been shown to be associated with physical performance and/or mobility function in older adults (13,14,17) and is sensitive to changes in voluntary neuromuscular activation capability over time (14). Factors that may be responsible for the reduced rate of EMG rise include the number of motor units recruited, the discharge rate of those motor units, and, potentially, the synchronization of discharges across motor units. With aging, it has been shown that maximal motor unit discharge rate declines (18,19) and the occurrence of rapid consecutive discharges (ie, doublets) are reduced (20). These age-related changes in motoneuronal behavior may be caused by reduced efferent drive from the corticospinal pathway (21), reduced motoneuronal conductivity (11,22), or poorer transmission of activation signals at the neuromuscular junction (23). There is also evidence of preferential loss of fast-conducting motor neurons with reinnervation of the orphaned muscle fibers by slower motor neurons, leading to a general slowing of muscle activation properties (24). Any of these changes, or a combination, may have contributed to the observed reductions in rate of EMG rise.
In an earlier cross-sectional study, we found no difference between the rate of EMG rise between healthy, high functioning older adults and middle-aged adults and concluded that impaired activation capacity is not an obligatory consequence of aging (13). Yet, in the present study, there was a clear age-related reduction of rate of EMG rise. This apparent discrepancy might be explained in part by the age and health status of the participants. In the earlier cross-sectional study, the average age of the older group was about 74 years, whereas in the present study, the average age was about 75 years at baseline and about 78 years at follow-up. Perhaps age-related neurophysiolocal changes accelerate with advancing age, such that neuromuscular function deteriorates considerably between the first and second half of the eighth decade. It may also be that emerging health conditions over the course of the longitudinal study contributed to reduction in neuromuscular function. Based on use of prescription medications and the presence of health conditions, there was a general decline in health status for the group over the course of the study. At follow-up testing, most of the older adults had been diagnosed with at least one medical condition and/or had begun taking prescription medication. Although most of the health conditions did not involve neuromuscular function, it is conceivable that some could have affected neuromuscular function indirectly. However, we found no significant differences in the change of rate of EMG rise or power when we directly compared participants with versus without diagnosed health conditions.
Power declined between the baseline and follow-up sessions at a rate of approximately 6% per year. This rate of loss is somewhat higher than earlier studies that reported an age-related loss of muscle performance of approximately 3.5% per year (25,26). The relatively high rate of loss for power may be due in part to the excellent health and functional status of our cohort at baseline. Therefore, the current participants may have had “more to lose” compared with typical older adults. Unlike power, 1RM strength did not change between baseline and follow-up. The finding that power declines earlier or more rapidly than strength is consistent with previous cross-sectional studies (25,27). A potential explanation for this finding is that rapid neuromuscular activation capability is more impaired than activation capability under slower or isometric contraction conditions (13,14). This means that with sufficient time to perform the task, older adults appear able to achieve full or nearly full neuromuscular activation. It is during rapid contractions that impaired neuromuscular performance is most evident and most likely to cause performance decrements. This is supported by our finding that impaired rate of EMG rise was strongly associated with impaired power production during the leg press movement. Contrary to our hypothesis, thigh muscle CSA was not significantly changed between baseline and follow-up assessment. Although anterior thigh muscle CSA showed a trend for reduction, the difference was only about 3% per year. This is roughly consistent with the findings of an earlier longitudinal study, which reported that whole-body lean mass declined at the rate of approximately 1% per year in a large cohort of older adults (5). Our findings further confirm the disassociation between muscle size and muscle performance that occurs with advancing age (7).
The approximately 3-year follow-up period in this study is shorter than most earlier longitudinal studies of physical performance. We chose this time period because our objective was to assess neural and muscle changes that are due specifically to normal aging. By minimizing the duration of the follow-up period, we were also more likely to minimize the confounding effects of changes in medical health, physical activity level, body composition, etc. However, the short study duration may also have prevented us from finding the hypothesized reduction of mobility function. Earlier studies have shown a link between weakness and loss of mobility function (12,13,28), but in the present study, we found that the SPPB score was similar at baseline and follow-up despite a decline in power production capability and rate of EMG rise. This finding may be explained by prior evidence demonstrating that the relationship between muscle performance and functional capability is characterized by thresholds or “cutpoints” (29,30). Individuals with muscle performance exceeding a certain threshold tend to demonstrate comparable functional capability, whereas individuals below that threshold demonstrate a substantial reduction of functional capability. Despite a loss of power at follow-up, our participants had apparently not yet reached a critical threshold for loss of mobility function. This finding suggests that loss of neuromuscular activation and power precedes the decline of mobility function. With a longer follow-up period or with a lower functioning cohort, we would have expected to find a decline in mobility function that was associated with impaired neuromuscular activation and power. Future studies should further evaluate the link between neuromuscular activation and mobility function.
There are some limitations to this study that should be acknowledged. The sample size is small, so it will be important to confirm these findings in future larger studies. Furthermore, the participants of the study were very healthy and high functioning at baseline, so these results may not generalize to lower functioning older adults. It is difficult to account for all of the lifestyle and health factors that might have contributed to altered activation capacity and power production. One potentially important factor, habitual physical factor, was not measured as part of this study. However, because our initial recruitment criteria screened out volunteers who had recently engaged in an exercise program prior to baseline testing, it is unlikely that participants would have had a dramatic reduction in physical activity level over the course of the study. It is also notable that some earlier longitudinal studies of strength loss with aging have found that physical activity is not a significant correlate (4,5).
It should also be acknowledged that although EMG is a powerful tool for neuromuscular assessment, it can be affected by a variety of nonneural factors (31). For example, the amount of subcutaneous adipose tissue and the consistency of electrode placement at the recording site will influence EMG signal characteristics (32). However, we found no change in subcutaneous adipose tissue between the baseline and follow-up time points, so that does not explain our findings. To mitigate the effect of variations in exact location of electrodes and other sources of variability, the rate of EMG rise was normalized to the maximal voluntary EMG magnitude during an isometric maximal voluntary contraction within each testing session. This is a widely used and accepted approach for reducing EMG signal variability between participants and across repeated sessions. Despite the technical issues posed by longitudinal measurement of EMG, our data show that changes in the rate of EMG rise account for approximately 60% of the variability in the change in power (Figure 3). Such a strong correlation supports the assertion that the EMG signal is representative of underlying neuromuscular function.
Conclusions
Maximal voluntary rate of EMG rise declines with advancing age and contributes to loss of muscle power. Future research should elucidate the neurophysiological mechanisms underlying impaired activation and power, so that interventions can be optimized.
Funding
This research was supported by the National Institute on Aging (AG-18844 to R.A.F.) and the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679). This material is based on work supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. D.J.C. was supported by the U.S. Department of Veterans Affairs Office of Rehabilitation Research and Development via Career Development Award (B7176W) and the University of Florida Claude D. Pepper Older Americans Independence Center (P30AG028740).
Acknowledgments
Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture or U.S. Department of Veterans Affairs.
References
- 1. Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greenlund LJ, Nair KS. Sarcopenia–consequences, mechanisms, and potential therapies. Mech Ageing Dev. 2003;124:287–299 [DOI] [PubMed] [Google Scholar]
- 3. Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–M724 [DOI] [PubMed] [Google Scholar]
- 4. Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217 [DOI] [PubMed] [Google Scholar]
- 5. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 6. Clark DJ, Fielding RA. Neuromuscular contributions to age-related weakness. J Gerontol A Biol Sci Med Sci. 2012;67:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834 [DOI] [PubMed] [Google Scholar]
- 8. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–551 [DOI] [PubMed] [Google Scholar]
- 10. Lamoureux EL, Sparrow WA, Murphy A, Newton RU. Differences in the neuromuscular capacity and lean muscle tissue in old and older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2001;56:M381–M385 [DOI] [PubMed] [Google Scholar]
- 11. McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–467 [DOI] [PubMed] [Google Scholar]
- 12. Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2010;65:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326 [DOI] [PubMed] [Google Scholar]
- 15. Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199 [DOI] [PubMed] [Google Scholar]
- 16. Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8 [DOI] [PubMed] [Google Scholar]
- 17. Laroche DP, Knight CA, Dickie JL, Lussier M, Roy SJ. Explosive force and fractionated reaction time in elderly low- and high-active women. Med Sci Sports Exerc. 2007;39:1659–1665 [DOI] [PubMed] [Google Scholar]
- 18. Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–852 [DOI] [PubMed] [Google Scholar]
- 19. Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol. 2008;104:739–746 [DOI] [PubMed] [Google Scholar]
- 20. Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95:2787–2795 [DOI] [PubMed] [Google Scholar]
- 21. Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res. 2009;198:489–500 [DOI] [PubMed] [Google Scholar]
- 22. Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J. The relationship of peripheral motor nerve conduction velocity to age-associated loss of grip strength. Aging (Milano). 1998;10:471–478 [DOI] [PubMed] [Google Scholar]
- 23. Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doherty TJ, Brown WF. Age-related changes in the twitch contractile properties of human thenar motor units. J Appl Physiol. 1997;82:93–101 [DOI] [PubMed] [Google Scholar]
- 25. Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age Ageing. 1994;23:371–377 [DOI] [PubMed] [Google Scholar]
- 26. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Izquierdo M, Ibañez J, Gorostiaga E, et al. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle-aged and older men. Acta Physiol Scand. 1999;167:57–68 [DOI] [PubMed] [Google Scholar]
- 28. Holsgaard-Larsen A, Caserotti P, Puggaard L, Aagaard P. Stair-ascent performance in elderly women: effect of explosive strength training. J Aging Phys Act. 2011;19:117–136 [DOI] [PubMed] [Google Scholar]
- 29. Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, Wolf DA. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol A Biol Sci Med Sci. 2002;57:B144–B152 [DOI] [PubMed] [Google Scholar]
- 30. Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457 [DOI] [PubMed] [Google Scholar]
- 31. De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163 [Google Scholar]
- 32. Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495 [DOI] [PubMed] [Google Scholar]