Abstract
Background
The traditional view of epithelial ovarian cancer asserts that all tumor subtypes share a common origin in the ovarian surface epithelium (OSE)
Design
A literature review was carried out to summarize the emerging understanding of extraovarian sources of epithelial ovarian carcinomas.
Results
Historically, there were no diagnostic criteria for documenting the origin of ovarian epithelial carcinomas. Moreover, there are no normal epithelial tissues in the ovary with morphologic similarities to these tumors. In fact, no precursor lesions have ever been reproducibly identified in the ovary. However, there is a strong correlation between extrauterine Müllerian tissue and the development of ovarian carcinomas, tumors of low malignant potential, and cystadenomas. The most recent support for this hypothesis comes from the careful analysis of risk-reducing bilateral salpingo-oopherectomy specimens from BRCA1 or BRCA2 mutation carriers. These studies showed that a significant majority of high-grade serous ovarian carcinomas, the most common subtype, arise from the fallopian tube fimbriae rather than the OSE.
Conclusions
Mounting evidence indicates that the vast majority of epithelial ovarian carcinomas are not ovarian in origin. Extrauterine Müllerian epithelium from various sites in the reproductive tract likely accounts for the diverse morphology and behavior of these tumors.
Keywords: coelomic epithelium, extrauterine Müllerian epithelium, fallopian tube fimbriae, ovarian carcinoma, primary peritoneal carcinoma
introduction
Worldwide, ovarian cancer is a deadly disease for which there is no effective means for early detection. There were nearly 215 000 incident cases in 2008 with 114 000 mortalities [1]. In general, the neoplasms arise from one of three cell types: epithelial cells, sex-cord stromal cells, or germ cells. Epithelial-derived tumors account for the predominant and most lethal form. This review will present past and current thinking on the histogenesis of these epithelial tumors from clinical and experimental perspectives, emphasizing relevance to their clinical management and to our understanding of their major risk factors.
cell of origin of ovarian epithelial tumors
Extrauterine pelvic tumors showing serous, endometrioid, clear cell, or mucinous differentiation have historically been thought to arise directly from the ovary. In retrospect, this is intriguing given the absence of corresponding normal tissues with similar morphologies in this organ. It seems even more surprising that early pathologists suggested that these tumors, which are distinct in most cases from mesotheliomas both morphologically and immunophenotypically, develop from coelomic mesothelium lining the ovarian surface. Further adding to this intrigue is the fact that normal cells from the same differentiation lineages as those of ovarian epithelial tumors are readily found in tissues and organs adjacent to the ovary. For example the entity of primary fallopian tube carcinoma was recognized as early as 1886 [2] and the papillary serous features of these tumors were well established by 1949 [3]. It would have been logical, given the close proximity of the fallopian tubes to the ovaries, as well as the fact that serous carcinomas almost always involve both the tubal fimbriae and ovaries, to suggest that all serous tumors of the tubo-ovarian region are of fimbrial origin. Instead, early pathologists speculated that all serous tumors of the tubo-ovarian area should be considered primary ovarian unless a tubal origin is evident based on its tissue distribution [3]. It is likely that the following arguments were instrumental in the establishment of this theory:
Co-existence of multiple histological subtypes
Although an origin from the fimbrial end of the fallopian tube could easily account for the serous subtype of ovarian tumors, it cannot account for the nonserous subtypes. However, serous and nonserous subtypes can sometimes co-exist, suggesting a common link in their histogenesis [4].
Distribution of benign tumors
Ovarian carcinomas have benign counterparts, called cystadenomas. Transition to histologically malignant areas can sometimes be observed within otherwise benign-looking cysts, further underscoring their similar differentiation lineage and supporting the view that ovarian cystadenomas and carcinomas share a common origin [5]. The frequent presence of small benign epithelial cysts confined to the ovary and identical in every respect to large cystadenomas except for their size is a strong argument supporting the view that the ovary can be a primary site of origin for at least some epithelial tumors.
Presence of ovarian-like tumors outside the ovary and fallopian tube
Benign serous, endometrioid, clear cell, and mucinous epithelial tumors are not only found within the ovary, but are also present outside the ovary where they are referred to as paratubal or paraovarian cystadenomas. In addition, carcinomas that are morphologically and clinically identical to ovarian carcinomas can sometimes occur without known involvement of either the ovary or the fallopian tube. These tumors, referred to as primary peritoneal carcinomas, can occur several years after a subject has undergone salpingo-oophorectomy for reasons other than cancer debulking, strongly supporting extraovarian and extrafimbrial origins [6].
müllerian metaplasia of coelomic epithelium
Early embryologists regarded the coelomic epithelium lining the ovarian surface as the precursor to all cells present within the adult ovaries including germ cells. The term ‘germinal epithelium’ was coined to underscore this alleged relationship. The idea that ovarian epithelial tumors arose from this epithelium was therefore highly plausible in that context. Given the characteristic resemblance of ovarian epithelial tumors to those arising in fallopian tube, endometrium, and endocervix, a theory was developed based on the notion that the rich hormonal milieu of the ovary can trigger metaplastic changes within the coelomic epithelium, causing a change in its differentiation lineage from mesothelium to tissue types similar to those lining either the fimbriae endometrium, or endocervix [7–9]. This theory readily accounts for the serous, endometrioid, clear cell, and mucinous differentiation seen in major ovarian carcinoma subtypes, respectively [8]. These epithelial types have a common embryological origin from the Müllerian ducts; hence, the term Müllerian metaplasia often used to designate these changes. The theory further suggests that such metaplastic changes are most likely to take place in coelomic epithelium located deep within the ovarian cortex such as within cortical invaginations because of presumed increased exposure to ovarian hormones [5, 9]. The presence of intraovarian Müllerian cysts and cystadenomas is explained based on the notion that such cortical invaginations may occasionally lose their connection to the ovarian surface, resulting in cortical inclusion cysts thought to be highly prone to Müllerian metaplasia. Furthermore, the theory accounts for primary peritoneal carcinomas by stipulating that the coelomic epithelium outside the ovary, similarly to the epithelium lining the ovarian surface, can undergo Müllerian metaplasia due to ovarian hormonal influences.
challenges to the coelomic hypothesis
Although an embryological relationship between the ovarian coelomic epithelium and underlying ovarian tissues is no longer favored, proponents of the coelomic epithelial theory argue that the Müllerian ducts arise from invagination of the coelomic epithelium, maintaining the notion of a link between Müllerian and coelomic epithelium and therefore accounting an alleged predisposition of the latter to undergo Müllerian metaplasia [7, 9, 10]. Recent evidence suggests that there may indeed be an embryological link between the coelomic epithelium and the Müllerian ducts, which arise from an epithelial anlage near the fetal kidney (mesonephros) [11, 12]. However, there is little evidence for an origin from wide invaginations of the coelomic epithelium [11, 12]. In addition, the coelomic hypothesis fails to account for the fact that a precursor lesion to ovarian carcinomas could not be clearly identified on the ovarian surface despite extensive histopathological examinations. Anecdotal findings of dysplastic epithelium within coelomic epithelium [5] were showcased as support for the coelomic hypothesis, but could not convincingly establish this site as an origin of tumor development and were too rare to be regarded as representing the main precursor lesion. Furthermore, examination of salpingo-oophorectomy specimens from individuals with familial ovarian cancer predisposition due to germline mutations in BRCA1 or BRCA2 led to the description of molecular and morphological changes associated with the carrier state for such mutations [13, 14], but did not lead to the identification of early cancers confined to the ovary. Many of these women were instead found to harbor readily identifiable precursor lesions in the fimbrial ends of the fallopian tube [15–24].
current views about the site of origin of epithelial tumors historically regarded as of primary ovarian origin
Fimbrial hypothesis
Early pathologists understood that they were under-diagnosing tumors of fimbrial origin at the benefit of ovarian carcinomas given the strict diagnostic criteria that they adopted for fimbrial carcinomas. The finding of dysplastic changes and early invasive cancers within the fimbriae of individuals with genetic predisposition to tumors historically regarded as high-grade ovarian serous carcinomas, plus the failure to identify similar changes in the ovarian surface epithelium (OSE) of such individuals, led to the realization of the extent to which the frequency of fimbrial carcinomas has been under-estimated in the past [25]. This resulted in fundamental changes in our approach to risk-reducing surgery for BRCA mutation carriers, which is currently focused on the fallopian tube instead of the ovary [26], and on the manner in which these surgical specimens are evaluated by pathologists [20]. However, the notion that carcinomas of the tubo-ovarian region arise in the fimbriae can only account for the serous subtype of ovarian carcinomas. The argument faced by early pathologists that some of these tumors show mixed serous and nonserous differentiation, suggesting a common origin for these multiple histological subtypes, remains valid. In addition, it is clear that the fimbriae are not always involved by serous carcinomas. The occasional development of serous carcinomas in BRCA1/2 mutation carriers who underwent risk-reducing salpingo-oophorectomy several years earlier underscores the fact that a fimbrial origin cannot account for all tumors currently referred to as primary peritoneal carcinomas [6, 27–29]. In addition, an origin from the fimbriae cannot account for intraovarian and para-tubal cystadenomas.
Dual coelomic and fimbrial origin
A simple and straightforward model suggests that early high-grade serous carcinomas, the subtype associated with BRCA1/2 mutation carriers, arise from the fallopian tube fimbriae while the emergence of the other subtypes, including low-grade serous carcinomas, originate from coelomic metaplasia. This model acknowledges that high-grade serous carcinomas, which were thought to have a dual origin from either the fallopian tube or the ovary as early as in the late nineteen century, most commonly arise in the former while the basic premises of the coelomic hypothesis remain applicable to low-grade lesions. Recent evidence, however, suggests a tubal origin for some low-grade serous carcinomas as well as ovarian cortical cysts [30–32].
Implantation of fimbrial epithelium on the ovarian surface
The fimbriae are in constant close proximity to the ovarian surface. They literally rub against this surface at ovulation and can easily intermix with the coelomic epithelium as a result of tubo-ovarian adhesions. A hypothesis recently favored in a major gynecological pathology textbook stipulates that implantation and occasional transformation of serous Müllerian epithelium from the fimbriae on to the ovarian coelomic epithelium predominantly accounts for intraovarian serous neoplasms [33]. Similar to the coelomic hypothesis, the model is consistent with the notion that such epithelium can invaginate and become entrapped within the ovarian stroma, accounting for the presence of intraovarian serous cysts, often called cortical inclusion cysts. This concept minimizes the importance of Müllerian metaplasia and instead emphasizes direct translocation of fimbrial epithelium on to the ovarian surface. However, an origin of serous intraovarian cysts from tubal precursors has never been demonstrated. In addition, serous epithelium admixed with coelomic epithelium on the ovarian surface should be a more common occurrence if this were an important mechanism of tumorigenesis. Moreover, this theory cannot account for the rarity of precursor lesions confined to the ovarian surface. Although fimbrial carcinomas could easily shed to the peritoneum, this concept cannot account for all primary peritoneal carcinomas because (i) these tumors do not involve the fimbriae and therefore could not have originated in this organ by definition, (ii) the strict diagnostic criteria for these tumors, which mandate thorough examination of the ovaries as well as the fallopian tubes, make it highly unlikely that all tumors diagnosed as primary peritoneal represent tubal primaries missed by pathologists due to mere sampling errors, (iii) the fact that several years can elapse following bilateral salpingectomy before a primary peritoneal carcinoma develops is more compatible with an extratubal origin, and (iv) viable implants of serous epithelium have not been described admixed with coelomic epithelium on peritoneal surfaces.
Extrauterine Müllerian hypothesis
Given the resemblance of the major subtypes of ovarian carcinomas to tumors arising in the fallopian tube, endometrium, or endocervix, and given that these three organs are embryologically derived from the Müllerian ducts, it seems logical to look for structures also derived from these ducts as potential sites of origin for all tumors historically regarded as of primary ovarian coelomic origin. Müllerian epithelial structures are in fact abundant in the soft tissues adjacent to the ovary and fallopian tube. Such structures, which include endosalpingiosis, endometriosis, and endocervicosis, represent non-neoplastic counterparts of serous, endometrioid/clear cell, and mucinous ovarian carcinomas, respectively. Although they have previously been referred collectively as ‘secondary Müllerian system’ [34], the term extrauterine Müllerian epithelium (EUME) was recently suggested to allow inclusion of the fallopian tube fimbriae, which is now established as an important site of tumor development [35].
It is well-documented that components of the EUME can give rise to not only serous, endometrioid and mucinous cystadenomas, but also to tumors of low malignant potential [36–41]. It is likely that the frequency of carcinomas arising from these structures, similarly to the frequency of fimbrial carcinomas, may have been grossly underestimated because invasive tumors arising from this primary site are likely to involve the ovary or the fallopian tube early on in the course of the disease given the close proximity to these organs, thus masquerading as ovarian or tubal carcinomas.
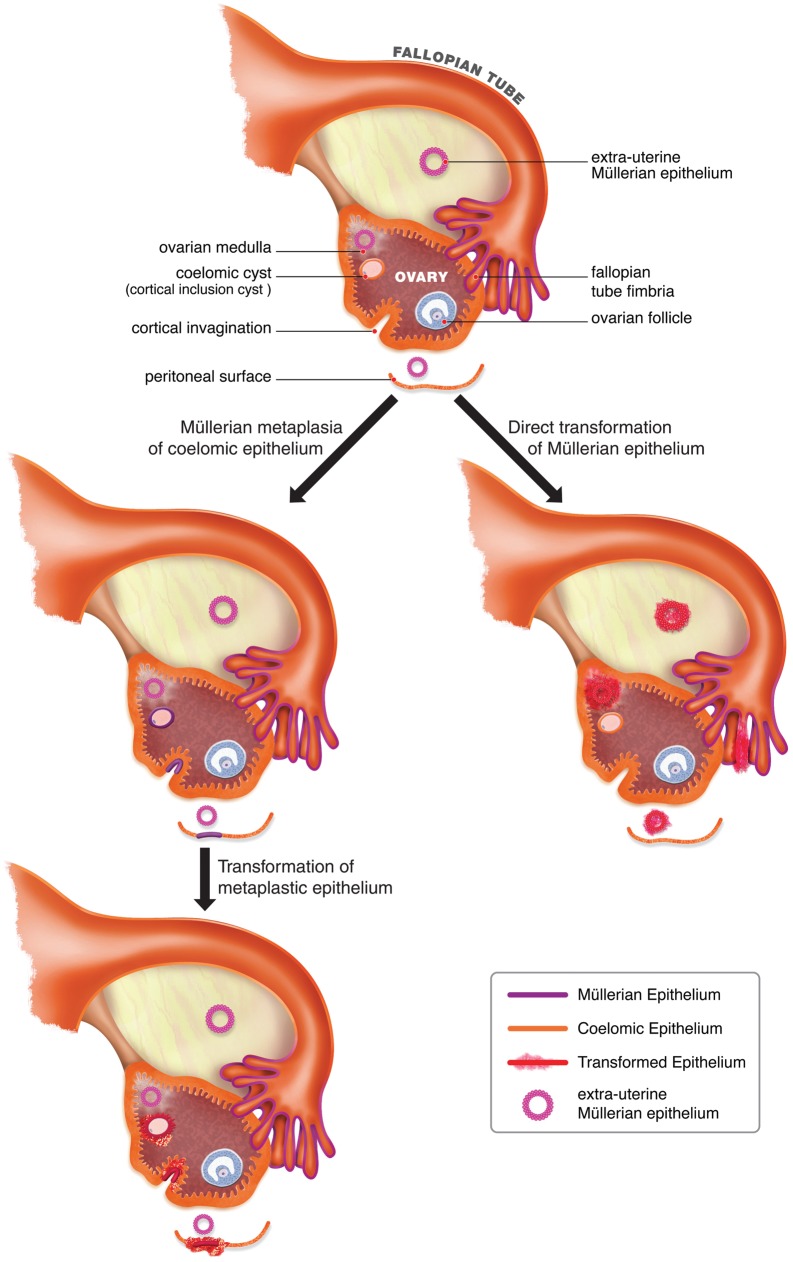
Figure 1 compares the main elements of the EUME hypothesis to those of the coelomic hypothesis. The EUME hypothesis regards EUME as the site of origin of all tumors traditionally referred to as ovarian, fimbrial, and primary peritoneal while the coelomic hypothesis regards these tumors as originating from multiple tissue types in spite of their clinico-pathological similarities. Also, the EUME hypothesis favors the view that tumors arise from Müllerian epithelium normally found within the tubo-ovarian area while the coelomic hypothesis stipulates an origin from coelomic epithelium that underwent Müllerian metaplasia as a necessary precursor step to malignant transformation. The EUME hypothesis accounts for all observations that puzzled early pathologists and led to the formulation of the coelomic hypothesis. Serous carcinomas may arise from either the fimbriae or from foci of endosalpingiosis while endometrioid and clear cell carcinomas arise from endometriosis and mucinous carcinomas arise from endocervicosis. This hypothesis not only readily accounts for paraovarian and paratubal cystadenomas given the presence of EUME in those sites, but also explains the presence of intraovarian Müllerian cysts, which can be regarded not as results of Müllerian metaplasia in pre-existing cortical inclusion cysts, but as examples of endosalpingiosis, endometriosis, and endocervicosis within the ovary. This hypothesis also accounts for primary peritoneal carcinomas because components of the EUME can be found sufficiently far from the ovary and fimbriae to give rise to tumors that spare both of these organs. While appealing, experimental support for this model is lacking and requires development of model systems to test these concepts.
Figure 1.
Diagram of ovarian and tubal anatomy depicting the coelomic and Müllerian models of ovarian cancer development. The coelomic hypothesis shown on the left argues that ovarian epithelial tumors arise from coelomic epithelium or its derivatives in cortical inclusion cysts after it has undergone metaplasia to acquire Müllerian characteristics, regarded as prerequisite for neoplastic transformation. The Müllerian hypothesis shown on the right favors the view that these tumors instead develop directly from pre-existing Müllerian structures in the tubo-ovarian area, referred to as extrauterine Müllerian epithelium, which includes the fallopian tube fimbriae, endosalpingiosis, endometriosis, and endocervicosis.
development of novel model systems
An unfortunate consequence of the widespread acceptance, until recently, that ovarian carcinomas were of coelomic origin has been the use of experimental models based on misconceptions about the exact origin of these tumors. Tremendous research efforts have focused on primary or immortalized in vitro culture of cells derived OSE using an approach first developed by Kruk et al. [42]. More recently, several mouse models were developed based on either transgenic technologies or introduction of viral vectors into the ovarian bursa as a means of targeting the OSE [43–49]. These models may have also involuntary targeted the fallopian tube and EUME. For example these anatomic sites may become genetically altered following intrabursal inoculations, an approach used in several models [45–49]. Likewise, it is possible that a model based on transgenic expression of SV40 large T antigen driven by the Müllerian inhibiting substance type 2 promoter also resulted in expression of this oncoprotein in the fallopian tube and EUME [44]. However, it is clear that newer in vitro and in vivo models that robustly mimic the histogenesis and biological characteristics of the human disease would not only better establish the true origin of these tumors, but also aid in the development of early detection tools, in the design of cancer prevention strategies based on the understanding of the effect of risk factors on normal precursor cells, and for testing of novel therapeutics.
cell culture models
Fallopian tube secretory epithelial cell lines
Serous carcinomas typically share characteristics that mimic a specific cell type found within this epithelium, the fallopian tube secretory epithelial cell (FTSEC) [50, 51]. There is therefore great interest in obtaining tubal/fimbrial cell cultures that retain secretory characteristics. A number of recent studies reported on the isolation and long-term culture of such cells [52–55]. In all cases, the cultured cells retained the expression of key Müllerian markers, including PAX8, a transcription factor that is essential for the development of the female reproductive tract [50, 56, 57]. Transformation of these FTSECs with defined genetic alterations relevant to ovarian cancer generally led to a transformed phenotype in vitro that resulted in growth of high-grade carcinomas in murine hosts that resemble human serous carcinomas [52, 54]. In one case, transformation led to the emergence of a high-grade lesion with mucinous differentiation [55], highlighting the plasticity of EUME and its ability to differentiate into various Müllerian epithelial lineages. Importantly, the tumors express the CA125 and HE4 biomarkers, exhibit transcriptional profiles that are highly similar to human serous ovarian carcinomas, and exhibit widespread copy number alterations typically seen in these tumors [52, 54]. A baboon tubal epithelial cell line was also reported as an alternative to human FTSECs and as a renewable source of cells for studies on downstream signaling pathways and neoplastic transformation in tubal epithelium [58].
Ex vivo and organoid models of fallopian tube epithelium
The development of model systems allowing examination of the molecular links between the biophysical chemistry of the menstrual cycle and the epidemiologic risks it poses is especially important now that the site of origin for serous carcinomas has shifted away from the ovarian surface, arguing against the notion first introduced by Fatallah [59] that the chronic breakage and repair of the ovarian surface associated with incessant ovulation is an important predisposing factor to malignant transformation. While the FTSEC lines described above are ideal for characterizing the role of specific cancer genes on tubal transformation, they grow as conventional monolayers lacking the polarity and the influence of ciliated and stromal cells residing in the normal fallopian tube. Toward this end, a human fallopian tube co-culture system of both secretory and ciliated cells was developed from primary human surgical specimens that recapitulate the morphological, ultrastructural, and immuno-phenotypic properties of the native tubal epithelium in situ [53, 60, 61]. A similar ex vivo model was developed using porcine oviduct epithelium [62]. Irradiation of these ex vivo cultures showed delayed repair of DNA double-strand breaks in the secretory cells compared with adjacent ciliated cells, suggesting that secretory cells are more susceptible to accumulation of mutagenic injury [61]. This would be consistent with the DNA damage observed in the early fallopian tube precursors of serous carcinomas called ‘p53 signatures’ [63].
In an attempt to address whether ovulation itself, as opposed to the hormonal factors associated with the menstrual cycle, may be a source of DNA damage seen in the fallopian tube fimbriae a 3D alginate hydrogel organoid culture of murine fallopian tube epithelium was developed from mice synchronized into different phases of their cycle [58]. The levels of phosphorylated-H2AX, a histone protein that gets phosphorylated at sites of double-strand DNA breaks, were three times higher in the organoid cultures from postovulatory mice compared with controls. This was accompanied by an infiltration of activated macrophages to the oviduct of ovulated mice [58]. The precise nature of the injury delivered to tubal epithelial cells as a consequence of ovulation remains to be defined. However, the development of robust ex vivo models like these and others will play a critical role in deciphering the connection between ovulation and early transformation events in the fallopian tube.
Culture of ovarian cystadenomas and tumors of low malignant potential
In vitro cultures of benign and low malignant potential ovarian epithelial tumors can also provide useful insights into the biology of the EUME [64, 65]. Such tumors can be readily cultured as primary explants and their in vitro longevity can be expanded over 50 or so population doublings using viral oncoproteins [66]. Not only can such cultures be immortalized by forced-expression of telomerase, but have also been known to become spontaneously immortalized in vitro, providing longitudinal models for investigations on the early events leading to malignant transformation [65–67].
mouse models
Models driven by cell-specific promoters
There were no mouse models targeting extraovarian Müllerian epithelium as the cell of origin until recently. Miyoshi et al. [68] utilized the promoter of the murine oviduct-specific glycoprotein (OGP) gene, expressed in the oviducts and uterus, to drive expression of the SV40 large T antigen. The transgenic mice spontaneously developed tumors in the female reproductive tract but not the ovaries. Ovariectomy suppressed T antigen expression and blocked tumorigenesis while estradiol administration to ovariectomized transgenic mice led to dramatic hyperplasia in the oviduct and uterus and to tumor development, suggesting that these tumors are estrogen dependent. This model may therefore be attractive for studies focused on understanding the well-established epidemiological link between the menstrual cycle activity as well as postmenopausal hormone replacement therapy and ovarian cancer risk [69]. Most recently, Kim et al. [70] conditionally knocked out Dicer1 and Pten, resulting in suppression of miRNA synthesis and activation of the PI3 K pathway, respectively. The mutant mice developed lesions with histologic and immunologic phenotypes consistent with high-grade serous carcinomas in the fallopian tube that spread to involve the ovary and then the peritoneum [70]. This model underscores the role of gene regulation through shRNAs in cancer development.
Models relating endometrioid carcinoma to endometriosis
Experimental support for a link between endometriosis and the development of endometrioid ovarian cancer came with the development of a mouse model of peritoneal endometriosis and endometrioid ovarian cancer based on the activation of an oncogenic Kras allele and deletion of Pten [45]. In this setting, the oncogenic Kras mutation induced endometriosis while superimposition of Pten loss propelled these precursor lesions towards invasive endometrioid carcinoma [45].
Models targeting determinants of estrus/menstrual cycle activity
Chodankar et al. [71] attempted to develop a model specifically for investigations of the association between the menstrual/estrus cycle and ovarian cancer risk by using a granulosa- cell-specific promoter to knock out Brca1, a gene that controls familial ovarian cancer predisposition, in cells that play a central role in controlling the menstrual cycle. The mutant mice showed a relative increase in the average length of the proestrus phase of their estrus cycle, which corresponds to the estrogen-dominated follicular phase of the human menstrual cycle [72]. Total circulating levels of estradiol were also elevated in the mutant mice, raising the possibility that loss of BRCA1 function increases epithelial tumor predisposition, at least in part, via increased estrogen stimulation unopposed by progesterone. Indeed, mutant mice with the highest proestrus to metestrus ratio had increased predisposition to ovarian epithelial tumors, although most tumors seen with this model were benign serous tumors consistent with serous cystadenomas as opposed to carcinomas. The conclusion that mutant mice were subjected to increased estrogen stimulation was further supported by the demonstration that they were taller and had stronger bones, two parameters that are controlled by this hormone [73].
The development of additional models driven by promoters directly relevant to high-grade serous ovarian carcinomas, for example the PAX8 promoter expressed in secretory fallopian tube epithelium, should prove particularly useful and advance our understanding of the genetic and physiologic factors that drive the development of ovarian cancers.
conclusion
Recent progress in our understanding of the exact site of origin of tumors traditionally referred to as of primary ovarian origin already has had a significant impact on our approaches to the clinical management of individuals with familial predisposition to these tumors. Although an exclusive origin from fallopian tube epithelium may argue for the merit of simple salpingectomy as an effective risk-reducing procedure for such individuals, remaining controversies dictate that caution should be exercised before recommending such additional profound changes. First, the notion that these tumors may also arise in EUME outside the fimbriae suggests that a simple salpingectomy may not offer maximal protection. In addition, data from experimental models summarized above suggests that although most tumors traditionally regarded as ovarian carcinomas may arise outside the ovary this organ remains an important driver of their development. This is consistent with the strong epidemiological evidence for an association between ovarian cancer risk and menstrual cycle activity. The availability of better experimental models that more robustly mimic the human disease should lead to a better understanding of the biology of these tumors. This in turn will facilitate the identification of novel biomarkers for their early detection, lead to more effective cancer prevention strategies, and should also lead to the identification of novel therapeutic targets.
funding
This work was supported by grants from the National Institutes of Health (R01 CA133117 and RO1 CA119078 to LD, and U01 CA152990, P50 CA105009, and R21 CA156021 to RD); the Ovarian Cancer Research Fund (RD); the Adelson Medical Research Foundation (RD); the Mary Kay Foundation (RD); The Robert and Debra First Fund (RD); the Gamel Ovarian Cancer Research Fund (RD); the Honorable Tina Brozman Foundation (RD), and a gift from the Ovarian Cancer Coalition of Greater California (LD).
disclosure
The authors have declared no conflicts of interest.
acknowledgements
We thank members of our laboratories for fruitful discussions and suggestions and Michael Cooper (Cooper Graphics: www.Cooper247.com) for medical illustration.
references
- 1.Boyle P, Levin B. Geneva, Switzerland: WHO Press; 2008. World cancer report 2008. [Google Scholar]
- 2.Orthmann EG. Ein primãres carcinoma papillare tubae dextrae, verbunden mit ovarial-abscess. Centrabl f Gynãk. 1886;10:816–818. [Google Scholar]
- 3.Finn WF, Javert CT. Primary and metastatic cancer of the fallopian tube. Cancer. 1949;2:803–814. doi: 10.1002/1097-0142(194909)2:5<803::aid-cncr2820020510>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Roberts DK, Marshall RB, Wharton JT. Ultrastructure of ovarian tumors. I. Papillary serous cystadenocarcinoma. Cancer. 1970;25:947–958. doi: 10.1002/1097-0142(197004)25:4<947::aid-cncr2820250432>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Scully RE. Pathology of ovarian cancer precursors. J Cell Biochem Suppl. 1995;23:208–218. doi: 10.1002/jcb.240590928. [DOI] [PubMed] [Google Scholar]
- 6.Pentheroudakis G, Pavlidis N. Serous papillary peritoneal carcinoma: unknown primary tumour, ovarian cancer counterpart or a distinct entity? A systematic review. Crit Rev Hematol/Oncol. 2010;75:27–42. doi: 10.1016/j.critrevonc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Auersperg N, Maines-Bandiera SL, Dyck H. Ovarian carcinogenesis and the biology of the ovarian surface epithelium. J Cell Physiol. 1997;173:261–265. doi: 10.1002/(SICI)1097-4652(199711)173:2<261::AID-JCP32>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Hertig AT, Gore H. Ovarian cystomas of germinal epithelial origin-a histogenetic classification. Rocky Mt Med J. 1958;55:47–50. [PubMed] [Google Scholar]
- 9.Wong AST, Auersperg N. Ovarian surface epithelium: family history and early events in ovarian cancer. Reprod Biol Endocrinol. 2003;1:1–8. doi: 10.1186/1477-7827-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auersperg N, Gilks CB. The origin of ovarian cancer: a developmental view. Gynecol Oncol. 2008;110:452–454. doi: 10.1016/j.ygyno.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salazar H, Godwin AK, Daly MB, et al. Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst. 1996;88:64–67. doi: 10.1093/jnci/88.24.1810. [DOI] [PubMed] [Google Scholar]
- 14.Werness BA, Afify AM, Bielat KL, et al. Altered surface and cyst epithelium of ovaries removed prophylactically from women with a family history of ovarian cancer. Hum Pathol. 1999;30:151–157. doi: 10.1016/s0046-8177(99)90269-1. [DOI] [PubMed] [Google Scholar]
- 15.Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 16.Colgan TJ, Murphy J, Cole DE, et al. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–1289. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn E, Kurman RJ, Vang R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma—evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–426. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leeper K, Garcia R, Swisher E, et al. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol. 2002;87:52–56. doi: 10.1006/gyno.2002.6779. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 21.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 22.Przybycin CG, Kurman RJ, Ronnett BM, et al. Are all pelvic (nonuterine) serous carcinomas of tubal origin. Am J Surg Pathol. 2010;34:1407–1416. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 23.Tone AA, Salvado S, Finlayson SJ, et al. The role of the fallopian tube in ovarian cancer. Clin Adv Hematol Oncol. 2012;10:296–306. [PubMed] [Google Scholar]
- 24.Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in 222 Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179–1183. doi: 10.1038/nm1196-1179. [DOI] [PubMed] [Google Scholar]
- 25.Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 26.Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14–24. doi: 10.1097/aog.0b013e3182783c2f. [DOI] [PubMed] [Google Scholar]
- 27.Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Levine DA, Argenta PA, Yee CJ, et al. Fallopian tube and primary peritoneal carcinomas associated with BRCA mutations. J Clin Oncol. 2003;21:4222–4227. doi: 10.1200/JCO.2003.04.131. [DOI] [PubMed] [Google Scholar]
- 29.Olivier RI, van Beurden M, Lubsen MAC, et al. Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up. Br J Cancer. 2004;90:1492–1497. doi: 10.1038/sj.bjc.6601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurman RJ, Vang R, Junge J, et al. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol. 2011;35:1605–1614. doi: 10.1097/PAS.0b013e318229449f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laury AR, Ning G, Quick CM, et al. Fallopian tube correlates of ovarian serous borderline tumors. Am J Surg Pathol. 2011;35:1759–1765. doi: 10.1097/PAS.0b013e318233b0f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Abushahin N, Pang S, et al. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Modern Pathol. 2011;24:1488–1499. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 33.Kurman RJ, Hedrick Ellenson L, Ronnett BM. Blaustein's Pathology of the Female Genital Tract. New York: Springer; 2011. [Google Scholar]
- 34.Lauchlan SC. The secondary mullerian system revisited. Int J Gynecol Pathol. 1994;13:73–79. doi: 10.1097/00004347-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Dubeau L. The cell of origin of ovarian epithelial tumors. Lancet Oncol. 2008;9:1191–1197. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez AA, Moore WF, Robboy SJ, et al. K-ras mutations in Mullerian inclusion cysts associated with serous borderline tumors of the ovary. Gynecol Oncol. 2001;80:201–206. doi: 10.1006/gyno.2000.6066. [DOI] [PubMed] [Google Scholar]
- 37.Carrick KS, Milvenan JS, Albores-Saavedra J. Serous tumor of low malignant potential arising in inguinal endosalpingiosis: report of a case. Int J Gynecol Pathol. 2003;22:412–415. doi: 10.1097/01.pgp.0000092155.33490.52. [DOI] [PubMed] [Google Scholar]
- 38.Chandraratnam E, Leong AS-Y. Papillary serous cystadenoma of borderline malignancy arising in a paraovarian paramesonephric cyst. Light microscopic and ultrastructural observations. Histopathology. 1982;7:601–611. doi: 10.1111/j.1365-2559.1983.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 39.Kadar N, Krumerman M. Possible metaplastic origin of lymph node "metastases" in serous ovarian tumor of low malignant potential (borderline serous tumor) Gynecol Oncol. 1995;59:394–397. doi: 10.1006/gyno.1995.9955. [DOI] [PubMed] [Google Scholar]
- 40.McCluggage WG, O'Rourke D, McElhenney C, et al. Mullerian papilloma-like proliferation arising in cystic pelvic endosalpingiosis. Hum Pathol. 2002;33:944–946. doi: 10.1053/hupa.2002.127437. [DOI] [PubMed] [Google Scholar]
- 41.Prade M, Spatz A, Bentley R, et al. Borderline and malignant serous tumor arising in pelvic lymph nodes: evidence of origin in benign glandular inclusions. Int J Gynecol Pathol. 1995;14:87–91. doi: 10.1097/00004347-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Kruk PA, Maines-Bandiera SL, Auersperg N. A simplified method to culture human ovarian surface epithelium. Lab Invest. 1990;63:132–136. [PubMed] [Google Scholar]
- 43.Clark-Knowles KV, Garson K, Jonkers J, et al. Conditional inactivation of Brca1 in the mouse ovarian surface epithelium results in an increase in preneoplastic changes. Exp Cell Res. 2007;313:133–145. doi: 10.1016/j.yexcr.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Connolly DC, Bao R, Nikitin AY, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 45.Dinulescu DM, Ince TA, Quade BJ, et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 46.Nikitin AF, Choi K-C, Eng JP, et al. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003;63:3459–3463. [PubMed] [Google Scholar]
- 47.Orsulic S, Li Y, Soslow RA, et al. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1:53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szabova L, Yin C, Bupp S, et al. Perturbation of Rb, p53, and Brca1 or Brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res. 2012;72:4141–4153. doi: 10.1158/0008-5472.CAN-11-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu R, Hendrix-Lucas N, Kuick R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/b-catenin and PI3 K/Pten signaling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–826. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 51.Tone AA, Begley H, Sharma M, et al. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin Cancer Res. 2008;14:4067–4078. doi: 10.1158/1078-0432.CCR-07-4959. [DOI] [PubMed] [Google Scholar]
- 52.Jazaeri AA, Bryant JL, Park H, et al. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia. 2011;13:899–991. doi: 10.1593/neo.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karst AM, Drapkin R. The new face of ovarian cancer modeling: better prospects for detection and treatment. F1000 Med Reprod. 2011;3:22. doi: 10.3410/M3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci USA. 2011;108:7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shan W, Mercado-Uribe I, Zhang J, et al. Mucinous adenocarcinoma developed from human fallopian tube epithelial cells through defined genetic modifications. Cell Cycle. 2012;11:2107–2113. doi: 10.4161/cc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowen NJ, Logani S, Dickerson EB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–337. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 57.Mittag J, Winterhager E, Bauer K, et al. Congential hypothyroid female Pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology. 2007;148:719–725. doi: 10.1210/en.2006-1054. [DOI] [PubMed] [Google Scholar]
- 58.King SM, Hilliard TS, Wu LY, et al. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627–642. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fathalla MF. Incessant ovulation–a factor in ovarian neoplasia? Lancet Oncol. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 60.Fotheringham S, Levanon K, Drapkin R. Ex vivo culture of primary human fallopian tube epithelial cells. J Vis Exp. 2011;9:51. doi: 10.3791/2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levanon K, Ng V, Piao HY, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miessen K, Sharbati S, Einspanier R, et al. Modelling the porcine oviduct epithelium: a polarized in vitro system suitable for long-term cultivation. Theriogenology. 2011;76:900–910. doi: 10.1016/j.theriogenology.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 64.Luo MP, Gomperts B, Imren S, et al. Establishment of long-term in vitro cultures of human ovarian cystadenomas and LMP tumors and examination of their spectrum of expression of matrix-degrading proteinases. Gynecol Oncol. 1997;67:277–284. doi: 10.1006/gyno.1997.4880. [DOI] [PubMed] [Google Scholar]
- 65.Yu J, Roy D, Dubeau L. Increased chromosomal stability of ovarian tumors of low malignant potential compared to cystadenomas. Br J Cancer. 2007;96:1908–1913. doi: 10.1038/sj.bjc.6603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velicescu M, Yu J, Herbert BS, et al. Aneuploidy and telomere attrition are independent determinants of crisis in SV40-transformed epithelial cells. Cancer Res. 2003;63:5813–5820. [PubMed] [Google Scholar]
- 67.Yu VM, Marion CM, Austria TM, et al. Role of BRCA1 in controlling mitotic arrest in ovarian cystadenoma cells. Int J Cancer. 2011;130:2495–2504. doi: 10.1002/ijc.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyoshi I, Takahashi K, Kon Y, et al. Mouse transgenic for murine oviduct-specific glycoprotein promoter-driven simian virus 40 large T-antigen: tumor formation and its hormonal regulation. Molec Reprod Dev. 2002;63:168–176. doi: 10.1002/mrd.10175. [DOI] [PubMed] [Google Scholar]
- 69.Pearce CL, Chung K, Pike MC, et al. Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer. 2009;115:531–539. doi: 10.1002/cncr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, Coffey DM, Creighton CJ, et al. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA. 2012;109:3921–3926. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chodankar R, Kwang S, Sangiorgi F, et al. Cell-Nonautonomous induction of ovarian and uterine serous cystadenomas in mice lacking a functional BRCA1 in ovarian granulosa cells. Curr Biol. 2005;15:561–565. doi: 10.1016/j.cub.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 72.Hong H, Yen H-Y, Brockmeyer A, et al. Changes in the mouse estrus cycle in response to Brca1 inactivation suggest a potential link between risk factors for familial and sporadic ovarian cancer. Cancer Res. 2010;70:221–228. doi: 10.1158/0008-5472.CAN-09-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yen HY, Gabet Y, Liu Y, et al. Alterations in Brca1 expression in mouse ovarian granulosa cells have short-term and long-term consequences on estrogen responsive organs. Lab Invest. 2012;92:802–811. doi: 10.1038/labinvest.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]