The advent of biologic molecules is one of the most important therapeutic advances to have occurred in the medical management of inflammatory bowel disease (IBD). From a Canadian perspective, the monoclonal antibodies directed against tumour necrosis factor-alpha (TNF-α), namely infliximab (Remicade, Johnson & Johnson, USA) and adalimumab (Humira, AbbVie Corporation, USA), are the only approved and commercially available biologics for the treatment of either Crohn disease (CD) or ulcerative colitis (UC) in Canada.
These molecules have a large and complex structure and are generated with the aid of DNA recombination technology. This complexity makes the development of biologically similar substitutes – as is common with smaller, less complex molecules – a challenge. However, following the expiry of the patent on Remicade, a biosimilar infliximab has been developed (CT-P13, Remsima [Celltrion, South Korea], Inflectra [Hospira Inc, USA]). In addition, another biosimilar infliximab has been developed by an Indian pharmaceutical company, Reliance Life Sciences.
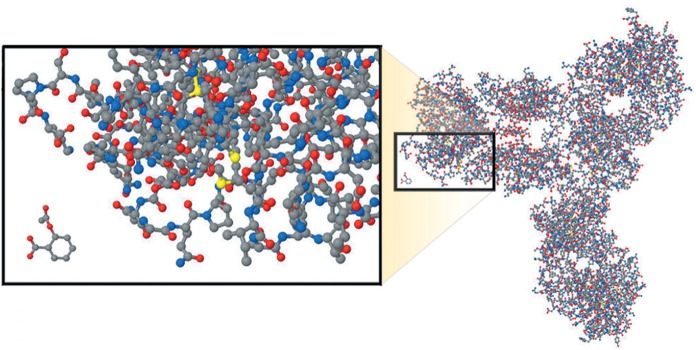
Standard, small-molecule pharmaceuticals are significantly different from their complex biological counterparts (Figure 1). Most synthesized pharmaceuticals have a molecular size of only a few hundred Daltons (Da) (eg, omeprazole is 345 Da). In comparison, infliximab is 149,000 Da. Moreover, monoclonal antibodies have a complex structure that is influenced by the vector and post-translational modification, among other factors (1–3). In addition, although the overall structure of a monoclonal antibody may be known, the manufacturing platform used by the manufacturer of the reference biologic drug (RBD) is not, due to the proprietary nature of the information. As such, a different biological system that is used to produce a biosimilar agent will likely translate into subtle differences that could be difficult to characterize. Such differences have the potential to translate into clinically relevant differences in efficacy, safety and immunogenicity. Therefore, it will be extremely challenging to ensure that a subsequent entry biologic (SEB) is, in fact, ‘equivalent’ to the RBD. Clinical equivalence can only be proven in clinical trials.
Figure 1).
Comparison between a biologic monoclonal antibody and an acetylsalicylic acid molecule. From Kozlowski S, Woodcock J, Midthun K, Behrman Sherman R. Developing the Nation’s Biosimilars Program. N Engl J Med 2011;365:385–8. Reprinted with permission from the Massachusetts Medical Society
It is important for the Canadian gastroenterology community to gain a full understanding of the important issues in the context of the development and entry into the marketplace of such biologic agents.
The objectives of the present document are to:
Provide a brief primer on the terminology germane to this issue.
Describe the current state of SEBs and the existing guidelines from Health Canada and other jurisdictions.
Provide perspective on the potential opportunity in the Canadian marketplace for SEBs in the arena of IBD.
Provide a brief overview of the existing data, generated from two trials conducted in rheumatoid arthritis and ankylosing spondylitis, for infliximab-Celltrion (Remsima).
Identify areas that will require careful thought and attention moving forward.
Provide a current position statement from the Canadian Association of Gastroenterology (CAG) regarding SEBs.
A PRIMER ON SEBs
Three terms exist in the lexicon that refer to essentially the same concept: follow-along protein product or biologics, biosimilars and SEBs. Which term is used is dependent on the jurisdiction in question. Health Canada refers to these molecules as SEBs, while in the United States they are referred to as ‘follow-on protein products’ and, in the European Union (EU), the term ‘biosimilar’ is used (4). Based on first principles, these biologic agents are considered to be ‘nonidentical’ but must be sufficiently similar to the reference product such that there is no clinically meaningful difference between them in terms of safety, purity and efficacy (5). The biological product that the SEB is intended to emulate is termed the ‘RBD’ (4,5). This represents an important distinction from small molecules for which structurally identical generic compounds can be developed and approved solely on the basis of chemical and manufacturing standards, and demonstration of pharmacokinetic equivalency.
Other important concepts to comprehend include interchangeability and substitutability. In the United States, the Patient Protection and Affordable Care Act contains a section called the Biologics Price Competition and Innovation Act, which describes an abbreviated licensing application process for SEBs. In this act, interchangeability exists when the SEB is “biosimilar to the reference product” and “can be expected to produce the same clinical result as the reference product in any given patient” and “the risk in terms of safety or diminished efficacy of alternating or switching between the biosimilar and the reference product must not be greater than the risk of using the reference product without such alternation or switch” (1,6). Therefore, interchangeability refers to the relative equality of a physician prescribing either an RBD versus an SEB for a given indication for which both agents are approved. The term ‘substitutability’ is often used in the same context, but is most commonly used in reference to pharmaceutical dispensing. With generic, small-molecule agents, pharmacies can often be allowed to automatically substitute for the less-expensive agent. As will be discussed below, Health Canada explicitly states that SEBs should not be automatically substituted when the RBD is prescribed by a physician. Unfortunately, the authority to make such decisions lies with provincial health authorities; therefore, the potential exists for decisions to be made, based on cost considerations, that are counter to Health Canada’s sensible assertion.
THE CURRENT STATE OF SEBs IN CANADA
The infliximab molecule made by Celltrion (CT-P13, Remsima, Inflectra) is by no means the first potential SEB. SEBs are, in fact, not new and globally a number of these agents currently exist. As such, international regulatory authorities have developed standards and guidelines pertaining to the development and licensing requirements for SEBs (4,7–10). In general, the principles underlying the guidelines in each respective jurisdiction are similar. Currently, there are no SEBs that have been approved under these guidelines in the United States, five are approved in Europe and only one in Canada (a recombinant human growth hormone [Omnitrope, Sandoz, Canada]). However, it is worth noting that Omnitrope was approved as an SEB before the finalization of the Health Canada guidelines (11). No SEB is currently in use or approved for the treatment of IBD in any jurisdiction globally, with the exception of infliximab-Celltrion, which is approved for the treatment of IBD in South Korea.
The importance of rapidly gaining an understanding and developing a systematic, evidence-based approach to the regulatory approval of SEBs is underscored by the fact that globally, several products referred to as biosimilar are already in use in countries such as India, Peru and Argentina. These include biosimilar erythropoietin and agents deemed biosimilar to rituximab (5,12–14). These products may or may not actually meet the criteria of being a biosimilar according to the more stringent Health Canada and Food and Drug Administration guidelines.
Some key criteria and policy statements contained in the Health Canada guidelines can be summarized as follows (the full list of requirements can be found in the Health Canada document) (4):
An SEB can only be considered if there is an existing approved RBD.
The SEB can be judged to be similar to the RBD by meeting an appropriate set of predetermined criteria (ie, similar biochemical structure, similar pharmacokinetic and pharmacodynamic properties and demonstration of similarity in clinical studies).
SEBs are not ‘generic biologics’ and authorization does not declare pharmaceutical or therapeutic equivalence to the RBD.
An SEB is to be considered a new biologic drug and is regulated no differently than the RBD. The SEB should not be used as the RBD for a later SEB submission (eg, if an SEB was approved for one indication, the SEB cannot be defined as the RBD for the SEB seeking approval for a different indication).
SEBs will not be labelled as interchangeable with the RBD.
An SEB should not be automatically substituted in place of a RBD by dispensing pharmacies.
As such, Health Canada will insist that SEBs be developed and studied in a manner similar to RBDs and will be required to be supported by nonclinical and clinical studies sufficient to support the SEB as a stand-alone product.
THE POTENTIAL OPPORTUNITY IN THE CANADIAN MARKET
The advent of TNF antagonists for the treatment of IBD has revolutionized how these complex and heterogeneous diseases are managed. In randomized controlled trials, these treatments have been associated with improved quality of life, reduced hospitalization and surgical rates, and may have the potential to alter the natural history of disease (15–17). Their superiority in CD relative to thiopurine antimetabolites was demonstrated in one randomized controlled trial with a follow-up period of one year (18).
However, it must be recognized that the cost of these therapies is significant and represents a significant burden to both public and private payers. In 2011, the total drug acquisition cost of biologic therapy for the treatment of IBD in Canada was approximately $460 million (19). However, drug acquisition costs account for only a portion of the total economic burden of IBD. Because these therapies are being used more frequently and earlier in the disease course, the market penetration is likely to increase the total cost of drugs. As such, provided SEBs will enter the market at a substantially lower cost, there is a potential opportunity to reduce cost to patients and payers that could enhance the availability of this class of therapy; SEBs could also drive down the cost of RBDs. In fact, cost considerations from the standpoint of private payers has begun to affect clinical decision making because adalimumab has been prioritized over infliximab by one Canadian payer (Green Shield Canada) on the basis of a perceived cost difference (20). Naturally, these considerations are wholly contingent on SEBs for IBD having a clear, demonstrated efficacy and safety record comparable with the RBDs.
EXISTING DATA FOR INFLIXIMAB-CELLTRION (REMSIMA, INFLECTRA)
There have been no clinical studies evaluating CT-P13 for treatment of CD or UC, and the available data for this molecule are still early in its evolution. It has been studied in a phase 3 clinical ‘equivalence’ trial with Remicade as the active comparator in patients with active rheumatoid arthritis (21). A 600-patient noninferiority designed study demonstrated a 2% difference in the American College of Rheumatology 20% response rate (60.9% CT-P13, 58.6% Remicade). The statistical assumption in this study was an equivalence margin of ±15% (95% CI). However, it is worth noting the comparison used in the area under the curve approach in their analysis. This methodology would not be acceptable to regulators evaluating an innovator molecule. Thus, the true ‘equivalence’ is worth questioning. The pharmacokinetics of CT-P13 was demonstrated to be equivalent to Remicade in a trial involving 200 patients with ankylosing spondylitis who were treated in combination with methotrexate (22). We can anticipate preliminary data in patients with IBD in the near future. As will be discussed below, some key questions will need to be addressed in terms of how these these data are interpreted.
WHAT ARE THE KEY QUESTIONS MOVING FORWARD?
There are several important questions that need to be thoughtfully addressed as the Canadian gastroenterology community moves forward in the arena of SEBs. The complexity of the potential issues that arise are beyond the scope of the present brief introduction and are reviewed in detail elsewhere (1). Some, but by no means all, of the key areas of future interest include:
How will clinical trials involving patients with IBD proceed and how will they be designed?
Health Canada, as well as other jurisdictions, requires a full supportive dossier of both nonclinical (ie, pharmacodynamic and pharmacokinetic) and clinical studies to support the biosimilarity and the clinical efficacy of the molecule. However, what is less clear is what the requirement will be with respect to the design of the clinical trial that is performed (superiority, noninferiority, etc). This is an important issue because a noninferiority study requires a large number of patients, which will be logistically challenging. It has been estimated that to exclude with 95% confidence that the SEB is not more than 7.5% inferior, 1500 patients would be required (1). As such, it seems plausible that regulatory decisions may be made based on trials of smaller size, increasing the likelihood that trials will fail to detect a clinically meaningful difference in therapeutic effect between the RBD and the SEB. An additional important consideration is whether regulatory agencies will require both induction and maintenance data or only induction data? As we have learned with existing TNF antagonists, attenuation of response with maintenance therapy is a key issue and it will be important to know whether SEBs will have similar performance characteristics in both induction and maintenance. An induction study alone will fail to inform us sufficiently.
Additionally, where will these clinical trials be conducted? If clinical trials are conducted in other regions of the world, such as Asia, a question will reasonably arise as to the generalizability of the results to a typical Canadian patient population, which may be ethnically more diverse. In fact, there are suggestions of important clinical differences of IBD in Asia that could be relevant (23).
The impact of immunogenicity on an SEB.
It is well known that immunogenicity is a clinically relevant phenomenon with both infliximab and adalimumab, and that immunogenicity impacts anti-TNF-α drug levels and clinical efficacy in both CD and UC (18,24–30). However, monoclonal antibodies are complex molecules and gaining a comprehensive understanding of the impact of immunogenicity to infliximab and adalimumab, as an example, has taken several years to accomplish. There will no guarantee that our understanding will easily be extrapolated to an SEB that may be subtly different in molecular structure. New assays need to be developed and studies undertaken to explore and understand this issue. Questions will necessarily arise as to whether antibodies directed against an RBD will cross-react with the SEB and vice versa. Inevitably, there will be many unanswered questions that will need to be explored. The clinical importance of this issue is well illustrated by immune-mediated changes that led to pure red cell aplasia in chronic renal failure patients being treated with a modified version of epoetin (Eprex, Janssen, USA), whereby a subtle production modification had significant and unforeseen consequences (31).
Will there be extrapolation across indications?
An SEB molecule will likely enter the marketplace for one indication as a prelude to possible approval for other indications. If biosimilarity is demonstrated sufficiently for, as an example, rheumatoid arthritis, will the nonclinical and clinical studies be sufficient to abbreviate the number and type of clinical studies that are required to gain approval for an alternative indication such as CD or UC? The Health Canada guidance document clearly indicates that this should not be the case (4). Interestingly, the Eurpean Medicines Agency and the United States Food and Drug Administration do appear to open the door for extrapolation across indications. Our previous experience in IBD has already dictated that extrapolation of indications can be problematic and generate unanticipated results (32). As such, it seems appropriate that Health Canada has taken a more rigorous stance on this issue.
CAG POSITION STATEMENTS REGARDING SEBs FOR IBD
The present brief review has highlighted some key issues that will require ongoing discussion and debate and, at this time, no definitive conclusions can be reached. Moreover, there will need to be a well-informed debate to help clarify the approach that societies, such as the CAG, and individual gastroenterologists must take. However, based on the above discussion, some position statements that are very similar to the position of Health Canada can be made by the authors on behalf of the CAG:
SEBs represent a potentially effective and cost saving option for the management of IBD that may serve to enhance access to biologic therapy.
SEBs should be regarded as stand-alone products, and should be supported by well-designed nonclinical and clinical studies in a population relevant to Canadian patients.
SEBs cannot be regarded as interchangeable with the RBD.
Prescriptions for RBDs should not be automatically substituted for less expensive SEBs by dispensing pharmacies.
SEBs should be supported by long-term pharmacovigilance data in a fashion similar to RBDs.
Companies bringing SEBs to the Canadian market should be committed to improving patient care by acquiring new scientific data beyond that which is required as a minimum to satisfy regulatory authorities and their commercial imperatives.
SUMMARY
Biosimilar monoclonal antibodies directed against TNF-α for the treatment of IBD are being developed, yet the complexity of these molecules, together with factors related to their manufacture, have the potential to translate into clinically relevant differences in efficacy, safety and immunogenicity in biosimilar agents. It is important for the Canadian gastroenterology community to gain a full understanding of the important issues in the context of the development and entry into the marketplace of such biologic agents.
Footnotes
CAG STATEMENT: This position paper has been developed under the direction of Drs Shane Devlin and Brian Bressler in accordance with the policies and procedures of the Canadian Association of Gastroenterology (CAG) and under the direction of CAG Clinical Affairs. It has been review by the CAG Practice Affairs and Clinical Affairs Committees and the CAG Board of Directors. The article was developed following a thorough consideration of medical literature and the best available evidence and clinical experience. It represents the consensus of a Canadian panel comprised of experts on this topic. This position paper aims to provide a reasonable and practical approach to care for specialists and allied health professionals obliged with the duty of bestowing optimal care to patients and families, and can be subject to change as scientific knowledge and technology advance and as practice patterns evolve. The position paper is not intended to be a substitute for physicians using their individual judgement in managing clinical care in consultation with the patient, with appropriate regard to all the individual circumstances of the patient, diagnostic and treatment options available and available resources. Adherence to these recommendations will not necessarily produce successful outcomes in every case.
DISCLOSURES: No industry or government relationships to report (HS).
ADVISORY BOARD MEMBERSHIP: AbbVie (AB, SD, BF, RF), AstraZeneca (BF), Celgene (BF), Centocor (BF, RF), Elan/Biogen (BF), Ferring (RF), Janssen-Ortho (AB, SD, RF), Merck/Schering (BF), Novartis (BF), Pfizer (BF), Prometheus (BF), Salax (BF), Shire (SD, RF), Takeda (AB, BF), Tillotts (BF), UCB Pharma (BF) and VSL3 (RF).
CONSULTATION FEES: Abbvie (BB, BF, RF), ActoGenix (BF), Albiero Pharma (BF), Amgen (BF), AstraZeneca (BF), Athersys (BF), Avaxia Biolgics Inc (BF), Axcan (BF), Boehringer Ingelheim (BF), Bristol-Myers Squibb (BF), Celgene (BF), Centocor (BF, RF), Elan/Biogen (BF), Ferring (BF, RF), Genetech (BB, BF), GiCare (BF), Gilead (BF), Given Imaging (BF), GlaxoSmithKline (BF), Ironwood (BF), Janssen-Ortho (BB, BF, RF), Merck/Schering (BF), Millennium Research Group (BF), Nektar (BF), Novo Nordisk (BF), Pfizer (BF), Prometheus (BF), Proctor & Gamble/Warner Chilcott (BF), Salax (BF), Serono (BF), Shire (BF, RF), Sigmoid (BF), Synergy (BF), Takeda (BB, BF), Teva (BF), Tillotts (BF), UCB Pharma (BF), Unity Pharma (BF), VSL3 (RF), Wyeth (BF), Zealand (BF), Zyngenia (BF).
EDUCATIONAL SUPPORT: AbbVie (AB).
RESEARCH GRANTS/CLINICAL TRIAL FUNDING: AbbVie (BB, BF, RF), ActoGenix (BF), Alba (RF), Amgen (BB), Bristol-Myers Squibb (BB, BF, RF), Centocor (BF, RF), CombinatoRx (BF), Elan/Biogen (BF), Genetech (BB, BF, RF), GlaxoSmithKline (BB, RF), Janssen-Ortho (BB, RF), Merck/Schering (SD, BF, RF), Millennium Research Group (BF, RF), Novartis (BF, RF), Proctor & Gamble/Warner Chilcott (RF), Protein Design Labs (BF), Qu Biologics (BB), Roche (RF), Takeda (BB), Tillots (BF), UCB Pharma (BF), VSL3 (RF), Wyeth (BF).
SPEAKERS BUREAU: AbbVie (AB, BB, SD, BF), Janssen-Ortho (AB, BB, SD, BF), Merck/Schering (SD), Shire (SD), Takeda (BB), UCB Pharma (BF).
OWNER/SHAREHOLDER: Metabolomic Technologies Inc (RF).
MEMBER OF CROHN’S DBAAC: British Columbia Ministry of Health (BB)
REFERENCES
- 1.Kay J, Feagan BG, Guirguis MS, et al. Health Canada/BIOTECanada Summit on regulatory and clinical topics related to subsequent entry biologics (biosimilars), Ottawa: May 14, 2012. Biologicals. 2012;40:517–27. doi: 10.1016/j.biologicals.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Crommelin DJ, Storm G, Verrijk R, et al. Shifting paradigms: Biopharmaceuticals versus low molecular weight drugs. Int J Pharm. 2003;266:3–16. doi: 10.1016/s0378-5173(03)00376-4. [DOI] [PubMed] [Google Scholar]
- 3.Misra M. Biosimilars: Current perspectives and future implications. Indian J Pharmacol. 2012;44:12–4. doi: 10.4103/0253-7613.91859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health Products and Food Branch, Health Canada Guidance for sponsors: Information and submission requirements for subsequent entry biologics (SEBs) < www.hc-sc.gc.ca/dhp-mps/brgtherap/applic-demande/guides/seb-pbu/notice-avis_seb-pbu_2010-eng.php> 2010 (Accessed May 31, 2013).
- 5.Russell AS, Ahluwalla V, Barnabe C, et al. Subsequent entry biologics/biosimilars: A viewpoint from Canada. Clin Rheumatol. 2012;31:1289–92. doi: 10.1007/s10067-012-2066-5. [DOI] [PubMed] [Google Scholar]
- 6.Kay J. Biosimilars: A regulatory perspective from America. Arthritis Res Ther. 2011;13:112. doi: 10.1186/ar3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Committee for Medicinial Products for Human Use, European Medicines Agency Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues. EMEA/CHMP/BWP/49348/2005. < www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003953.pdf> 2006 (Accessed May 31, 2013).
- 8.Committee for Medicinial Products for Human Use, European Medicines Agency Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. EMEA/CHMP/BMWP/42832/2005. < www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdf> 2006 (Accessed May 31, 2013).
- 9.Food and Drug Administration Guidance for industry: Scientific considerations in demonstrating biosimilarity to a reference product (draft) < www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf> 2012 (Accessed May 31, 2013).
- 10.Food and Drug Administration Guidance for industry: Quality considerations in demonstrating biosimilarity to a reference product (draft) < www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf> 2012 (Accessed February 10, 2013).
- 11.Klein AV. The first subsequent entry biologic authorized for market in Canada: The story of Omnitrope, a recombinant human growth hormone. Biologicals. 2011;39:278–81. doi: 10.1016/j.biologicals.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Storring PL, Tiplady RJ, Gaines, et al. Epoetin alfa and beta differ in their erythropoietin isoform compositions and biological properties. Br J Haematol. 1998;100:79–89. doi: 10.1046/j.1365-2141.1998.00521.x. [DOI] [PubMed] [Google Scholar]
- 13.Skibeli V, Nissen-Lie G, Torjesen P. Sugar profiling proves that human serum erythropoietin differs from recombinant human erythropoietin. Blood. 2001;98:3626–34. doi: 10.1182/blood.v98.13.3626. [DOI] [PubMed] [Google Scholar]
- 14.Combe C, Tredree RL, Schellekens H. Biosimilar epoetins: An analysis based on recently implemented European medicines evaluation agency guidelines on comparability of biopharmaceutical proteins. Pharmacotherapy. 2005;25:954–62. doi: 10.1592/phco.2005.25.7.954. [DOI] [PubMed] [Google Scholar]
- 15.Loftus EV, Feagan BG, Colombel JF, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: Patient-reported outcomes of the CHARM trial. Am J Gastroenterol. 2008;103:3132–41. doi: 10.1111/j.1572-0241.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 16.Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: Results from the CHARM study. Gastroenterology. 2008;135:1493–9. doi: 10.1053/j.gastro.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250–60. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 18.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 19.Rocchi A, Benchimol EI, Bernstein CN, et al. Inflammatory bowel disease: A Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–7. doi: 10.1155/2012/984575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green Shield Canada < https://www.cutrust.com/employee/downloads/Benefits/drugforms/Drug%20Special%20Authorization%20Request%20Form%20for%20TNF%20Antagonists%20and%20Similar%20Biologics%20(2012-02).pdf> (Accessed March 14, 2013).
- 21.Yoo D, Miranda P, Piotrowski M. A randomized, double-blind, phase 3 study demonstrates clinical equivalence of CT-P13 to infliximab when co-administered with methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:359. doi: 10.1136/annrheumdis-2012-203090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park W, Hrycaj P, Kovalenko V. A randomized, double-blind, phase 1 study demonstrates equivalence in pharmacokinetics, safety, and efficacy of CT-P13 and infliximab in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:111. doi: 10.1136/annrheumdis-2012-203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prideaux L, Kamm MA, De Cruz PP, et al. Inflammatory bowel disease in Asia: A systematic review. J Gastroenterol Hepatol. 2012;27:1266–80. doi: 10.1111/j.1440-1746.2012.07150.x. [DOI] [PubMed] [Google Scholar]
- 24.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–53. doi: 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 25.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 26.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137:1628–40. doi: 10.1053/j.gastro.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 27.Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–31. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–54. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: A predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 30.Panaccione R, Ghosh S, Middleton S, et al. Infliximab, azathioprine, or infliximab + azathioprine for treatment of moderate to severe ulcerative colitis: The UC Success Trial. Gastroenterology. 2011;140:A-202. (Abst) [Google Scholar]
- 31.McKoy JM, Stonecash RE, Cournoyer D, et al. Epoetin-associated pure red cell aplasia: Past, present, and future considerations. Transfusion. 2008;48:1754–62. doi: 10.1111/j.1537-2995.2008.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn’s disease: A randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088–94. doi: 10.1053/gast.2001.28674. [DOI] [PubMed] [Google Scholar]