Abstract
BACKGROUND:
Many consider histology to be the gold standard for Helicobacter pylori detection. Because the number and distribution of H pylori organisms vary, particularly in patients taking proton pump inhibitors (PPIs), the American Gastroenterological Association recommends discontinuing PPIs two weeks before endoscopy, and taking biopsies from both the body and antrum.
OBJECTIVE:
To assess the influence of clinical practice on the histopathological detection of H pylori infection.
METHODS:
Electronic patient records were evaluated for the sites of gastric sampling and PPI use at endoscopy. One hundred fifty cases with biopsies taken from both antrum and body were randomly selected for pathological re-review with special stains. The gastric regions sampled, H pylori distribution and influence of clinical factors on pathological interpretation were assessed.
RESULTS:
Between 2005 and 2010, 10,268 biopsies were taken to detect H pylori. Only one region was sampled in 60% of patients (antrum 47%, body 13%). Re-review of biopsies taken from both antrum and body indicated that the correct regions were sampled in only 85 (57%) patients. Of these, 54 were H pylori positive and 96 were H pylori negative. H pylori was present in the antrum in only 15% of the patients and body only in 21%. Of 96 H pylori-negative patients, two were reinterpreted as positive. Forty-seven per cent of patients were taking PPIs at endoscopy, contributing to both false-negative and false-positive diagnoses.
CONCLUSION:
Despite national and international guidelines for managing H pylori infection, the American Gastroenterological Association guidelines are infrequently adhered to, with PPIs frequently contributing to false diagnosis; sampling one region only increases the likelihood of missing active infection by at least 15%.
Keywords: Endoscopic accuracy, Diagnostic accuracy, Helicobacter pylori, Histopathological diagnosis, Management guidelines
Abstract
HISTORIQUE :
Bien des gens considèrent l’histologie comme la référence pour déceler l’infection à Helicobacter pylori. Puisque le nombre et la répartition des organismes à H pylori varient, notamment chez les patients prenant des inhibiteurs de la pompe à protons (IPP), l’American Gastroenterological Association recommande d’arrêter de prendre des IPP deux semaines avant l’endoscopie et de prélever des biopsies à la fois dans le corps et l’antre.
OBJECTIF :
Évaluer l’influence de la pratique clinique sur la détection histopathologique de l’infection à H pylori.
MÉTHODOLOGIE :
Les chercheurs ont évalué les dossiers électroniques des patients pour déterminer les foyers de prélèvement gastrique et l’utilisation des IPP à l’endoscopie. Ils ont sélectionné au hasard 150 cas qui avaient subi des biopsies prélevées dans l’antre et dans le corps afin de procéder à un nouvel examen pathologique à l’aide de colorations spéciales. Ils ont évalué les foyers gastriques des prélèvements, la répartition du H pylori et l’influence des facteurs cliniques sur l’interprétation pathologique.
RÉSULTATS :
Entre 2005 et 2010, 10 268 biopsies ont été prélevées pour déceler le H pylori. Le prélèvement a été effectué dans un seul foyer chez 60 % des patients (antre 47 %, corps 13 %). Le nouvel examen des biopsies prélevées dans l’antre et dans le corps a indiqué que les prélèvements provenaient des bons foyers chez seulement 85 (57 %) des patients. De ce nombre, 54 étaient positifs au H pylori et 96 y étaient négatifs. Le H pylori était présent dans l’antre de seulement 15 % des patients et dans le corps de seulement 21 % des patients. Chez les 96 patients négatifs au H pylori, deux ont été réinterprétés comme positifs. Quarantesept pour cent des patients prenaient des IPP à l’endoscopie, contribuant à la fois à des diagnostics faux négatifs et faux positifs.
CONCLUSION :
Malgré les lignes directrices nationales et internationales sur la prise en charge de l’infection à H pylori, les lignes directrices de l’American Gastroenterological Association sont peu respectées, et les IPP contribuent souvent à un faux diagnostic. Le prélèvement dans un seul foyer accroît d’au moins 15 % la possibilité de rater l’infection active.
Helicobacter pylori infection remains clinically relevant, particularly in centres that serve the general population with a high mean age and a high number of immigrants (1–3). The infection plays an active role in many diseases, including peptic ulcer disease, gastric mucosa-associated lymphoid tissue (MALT) lymphoma and adenocarcinoma, dyspepsia, iron-deficiency anemia, idiopathic thromobocytopenic purpura and, in some patients, coronary artery disease (4–18). Active infection should be eradicated in patients with uninvestigated dyspepsia, active peptic ulcer disease, a high risk of gastric cancer and gastric MALT lymphoma (18,19). Importantly, to reduce the risk of gastric atrophy, active H pylori infection should be investigated and eradicated before administering proton pump inhibitors (PPIs) to patients with gastroesophageal reflux disease (20).
Although several tests are available, many consider histopathological diagnosis of H pylori infection to be the ‘gold standard’ (21,22). Obtaining corpus biopsies in addition to antral biopsies has been shown to increase diagnostic accuracy (22,23); however, because PPIs decrease H pylori density, distribution and shape (24,25), their use renders the bacteria more difficult to detect. Therefore, the diagnostic accuracy of histopathology is dependent on which regions are sampled, pathological interpretation (26) and whether the patient is taking PPIs.
The American Gastroenterological Association (AGA) and American College of Gastroenterology (ACG) recommend discontinuing PPIs two weeks before endoscopy, and taking biopsies from both the body and antrum (9,18). The present study evaluated both daily endoscopy practice and its influence on the histopathological diagnosis of H pylori infection. We aimed to establish whether gastroenterologists regularly sample the gastric antrum and body; the frequency of PPI use at endoscopy; and how these endoscopic practices influenced pathological interpretation. In patients who were H pylori positive, we also evaluated the effect of biopsy site on diagnosis. The present study addressed the influence of everyday practice on diagnostic efforts.
METHODS
Selection of biopsy specimens
The pathology files at Toronto General Hospital (Toronto, Ontario) were reviewed for biopsy specimens submitted specifically for H pylori diagnosis between 2005 and 2010. The anatomical sites from where the biopsies were taken were noted to establish whether AGA guidelines had been followed for sampling the body and antrum.
Biopsies from 150 patients, in which both the antrum and corpus had been sampled at endoscopy, were randomly chosen for further clinical and pathological analysis. In this group of 150 patients, it was established whether both regions were correctly sampled (by analysis of the histology of the gastric mucosa – transitional mucosa was grouped with antral mucosa), and the density of H pylori within each region. The electronic patient record system was used to evaluate whether the patients were taking PPIs at the time of endoscopy.
To investigate the sampling pattern when the endoscopy report indicated that only one region was sampled, the histology pattern in 200 consecutive specimens that satisfied that criterion was reviewed.
Histological and immunohistochemical staining
Hematoxylin and eosin sections were retrieved from the archives and two 4 μm sections were cut from each block. These were stained with dual silver/periodic acid-Schiff (27) and anti-H pylori rabbit polyclonal antibody (760–2645, Ventana Medical Systems Inc, USA; according to manufacturer’s instructions), respectively. Each set of three slides was separately randomized and coded for use within the study.
Histopathological examination of the slides
The slides were examined independently by four expert gastrointestinal pathologists and one trainee histopathologist. Observers were blinded to the clinical information of the patients, and had no knowledge of their own or their colleagues’ interpretation of the other slides.
A visual analogue scale graded from 0 (absent) to 5 (high) was used to score H pylori, acute inflammation and chronic inflammation on each slide (28). A score of 1 indicated that only one or two bacteria were observed in the entire biopsy specimen, while a score of 5 indicated that the surface was covered with bacteria and bacterial aggregates. A biopsy specimen was considered to be positive when all observers agreed that bacteria with the characteristic morphological features of H pylori were present in at least one of the special stain (dual silver/periodic acid-Schiff- or immunohistochemistry-stained) slides. Slides exhibiting discrepancy among observers were resolved by joint re-examination to reach consensus interpretation.
Data analysis
All scores were entered into a database and analyzed using Stata/SE version 11.2 (StataCorp, USA). The Fisher’s exact test or, when appropriate, the χ2 test (both two-tailed), were used for comparison of proportions; P<0.05 was considered to be statistically significant.
RESULTS
Evaluation of overall sampling pattern
From 2005 to 2010, gastric biopsies from 10,268 patients were specifically examined for H pylori infection in the pathology department of Toronto General Hospital. Specimens received from 6143 patients (60%) had biopsies taken from one region only, 4827 (47%) patients had biopsies taken from the antrum only and 1316 (13%) patients had biopsies taken from the body only. Thus, biopsies from both the antrum and corpus were taken only in 40% of the patients at endoscopy.
Evaluation of sampling accuracy
Biopsies in which the endoscopy report indicated that two regions were sampled:
In the 150 biopsy sets randomly chosen from patients in whom the endoscopy report indicated that biopsies had been taken from both the antrum and body, 701 individual biopsies (265 [38%] antrum, 393 [56%] body and 43 [6%] cardia) were reviewed. Each patient had a minimum of two biopsies available for examination (mean 6, median 4, range two to 12 biopsies).
Although the endoscopy report indicated that antral and body biopsies were obtained from each of these patients, histopathological review confirmed that biopsies originated from both regions in only 85 (57%) patients. In the remaining biopsies, the gastric region sampled was body only in 26 patients (17%), antrum only in 16 patients (11%), antrum and cardia in eight patients (5%), and body and cardia in 15 patients (10%) (Table 1).
TABLE 1.
Gastric regions sampled in biopsies in which endoscopy indicated antrum and corpus biopsies were obtained*
| Gastric region | n (%) |
|---|---|
| Antrum and body | 85 (56.67) |
| Antrum and cardia | 8 (5.33) |
| Body and cardia | 15 (10) |
| Antrum only | 16 (10.67) |
| Body only | 26 (17.33) |
| Total | 150 (100) |
Only one patient had biopsies taken as recommended by the updated Sydney System (2 antrum, 2 corpus, 1 incisura)
Biopsies in which the endoscopy report indicated that only one region was sampled:
In the 200 consecutive specimens the endocopy report indicated antral biopsies were obtained from 178 patients (89%) and oxyntic biopsies were obtained from 22 patients (11%).
When the endoscopy report indicated antral biopsies were obtained, histology review confirmed that the biopsies were from the antrum in only 119 (66.85%). In the remaining specimens, the gastric region sampled was antrum and body in 47 (26.40%), antrum and duodenum in one (0.56%), body in 10 (5.62%) and transitional mucosa in one (0.56%).
When the endoscopy report indicated oxyntic biopsies were obtained, histology review confirmed oxyntic mucosa in 18 (81.82%). In the remaining specimens, three (13.63%) had antral biopsies, and one (4.56%) had antral and oxyntic mucosa.
Therefore, an extrapolation suggested that that 47% of patients in the entire cohort had biopsies from one region of the stomach only (30% [one-half of specimens in which endoscopy indicated only one region was sampled] and 17% of patients in which the endoscopy report indicated both regions were sampled but incorrect sampling was determined pathologically). The largest source of error appeared to be that endoscopists did not take biopsies far enough distally to ensure that the gastric antrum was actually sampled in the biopsies.
Evaluation of histopathological sensitivity
From the histological re-assessment, 56 (37%) patients were positive for H pylori and 94 (63%) were negative. At routine signout, six of 56 (10.7%) patients had incorrectly been interpreted as H pylori negative. The biopsies from patients with a false-negative diagnosis either did not have any active inflammation or only had mild active inflammation. The average number of biopsies taken in patients with a false negative was three (range one to seven). In comparison, the mean number of biopsies taken in patients with a true-positive diagnosis was five (range two to 11).
Of 94 patients negative for H pylori on reassessment, two (2%) had been interpreted as positive for the infection at routine signout. Biopsies from these patients had mild active inflammation only. Active inflammation was focal and, in one case, was limited to an area with intestinal metaplasia. The sensitivity, specificity, negative predictive value and positive predictive value of histopathological analysis for H pylori in the present study were 89.29%, 97.87%, 93.88% and 96.15%, respectively.
The influence of sampling pattern (regions examined) on diagnostic accuracy
The antrum and body had been correctly sampled in 34 of 56 (61%) patients positive for H pylori infection. Bacteria were identified in the antrum only in five (15%) patients, and in the body only in seven (21%) patients, as shown in Figure 1. This confirms that obtaining biopsies from both the antrum and the body increases the probability of correctly diagnosing active infection.
Figure 1).
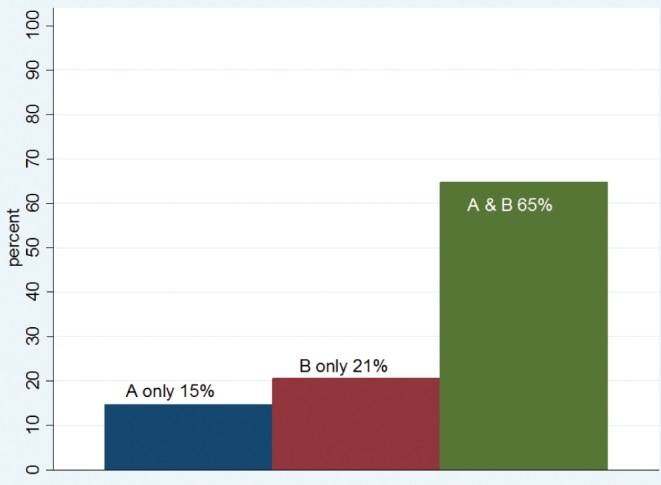
Bacteria distribution in biopsy sets that had both antrum (A) and body (B). A & B Antrum and body
In patients who received a true-positive diagnosis, the antrum and body were correctly sampled in 33 of 50 (66%) cases. In contrast, the antrum and body were correctly sampled in only one of six (17%) patients who received a false-negative diagnosis (P=0.0194), as shown in Table 2 and Figure 2.
TABLE 2.
Biopsy patterns in patients with a true-positive diagnosis compared with a false-negative diagnosis
| Site | True positive | False negative |
|---|---|---|
| Antrum and body | 33 (66) | 1 (16.67) |
| Antrum and cardia | 4 (8) | 1 (16.67) |
| Body and cardia | 3 (6) | 0 (0) |
| Antrum only | 7 (14) | 2 (33.33) |
| Body only | 3 (6) | 2 (33.33) |
| Total | 50 (100) | 6 (100) |
Data presented as n (%). False-negative results were from biopsy sets with few bacteria per biopsy, or from biopsy sets where the bacteria were present in one region. The antrum and body were correctly sampled in 33 of 50 (66%) patients correctly diagnosed with the infection. In contrast, the antrum and body were correctly sampled in only one of six (17%) patients who received a false-negative diagnosis (P=0.0194)
Figure 2).
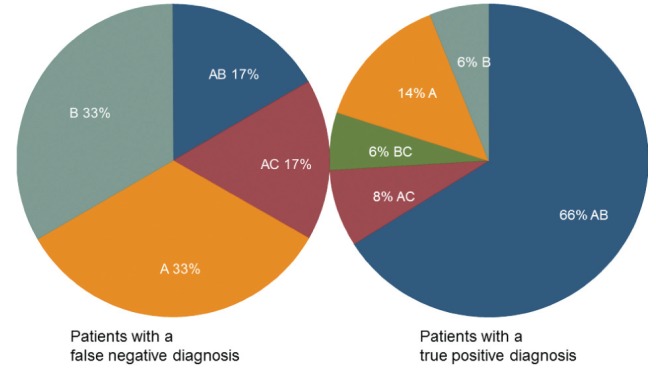
The influence of regions sampled on diagnostic accuracy. Biopsy material from both the antrum (A) and body (B) were more likely to be sampled in patients with a true-positive diagnosis (P=0.0194). AB Antrum and body; AC Antrum and cardia; BC Body and cardia
Overall, sampling bias was apparent in many of the biopsy sets and was a major contributor to a false-negative diagnosis. H pylori density was generally low in biopsies with a false-negative reading (mean score of 1 [scale 0 to 5]). In these biopsy sets, only a few bacteria were present per biopsy, or the bacteria were distributed only focally within each fragment or were present in only a few of the available biopsy fragments (sampling bias for the infection).
PPI use and diagnostic accuracy
Seventy-one patients (47%) were taking PPIs at the time of endoscopy, as shown in Table 3. Of the patients with a false-negative diagnosis, four of six (67%) were taking PPIs at the time of biopsy. In contrast, of the 50 patients correctly diagnosed positive for the infection (ie, true positives), 22 (44%) were taking PPIs at the time of endoscopy, as shown in Figure 3 (P=0.29). Parietal cell hypertrophy was observed in only one patient. Similarly, 44 of 92 (48%) patients with a true-negative diagnosis were taking PPIs, and histopathological examination confirmed PPI use in 17 (39%).
TABLE 3.
The frequency of proton pump inhibitor (PPI) use among patients
| PPI use |
Category
|
Total | |||
|---|---|---|---|---|---|
| False negative | False positive | True negative | True positive | ||
| No, n | 2 | 1 | 48 | 28 | 79 |
| Yes, n (%) | 4 (67) | 1 (50) | 44 (48) | 22 (44) | 71 (47) |
| Total, n | 6 | 2 | 92 | 50 | 150 |
Figure 3).
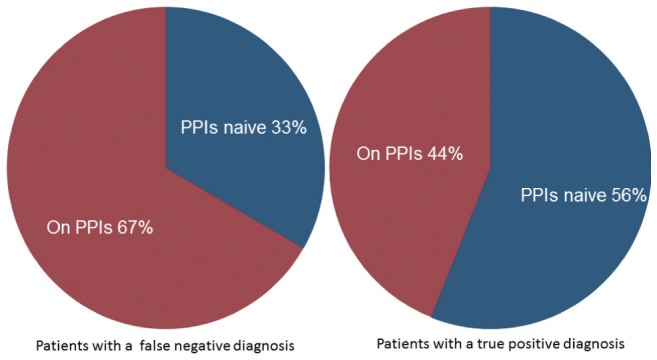
Frequency of proton pump inhibitor (PPI) use among patients
Furthermore, many of these patients exhibited chronic active inflammation. This suggests that PPI use is an important contributor to the inability to detect H pylori in patients with an otherwise typical H pylori pattern of chronic active inflammation.
DISCUSSION
Despite national and international guidelines (18,21) for the clinical management of H pylori infection, H pylori bacteria can be – and frequently are – missed on endoscopy. Factors that may cause a false-negative result include sampling the wrong area, taking an inadequate number of biopsies and/or continued use of PPIs at the time of endoscopy. All of the above factors are encountered in daily practice, including in the present study. As a result, each year, the incorrect diagnosis is made and many patients are inappropriately managed.
An important question is the probability of an incorrect diagnosis in patients undergoing endoscopy with subsequent histological assessment of the biopsy. In the present study, 5% (one in 20) were misclassified during routine practice. In the false-negative group, the microorganisms were either rarely present, with only one or two bacteria per slide, or the distribution of microorganisms was in only a few biopsies per set, which may have been restricted to one region (antrum or body). In the absence of significant active inflammation, chronic inflammation was interpreted to be negative for H pylori, irrespective of severity. These results are consistent with previous studies that show H pylori may be present in biopsy specimens that are histologically close to normal, especially in the gastric body (23,29,30). H pylori is more likely to be missed if the classical histological picture is not present on the hematoxylin and eosin sections (31). This is particularly exaggerated with PPI use, for which bacterial density is low and typically more proximal (21,22) (Figure 4).
Figure 4).
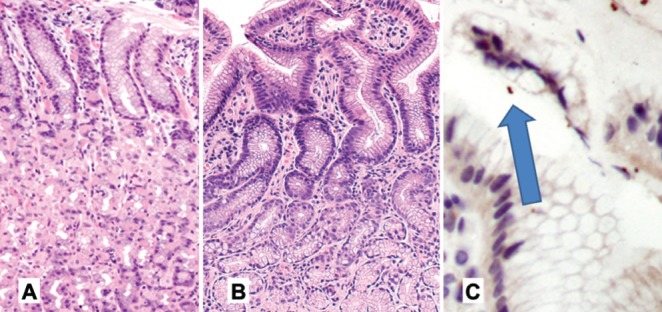
With proton pump inhibitor use, Helicobacter pylori-associated inflammation is absent or significantly reduced (A), oxyntic mucosa (hematoxylin and eosin, original magnification ×5).B Antral mucosa (hematoxylin and eosin, original magnification ×10). In addition, fewer bacteria (arrow) are present (C); H pylori monoclonal antibody (original magnification ×10)
The present study confirms that obtaining biopsies from both the antrum and the body increases the probability of diagnosing active infection (32). These data are consistent with previous data in which a combination of a biopsy from angulus incisura and one from the greater curvature of the corpus is needed to correctly identify all treatment failures (23). To prevent missing a true-positive result when intestinal metaplasia is present at the incisura, it is optimal that corpus biopsies, as well as biopsies from the antrum closer to the pylorus than the incisura, are taken in addition to a biopsy from the incisura angularis (33).
With the decline in the incidence of H pylori infection in the Western world, the need to exclude other diseases, such as Barrett’s esophagus, has taken precedence in routine clinical and pathological practice. The sampling behaviour among gastroenterologists in the present study may reflect this change, or perhaps a lack of awareness of the need to take both corpus and antrum biopsies to accurately exclude active infection. As such, the problem of false-negative diagnoses in the present study (10.7%) is likely to be an underestimate, given that the present study only re-examined biopsy sets that included both antrum and corpus from each patient. We excluded 60% of biopsy sets taken exclusively for H pylori diagnosis because the endoscopist had sampled only one region (antrum only in 47% and body only in 13%). Of the remaining patients, only 57% sampled both the antrum and body (with antrum alone sampled in 11% and body alone in 26%), as shown in Table 2. Thus, when both gastric regions were sampled, a large source of error was that biopsies were not taken far enough distally to ensure that the antrum was adequately sampled. In patients positive for the infection, inadequate sampling of both body and antrum can result in a miss rate of 15% when only antral biopsies are taken (organisms migrate proximally under a variety of circumstances, including in PPI use [21,22]), and a miss rate of 21% if only oxyntic biopsies are reviewed.
In the present study, biopsies from two patients (2%) were incorrectly interpreted to be positive for the infection (ie, false positive). Active inflammation in these biopsies was mild and focal. The apices of mucous cells may be cut in a plane such that they may resemble a curved organism (Figure 5). Also, chains of cocci may be apparent and these may occasionally be confused with H pylori. It is possible that in some instances cocci represent degenerate forms of the organisms (34), although they can also be of both oral and duodenal origin. Both patients subsequently received triple therapy for H pylori infection with no side effects; so they were not seriously affected by the false-positive diagnosis. Nonetheless, it is important to point out the risks of unnecessary antibiotic use with respect to idiosyncratic or allergic reactions that are potentially fatal (35,36).
Figure 5).
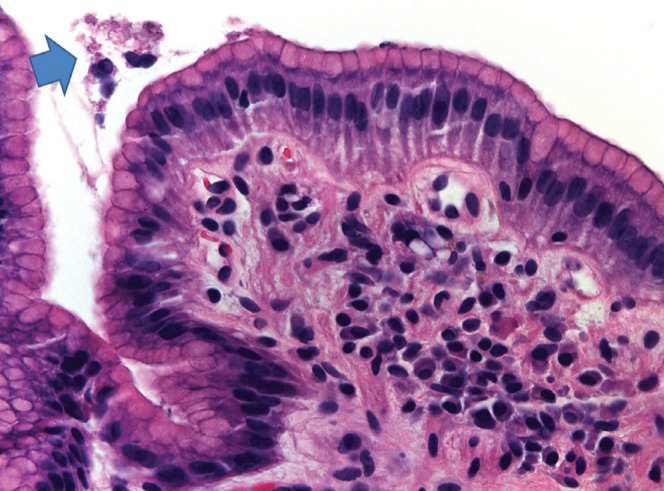
Apices of mucous cells may be cut in a plane such that they may resemble a curved organism. Hematoxylin and eosin stain, original magnification ×40
While it is possible to blame the operator (in this case, the pathologist), one also must question the context in which the mistake was made. False-negative and false-positive diagnoses are particularly observed in patients using PPIs and in patients who recently received antibiotics (31), but also if biopsies are only taken from one part of the stomach instead of both antrum and oxyntic mucosa (36).
The AGA and ACG guidelines emphasize the need to test symptomatic patients for H pylori before prescribing PPIs (9,21). PPIs were being used by 47% of patients at the time of endoscopy, with a higher frequency among patients who received a false-negative diagnosis (67% among patients with false-negative diagnosis versus 48% among patients with a true-negative diagnosis [P=0.37]). H pylori flourishes in a pH range of 3.5 to 5 (37) and, therefore, PPI use decreases the number of bacteria present, as well as facilitating proximal migration of the bacteria (38). The lower bacterial count can increase the probability of a false-negative diagnosis given the small number of bacteria present. Therefore, PPI use may be a major contributor to the inability to detect H pylori histologically in patients who have an active infection, and gastrointestinal signs and symptoms known to be related to H pylori such as ulcer-like dyspepsia. Furthermore, many patients on PPIs experience chronic gastric inflammation (39). Because pathologists rely on inflammation as an indicator of active infection, this can increase the probability of a false-positive diagnosis. This suggests that PPI use is a major contributor to both false-negative and false-positive diagnoses.
In the Canadian population, individuals belonging to high-risk groups for H pylori infection total more than four million, based on birth origin and/or area of residence (www.cdhf.ca/digestive-disorders/statistics.shtml#hpylori). H pylori infection is a potentially modifiable risk factor in many chronic diseases (gastric and nongastric) (40–42) and it is likely that H pylori infection accounts for a substantially under-recognized global burden of disease. In addition to identifying the true positive population, diagnostic accuracy is important to exclude false positives because inappropriate H pylori eradication treatment can precipitate pseudomembranous colitis, particularly in elderly patients (43,44). In addition, patients with H pylori-negative duodenal ulcers appear to experience a significantly worse outcome when treated empirically (45).
The number and location of the gastric biopsies is important for the accurate identification of H pylori because biopsies series taken from the antrum or body would result in only a significant false-negative diagnosis. If endoscopists truly wish to maximize their yield of H pylori, they must note that the highest yield is from biopsies taken from the antrum. To obtain antral mucosa, biopsies must be taken distally enough to obtain histological antral mucosa, which needs to be quite close to the pylorus. This still results in a false-negative rate of 13%, emphasizing the need for biopsies from oxyntic mucosa as well as antral mucosa. Furthermore, if the antral mucosa is not sampled, either deliberately or accidentally, the false-negative rate for Helicobacter, if present, rises to 30%. Routine sampling for Helicobacter should, therefore, always include biopsies from both the antral and oxyntic mucosa.
Given the design of the present study, one question that could not be answered was the true false-negative rate. In the present study, it was apparent that the morphological appearance of H pylori infection (chronic active gastritis that could be maximal in either the antral or oxyntic mucosa), was accompanied by parietal cell hypertrophy, indicative of hypergastrinemia, but practically of PPI use. It would, therefore, be sensible for the endoscopist to indicate whether the patient is on, or has recently been on PPIs or antibiotics, or had recently undergone Helicobacter eradication therapy, all of which can reduce the number of organisms to low or undetectable levels. Pathologists should be aware that the presence of an inflammatory pattern compatible with H pylori, and PPI-related changes in parietal cells, may be indicative of a false-negative biopsy. In addition, pathologists should also consider lymphocytic gastritis in which the bacterial load is low, especially in the oxyntic mucosa; lymphocytic gastritis is a significantly less common cause of gastritis that responds to Helicobacter eradication therapy (46). Finally, atrophic gastritis, especially with extensive intestinal metaplasia, may also result in a false-negative set of biopsies (33).
The joint AGA and ACG guidelines are provided to direct best possible care. Despite widespread dissemination and teaching of the guidelines, they are not adhered to in everyday practice. Practicing endoscopists need to be aware that they need to take biopsies from both (distal) antrum and oxyntic mucosa (ideally greater and lesser curve of both), and both clinicians and pathologists need to be aware that PPI use appears to be an important cause of a false-negative H pylori diagnosis, especially when the pattern of inflammation present suggests that organisms should be present.
REFERENCES
- 1.Bastos J, Peleteiro B, Pinto H, et al. Prevalence, incidence and risk factors for Helicobacter pylori infection in a cohort of Portuguese adolescents (EpiTeen) Dig Liver Dis. 2013;45:290–5. doi: 10.1016/j.dld.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Porras C, Nodora J, Sexton R, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701) Cancer Causes Control. 2013;24:209–15. doi: 10.1007/s10552-012-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones N, Chiba N, Fallone C, et al. Helicobacter pylori in First Nations and recent immigrant populations in Canada. Can J Gastroenterol. 2012;26:97–103. doi: 10.1155/2012/174529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soll AH. Consensus conference. Medical treatment of peptic ulcer disease. Practice guidelines. Practice Parameters Committee of the American College of Gastroenterology. JAMA. 1996;275:622–9. doi: 10.1001/jama.275.8.622. [DOI] [PubMed] [Google Scholar]
- 5.Rathbone M, Rathbone B. Helicobacter pylori and gastric cancer. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 2011;185:83–97. doi: 10.1007/978-3-642-03503-6_5. [DOI] [PubMed] [Google Scholar]
- 6.Gisbert JP, Calvet X. Review article: common misconceptions in the management of Helicobacter pylori-associated gastric MALT-lymphoma. Aliment Pharmacol Ther. 2011;34:1047–62. doi: 10.1111/j.1365-2036.2011.04839.x. [DOI] [PubMed] [Google Scholar]
- 7.Wotherspoon AC, Dogan A, Du MQ. Mucosa-associated lymphoid tissue lymphoma. Curr Opin Hematol. 2002;9:50–5. doi: 10.1097/00062752-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Endoscopy. 2012;44:74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NIH Consensus Conference Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65–9. [PubMed] [Google Scholar]
- 10.Corrado E, Rizzo M, Coppola G, et al. An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb. 2010;17:1–11. doi: 10.5551/jat.2600. [DOI] [PubMed] [Google Scholar]
- 11.Niccoli G, Franceschi F, Cosentino N, et al. Coronary atherosclerotic burden in patients with infection by CagA-positive strains of Helicobacter pylori. Coron Artery Dis. 2010;21:217–21. doi: 10.1097/MCA.0b013e3283399f36. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Franceschi F, Nishizawa T, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2011;16(Suppl 1):65–9. doi: 10.1111/j.1523-5378.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 13.Malfertheiner P, Selgrad M. Helicobacter pylori infection and current clinical areas of contention. Curr Opin Gastroenterol. 2010;26:618–23. doi: 10.1097/MOG.0b013e32833efede. [DOI] [PubMed] [Google Scholar]
- 14.Figura N, Franceschi F, Santucci A, et al. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2010;15(Suppl 1):60–8. doi: 10.1111/j.1523-5378.2010.00778.x. [DOI] [PubMed] [Google Scholar]
- 15.Moyaert H, Franceschi F, Roccarina D, et al. Extragastric manifestations of Helicobacter pylori infection: Other Helicobacters. Helicobacter. 2008;13(Suppl 1):47–57. doi: 10.1111/j.1523-5378.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 16.Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, et al. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: Preliminary results. Neurobiol Aging. 2012;33:1009.e11–9. doi: 10.1016/j.neurobiolaging.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Dobbs RJ, Dobbs SM, Weller C, et al. Helicobacter hypothesis for idiopathic Parkinsonism: Before and beyond. Helicobacter. 2008;13:309–22. doi: 10.1111/j.1523-5378.2008.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chey WD, Wong BC. Practice Parameters Committee of the American College of G. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 19.Cekin AH, Taskoparan M, Duman A, et al. The role of Helicobacter pylori and NSAIDs in the pathogenesis of uncomplicated duodenal ulcer. Gastroenterol Res Pract. 2012;2012:189373. doi: 10.1155/2012/189373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundell L, Havu N, Miettinen P, et al. Changes of gastric mucosal architecture during long-term omeprazole therapy: Results of a randomized clinical trial. Aliment Pharmacol Ther. 2006;23:639–47. doi: 10.1111/j.1365-2036.2006.02792.x. [DOI] [PubMed] [Google Scholar]
- 21.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection – the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 22.Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: A topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994;40:342–5. doi: 10.1016/s0016-5107(94)70067-2. [DOI] [PubMed] [Google Scholar]
- 23.el-Zimaity HM, al-Assi MT, Genta RM, Graham DY. Confirmation of successful therapy of Helicobacter pylori infection: Number and site of biopsies or a rapid urease test. Am J Gastroenterol. 1995;90:1962–4. [PubMed] [Google Scholar]
- 24.Logan RP, Walker MM, Misiewicz JJ, et al. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995;36:12–6. doi: 10.1136/gut.36.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein NS. Chronic inactive gastritis and coccoid Helicobacter pylori in patients treated for gastroesophageal reflux disease or with H pylori eradication therapy. Am J Clin Pathol. 2002;118:719–26. doi: 10.1309/LJ4D-E2LX-7UMR-YMTH. [DOI] [PubMed] [Google Scholar]
- 26.el-Zimaity HM. Accurate diagnosis of Helicobacter pylori with biopsy. Gastroenterol Clin North Am. 2000;29:863–9. doi: 10.1016/s0889-8553(05)70153-9. [DOI] [PubMed] [Google Scholar]
- 27.el-Zimaity HM, Wu J, Akamatsu T, Graham DY. A reliable method for the simultaneous identification of H pylori and gastric metaplasia in the duodenum. J Clin Pathol. 1999;52:914–6. doi: 10.1136/jcp.52.12.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.el-Zimaity HM, Graham DY, al-Assi MT, et al. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- 29.Collins JS, Hamilton PW, Watt PC, et al. Superficial gastritis and Campylobacter pylori in dyspeptic patients – a quantitative study using computer-linked image analysis. J Pathol. 1989;158:303–10. doi: 10.1002/path.1711580407. [DOI] [PubMed] [Google Scholar]
- 30.El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: Role of the Sydney System. Hum Pathol. 1999;30:72–7. doi: 10.1016/s0046-8177(99)90303-9. [DOI] [PubMed] [Google Scholar]
- 31.El-Zimaity HM, Segura AM, Genta RM, Graham DY. Histologic assessment of Helicobacter pylori status after therapy: Comparison of Giemsa, Diff-Quik, and Genta stains. Modern Pathol. 1998;11:288–91. [PubMed] [Google Scholar]
- 32.Lan HC, Chen TS, Li AF, et al. Additional corpus biopsy enhances the detection of Helicobacter pylori infection in a background of gastritis with atrophy. BMC Gastroenterol. 2012;12:182. doi: 10.1186/1471-230X-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Andersen LP, Rasmussen L. Helicobacter pylori-coccoid forms and biofilm formation. FEMS Immunol Medical Microbiol. 2009;56:112–5. doi: 10.1111/j.1574-695X.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 35.Renaudin JM, Beaudouin E, Ponvert C, et al. Severe drug-induced anaphylaxis: Analysis of 333 cases recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy. 2013;68:929–37. doi: 10.1111/all.12168. [DOI] [PubMed] [Google Scholar]
- 36.James A, Blagojevic J, Benham SW, et al. Azathioprine hypersensitivity presenting as septic shock with encephalopathy. BMJ Case Rep. 2013. Mar, doi:pii: bcr2012008340. 10.1136/bcr-2012-008340. [DOI] [PMC free article] [PubMed]
- 37.Meyer-Rosberg K, Scott DR, Rex D, et al. The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology. 1996;111:886–900. doi: 10.1016/s0016-5085(96)70056-2. [DOI] [PubMed] [Google Scholar]
- 38.El-Zimaity H. Gastritis and gastric atrophy. Curr Opin Gastroenterol. 2008;24:682–6. doi: 10.1097/MOG.0b013e328311d1cc. [DOI] [PubMed] [Google Scholar]
- 39.Nordenstedt H, Graham DY, Kramer JR, et al. Helicobacter pylori-negative gastritis: Prevalence and risk factors. Am J Gastroenterol. 2013;108:65–71. doi: 10.1038/ajg.2012.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929–36. doi: 10.1001/archinternmed.2011.533. [DOI] [PubMed] [Google Scholar]
- 41.Moayyedi P, Soo S, Deeks JJ, et al. WITHDRAWN: Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2011:CD002096. doi: 10.1002/14651858.CD002096.pub5. [DOI] [PubMed] [Google Scholar]
- 42.Yeh JM, Kuntz KM, Ezzati M, Goldie SJ. Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. J Int Cancer. 2009;124:157–66. doi: 10.1002/ijc.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harsch IA, Hahn EG, Konturek PC. Pseudomembranous colitis after eradication of Helicobacter pylori infection with a triple therapy. Medical Sci Monit. 2001;7:751–4. [PubMed] [Google Scholar]
- 44.Kubo N, Kochi S, Ariyama I, et al. [Pseudomembranous colitis after Helicobacter pylori eradication therapy]. Kansenshogaku zasshi. J Japanese Assoc Infect Dis. 2006;80:51–5. doi: 10.11150/kansenshogakuzasshi1970.80.51. [DOI] [PubMed] [Google Scholar]
- 45.Bytzer P, Teglbjaerg PS. Helicobacter pylori-negative duodenal ulcers: Prevalence, clinical characteristics, and prognosis – results from a randomized trial with 2-year follow-up. Am J Gastroenterol. 2001;96:1409–16. doi: 10.1111/j.1572-0241.2001.03774.x. [DOI] [PubMed] [Google Scholar]
- 46.Niemela S, Karttunen T, Kerola T, Karttunen R. Ten year follow up study of lymphocytic gastritis: Further evidence on Helicobacter pylori as a cause of lymphocytic gastritis and corpus gastritis. J Clin Pathol. 1995;48:1111–6. doi: 10.1136/jcp.48.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]


