Abstract
Objectives:
Little is known about the impact of HIV infection on biological ageing in sub-Saharan Africa. The study aimed to assess biological ageing in South African HIV-infected adults and HIV-seronegative individuals using two validated biomarkers, telomere length and CDKN2A expression (a mediator of cellular senescence).
Design:
A case–control study.
Methods:
Two hundred and thirty-six HIV-infected adults aged at least 30 years and 250 age and sex frequency matched HIV-seronegative individuals were recruited from clinics in township communities in Cape Town. Biological ageing was evaluated by measurement of telomere length and CDKN2A expression in peripheral blood leukocytes.
Results:
The median ages of the HIV-infected and HIV-seronegative participants were 39 and 40 years, respectively. Among HIV-infected participants, 87.1% were receiving antiretroviral therapy (ART), their median CD4+ cell count was 468 cells/μl and 84.3% had undetectable viral load. Both biomarkers were validated against chronological age in HIV-seronegative individuals. Telomere length was significantly shorter in HIV-infected individuals than in HIV-seronegative individuals (mean relative T/S ratio ±SE:0.91 ± 0.007 vs. 1.07 ± 0.008, P < 0.0001). CD2NKA expression was higher in HIV-infected participants than in HIV-seronegative individuals (mean expression: 0.45 ± 0.02 vs. 0.36 ± 0.03, P = 0.003). Socioeconomic factors were not associated with biological ageing in HIV-infected participants. However, in participants on ART with undetectable viral load, biomarker levels indicated greater biological ageing in those with lower current CD4+ cell counts.
Conclusion:
Telomere length and CDKN2A expression were both consistent with increased biological ageing in HIV-infected individuals. Prospective studies of the impact of HIV on biological ageing in sub-Saharan Africa are warranted.
Keywords: accelerated ageing, Africa, biomarkers of ageing, CDKN2A, HIV, telomeres
Introduction
HIV-infected individuals are at an increased risk of age-related non-AIDS morbidity and mortality compared with HIV-uninfected persons [1]. It is speculated that HIV-infected individuals may not only be ageing chronologically but also undergoing accelerated biological ageing mediated by increased cellular senescence [2]. Chronological age is an imprecise measure of biological ageing, due to inter-individual differences in rates of ageing. The disconnection between chronological age and lifespan has led to a search for effective and validated biomarkers of ageing (BoA), defined as ‘biological parameters of an organism that either alone or in some multivariate composite will better predict functional capability at some late age, than will chronological age’ [3].
Data from industrialized countries on the impact of HIV on accelerated ageing may be confounded by differential risk exposure by HIV status to risk factors, such as smoking and alcohol consumption. Moreover, lower socioeconomic status and poor diet are also associated with accelerated biological ageing [4]. Thus, the NIH Office of AIDS Research has highlighted the need for carefully designed studies of HIV and ageing that takes these factors into account [5]. Assessment of biological ageing in HIV infection in sub-Saharan Africa may also be influenced by coexisting morbidities, malnutrition, a high prevalence of enteric pathogens and opportunistic coinfections and epigenetic variation [6,7]. Thus, data related to biological ageing and HIV obtained from well resourced settings may not be directly translatable to African populations. However, few data are available from sub-Saharan Africa populations [8] where three million people aged 50 years and older are living with HIV [9]. If premature biological ageing is associated with HIV, then age-related morbidity in HIV-infected individuals is likely to place a significant burden on healthcare systems in sub-Saharan Africa.
Telomeric DNA length is a widely used BoA. Telomeres are nucleoprotein complexes at the ends of eukaryotic chromosomes. Their DNA component shortens with somatic cell division, and upon reaching a critically short length, a DNA damage signal leads to cell cycle arrest, resulting in replicative senescence [10,11]. Telomere shortening is associated with increasing chronological age, and a wide range of diseases, including cardiovascular disease [12] and renal dysfunction [13]. Telomere attrition is affected by psychosocial confounders, genetics and potentially by nucleoside reverse transcriptase inhibitors (NRTIs) [14]. Expression levels of the cell cycle regulator CDKN2A may represent a more robust BoA [15]. CDKN2A acts as a tumour suppressor and maintains cells in a state of growth arrest both in replicative and stress-induced premature senescence. Increasing levels of CDKN2A transcriptional expression occur with increasing age both in solid organs and peripheral blood leukocytes (PBLs) [16]. In the former, increasing CDKN2A expression correlates directly with decreasing function.
This cross-sectional study conducted in South Africa was undertaken to provide a rapid assessment of whether there was evidence that HIV-infected individuals had advanced biological ageing compared with HIV-seronegative individuals by comparing telomere length and CDKN2A expression.
Materials and methods
Ethics statement
The study was approved by the Ethics Committees of the London School of Hygiene and Tropical Medicine and the University of Cape Town Faculty of Health Sciences, and was adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Study participants
HIV-infected individuals aged at least 30 years were enrolled from a community-based HIV treatment centre in Nyanga district in Cape Town [17]. All participants had a confirmed serological diagnosis of HIV and either about to commence antiretroviral therapy (ART; ART-naive) or were already on first-line ART. HIV-seronegative participants were recruited using frequency-matching by sex and 5-year age categories. HIV-seronegative individuals were enrolled from participants confirmed to be HIV-seronegative attending an HIV prevention trials centre (Emavundleni Centre). These two centres were chosen, as attendees were drawn from the same community and therefore likely to have similar sociodemographic characteristics.
Data and sample collection
Sociodemographic information and medical history were obtained by questioning participants in their first language (Xhosa or English). Data collected included factors known to affect ageing [e.g. indoor/outdoor occupation as a proxy for ultraviolet (UV) exposure]. Clinical information was available relating to current and nadir CD4+ cell counts, peak and current HIV plasma viral load and WHO clinical status. Venous blood was collected at the time of participant interview to measure telomere length and CDKN2A expression in PBL.
DNA/RNA extraction
DNA was extracted from PBLs using the Maxwell Automated Purification System according to manufacturer's instructions (Promega, Madison, Wisconsin, USA). DNA concentration and purity was quantified by Nanodrop Spectrophotometer (ThermoFisher Scientific, Waltham, Massachusetts, USA). RNA was extracted using Trizol reagent (Invitrogen, Life Technologies, Grand Island, New York, USA) following manufacturer's guidelines. DNA/RNA extraction was performed in Cape Town and samples shipped on dry ice to the University of Glasgow.
Telomere length determination
Telomere lengths were determined by quantitative PCR (qPCR) following the method of Cawthon [18]. Telomere length determination was performed blindly using a Roche Light Cycler (Roche Diagnostics, Indianapolis, Indiana, USA) LC480. Briefly, telomere length analyses were performed in triplicate for each sample, using a single-copy gene amplicon primer set (acidic ribosomal phosphoprotein, 36B4) and a telomere-specific amplicon primer set [19]. Quality control parameters for the amplifications comprised a cut-off of 0.15 for the standard deviation (SD) of the threshold cycle (Ct) for sample replicates. At a SD above 0.15, the sample was reanalysed. The average SD across plates was 0.05. Relative telomere length was estimated from Ct scores using the comparative Ct method after confirming that telomere and control gene assays yielded similar amplification efficiencies. This method determines the ratio of telomere repeat copy number to single copy gene number (T/S) ratio in experimental samples relative to a control sample DNA. This normalized T/S ratio was used as the estimate of relative telomere length (Relative T/S). The interassay variation was assessed by comparing the relative telomere estimates (T/S ratio) estimates across assays for the positive controls, assayed on every assay plate. The average interassay coefficient of variance was 0.6% for telomere length and 0.23% for 36B4, with coefficient of variances comparable to previous data from this laboratory [20,21].
CDKN2A expression determination
Relative quantitative real-time PCR (qRT-PCR) was used to estimate mRNA levels corresponding to the candidate senescence associated gene CDKN2A in line with established methodology [19,22]. Expression levels were measured against a reference hypoxanthine phosphoribosyltransferase (HPRT) housekeeping gene on an ABI Prism(R) 7500 Sequence Detection System. Sequences of human TaqMan Primer/Probe sets were designed by Primer Express algorithm (Applied Biosystems, Austin, Texas, USA). The comparative threshold cycle method (ΔΔCT) [23] was employed to quantify relative gene expression.
Statistical analysis
Analyses were performed using Stata 12 (Stata Corp, College Station, Texas, USA). Clinical and biochemical data were summarized as the median with interquartile range (IQR) or mean with standard error (SE), as appropriate. Analyses were conducted on log10-transformed values of telomere length and mean CDKN2A expression to satisfy the assumption of normally distributed residuals. Results are displayed back-transformed to the original scale. Univariable analyses were performed to assess the relationships between mean telomere length, CDKN2A expression, HIV status and other clinical/demographic categories. Multivariable linear regression was used to examine the relationships of biomarker expression with HIV status adjusting for confounders identified in the univariable analysis and for a priori defined confounders (age and sex).
Results
Participant characteristics
Characteristics of the 236 HIV-infected individuals and 250 age/sex frequency matched HIV-seronegative individuals are reported in Table 1. All participants were of African ancestry. Telomere data were available for all participants, and CDKN2A data for 444 participants (91.4%). The majority (75%) of the study population was female. The median age of the HIV-infected population was 39 years (IQR 35–46 years), similar to the HIV-seronegative group (median 40 years (IQR 35–49 years)) (P = 0.17). HIV-infected participants tended to be of higher socioeconomic status and were less likely to smoke or consume alcohol (Table 1). Cases also had a lower mean BMI and were more likely to have current or previous tuberculosis (TB) than HIV-seronegative individuals. Overall, 87.1% of HIV-infected participants were receiving ART and the current CD4+ cell count among these participants was 468 cells/μl (IQR 325–607 cells/μl) and 84.3% had an undetectable viral load (<50 copies/ml). All participants on ART received a regimen that contained an NRTI.
Table 1.
Characteristics of study population.
| Variable | HIV-infected participants (236) % (n) | HIV-seronegative participants (250) % (n) | P |
| Age (years) | 39 (35–46)a | 40 (35–49)a | 0.17 |
| Age (years) by group | |||
| 30–39 | 50.4 (119) | 46.4 (116) | |
| 40–49 | 32.6 (77) | 32.0 (80) | 0.41 |
| >50 | 17.0 (40) | 21.6 (54) | |
| Male sex | 25.4 (60) | 24.0 (60) | 0.72 |
| Education | |||
| <High school | 11.9 (28) | 17.6 (44) | 0.08 |
| High school or tertiary education | 88.1 (208) | 82.4 (206) | |
| Income | |||
| <US$ 125/month | 56.8 (134) | 67.2 (168) | 0.02 |
| >US$ 125/month | 43.2 (102) | 32.8 (82) | |
| Housing | |||
| Informal | 47.9 (113) | 40.4 (101) | 0.10 |
| Formal | 52.1 (123) | 59.6 (149) | |
| Water supply | |||
| Shared | 75.0 (177) | 74.0 (185) | 0.80 |
| Own | 25.0 (59) | 26.0 (65) | |
| Alcohol (amount per week) | |||
| Nil | 69.5 (164) | 56.4 (141) | |
| Up to 1 l/week | 20.3 (48) | 21.6 (54) | 0.001 |
| >1 l/week | 10.2 (24) | 22.0 (55) | |
| Duration of smoking (years) | |||
| Nil | 84.8 (200) | 72.0 (180) | |
| <10 years | 5.1 (12) | 12.8 (32) | 0.002 |
| >10 years | 10.2 (24) | 15.2 (38) | |
| Illicit drugs | |||
| Never taken | 97.4 (222) | 98.0 (240) | |
| Ever taken | 2.6 (6) | 2.0 (5) | 0.67 |
| BMI (kg/m2) | 27.8 ± 0.4 | 31.5 ± 0.6 | <0.0001 |
| Comorbidity (including hypertension) | |||
| None | 64.4 (152) | 55.2 (138) | |
| One or more | 35.6 (84) | 44.8 (112) | 0.04 |
| TB status | |||
| No history | 32.6 (77) | 87.6 (219) | |
| Current | 4.2 (10) | 0.4 (1) | <0.0001 |
| Previous | 63.1 (149) | 12.0 (30) | |
| HIV characteristics % (n) or median (IQR) | |||
| WHO stage | |||
| 1/2 | 27.1 (67) | ||
| 3/4 | 72.3 (181) | ||
| ART naive | 12.9 (32) | ||
| CD4+ cell count in ART-naive group (n = 32) | 182 (84–202) | ||
| Log10VL in ART-naive group (n = 21) | 4.88 (4.21–5.18) | ||
| Current CD4+ cell count in ART group (cells/μl) | 468 (325–607) | ||
| Nadir CD4+ cell count in ART group | 128 (76–171) | ||
| % with undetectable VLb in ART group (cells/μl) | 84.3 (172) | ||
| Peak log10VL in ART group | 4.56 (3.84–4.98) | ||
| Duration of ART, months | 58 (34–75) | ||
ART, antiretroviral therapy; TB, tuberculosis; VL, viral load.
aValues expressed as median (interquartile range).
bUndetectable VL refers to VL <50 copies/ml.
Biological age and chronological age in HIV-seronegative individuals
Telomere length and CDKN2A levels were validated against chronological age in HIV-seronegative individuals. As expected, there was a negative association between chronological age and telomere length (Pearson r = −0.13, P = 0.05), and a positive association with CDKN2A expression (r = 0.16, P = 0.02). The relationship between CDKN2A expression and age was similar in men and women. However, for telomere length, age-related attrition was somewhat greater in men than in women, but this difference did not reach statistical significance (r = −0.25 vs. r = −0.09, P-interaction = 0.13).
Biological age and HIV status
Telomere length was significantly shorter in HIV-infected individuals than in HIV-seronegative individuals [mean relative T/S ratio (Rel T/S) ± SE: 0.91 ± 0.007 vs. 1.07 ± 0.008, P < 0.0001, Fig. 1a]. Telomere length decreased with chronological age in HIV-infected individuals (r = −0.15, P = 0.03). Mean CDKN2A expression was higher in HIV-infected participants than in HIV-seronegative individuals (0.45 ± 0.02 vs. 0.36 ± 0.03, P = 0.003, Fig. 1b, Table 2), and there was little evidence of correlation between chronological age and CDKN2A expression in HIV-infected individuals (r = 0.09, P = 0.17). No interactions were detected with HIV status when assessing the relationship between biomarkers and chronological age (data not shown).
Fig. 1.
Assessment of biomarkers (telomere length and CDKN2A) in peripheral blood leukocytes.
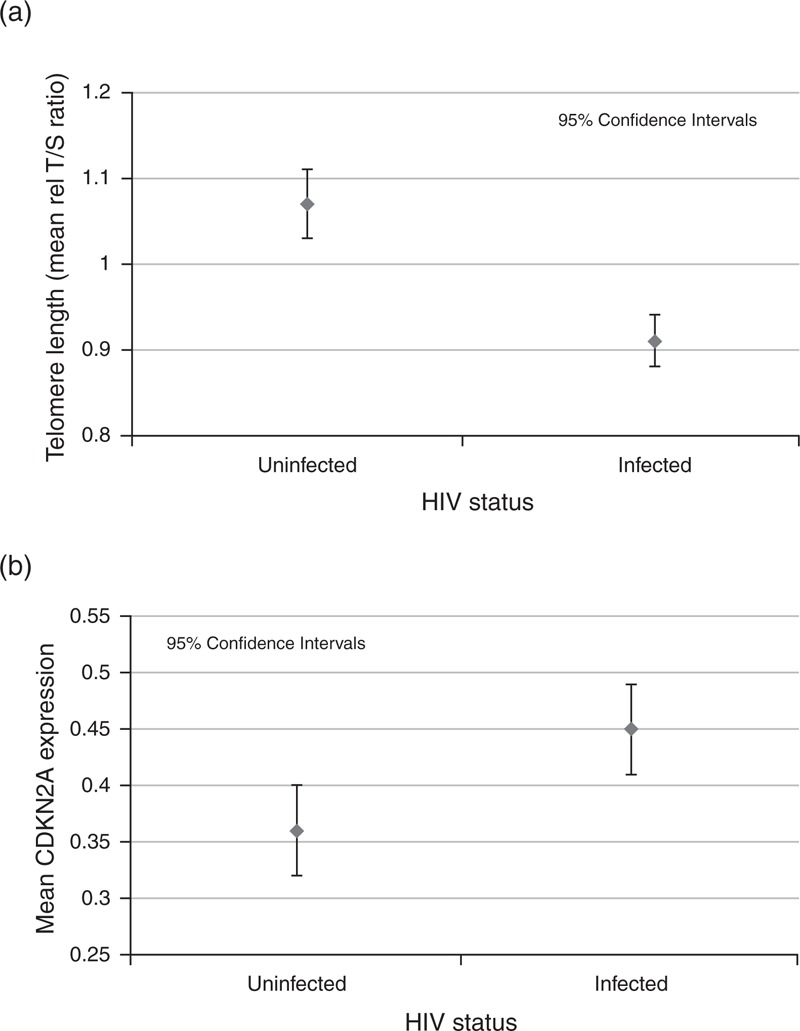
(a) Measurement of telomere length in peripheral blood leukocytes in HIV-seronegative and HIV-infected individuals. Telomere length measured as mean relative T/S ratio: RelT/S ± SE: 0.91 ± 0.007 vs. 1.07 ± 0.008, P < 0.0001. (b) Measurement of CDKN2A expression in HIV-seronegative and HIV-infected individuals. Relative expression 0.45 ± 0.02 vs. 0.36 ± 0.03, P = 0.003.
Table 2.
Association of HIV states with telomere length and CDKN2A expression.
| Clinical group | N | Telomere length Mean Rel T/S (95% CI) | P | N | Mean CDKN2A expression (95% CI) | P |
| HIV-seronegative | ||||||
| 250 | 1.07 (1.04–1.11) | <0.0001a | 217 | 0.35 (0.32–0.39) | 0.006a | |
| HIV-infected; | ||||||
| On ART | 204 | 0.91 (0.87–0.94) | 199 | 0.45 (0.40–0.50) | ||
| HIV-infected; ART-naive | ||||||
| 32 | 0.89 (0.81–0.98) | 0.71b | 28 | 0.46 (0.34–0.62) | 0.80b | |
ART, antiretroviral therapy; CI, confidence interval.
aP value for HIV-seronegative individuals vs. HIV-infected participants overall.
bP value between HIV group (i.e. on ART and ART-naive).
Among the HIV-infected patients, there was no evidence that either telomere length or CDKN2A expression was associated with ART status (P = 0.71 telomere length; P = 0.80 CDKN2A; Table 2).
Among the 172 participants on ART with viral suppression, current CD4+ cell count was positively associated with telomere length and negatively associated with CDKN2A expression (P-trend = 0.02 telomere length; P-trend = 0.05 CDKN2A; Table 3). There was no evidence of an association between these BoA and CD4+ cell count in patients with detectable viral load (data not shown).
Table 3.
Association of biomarkers with HIV-related covariates.
| Variable | N = 172 | Telomere length Mean Rel T/S (95% CI) | P | N = 168 | Mean CDKN2A (95% CI) | P |
| WHO stage | ||||||
| 1/2 | 33 | 0.89 (0.81–0.97) | 32 | 0.44 (0.35–0.56) | ||
| 3/4 | 139 | 0.92 (0.88–0.96) | 0.48 | 136 | 0.46 (0.41–0.51) | 0.81 |
| Duration of ART (months) | ||||||
| 0–36 | 40 | 0.86 (0.80–0.93) | 0.10* | 39 | 0.51 (0.41–0.63) | 0.17* |
| 36–72 | 80 | 0.91 (0.87–0.97) | 79 | 0.45 (0.39–0.52) | ||
| >72 | 52 | 0.95 (0.88–1.01) | 50 | 0.42 (0.35–0.51) | ||
| Current CD4+ cell count (cells/μl) | ||||||
| <200 | 7 | 0.85 (0.70–1.02) | 0.02* | 7 | 0.63 (0.38–1.03) | 0.05* |
| 201–350 | 37 | 0.83 (0.77–0.91) | 37 | 0.52 (0.42–0.64) | ||
| >351 | 128 | 0.93 (0.90–0.98) | 124 | 0.43 (0.38–0.48) | ||
| Nadir CD4+ cell count (cells/μl) | ||||||
| <200 | 153 | 0.91 (0.87–0.94) | 0.58 | 151 | 0.46 (0.41–0.51) | 0.80 |
| >201 | 19 | 0.93 (0.83–1.05) | 17 | 0.44 (0.32–0.61) | ||
| Peak viral load | ||||||
| <10 000 copies | 52 | 0.92 (0.86–0.99) | 51 | 0.50 (0.42–0.61) | ||
| >10 000 copies | 120 | 0.91 (0.87–0.95) | 0.68 | 117 | 0.44 (0.38–0.48) | 0.27 |
| TB status | ||||||
| No history | 48 | 0.91 (0.84–0.97) | 47 | 0.47 (0.39–0.57) | ||
| Current /previous history | 124 | 0.91 (0.87–0.95) | 0.92 | 121 | 0.45 (0.40–0.51) | 0.69 |
Linear regression adjusted for age in participants on ART with suppressed VL (i.e. <50 copies/ml). ART, antiretroviral therapy; CI, confidence interval; TB, tuberculosis; VL, viral load. *P value test for trend.
Biological age and sociodemographic characteristics
As sociodemographic characteristics may be associated with biological ageing [4,24], we analysed their association with the two BoA, stratified by HIV status (Table 4a,b). HIV-infected participants aged at least 50 years had significantly shorter telomeres than HIV-seronegative individuals in the same age group (mean RelT/S 0.84 vs. 1.01, P = 0.03; Table 4a). There were no significant associations between telomere length and other sociodemographic variables for HIV-infected participants. In HIV-seronegative individuals, an association with alcohol consumption and telomere length was detected, with those who did not consume alcohol and those who consumed more than 1 l/week having the shortest telomere lengths (P = 0.04) (Table 4a). For CDKN2A expression, the only evidence of an association was among HIV-seronegative individuals, in whom CDKN2A expression was higher among those with lower incomes than among those with higher incomes (mean RelT/S 0.39 vs. 0.30, P = 0.03; Table 4b).
Table 4.
(a) Mean telomere length (RelT/S) in HIV-infected participants and HIV-seronegative individuals, adjusted for age.
| Variable | N | HIV-infected participants Telomere length Mean Rel T/S (95% CI) | P | N | HIV-seronegative participants Telomere length Mean Rel T/S (95% CI) | P |
| Age (years) | ||||||
| 30–39 | 119 | 0.92 (0.88–0.96) | 116 | 1.10 (1.04–1.15) | ||
| 40–49 | 77 | 0.93 (0.88–0.98) | 0.11 | 80 | 1.09 (1.02–1.15) | 0.09* |
| >50 | 40 | 0.84 (0.77–0.91) | 54 | 1.01 (0.94–1.08) | ||
| Sex | ||||||
| Male | 60 | 0.92 (0.86–0.99) | 60 | 1.04 (0.97–1.12) | ||
| Female | 176 | 0.90 (0.87–0.94) | 0.56 | 190 | 1.08 (1.04–1.12) | 0.30 |
| Income/month (US$) | ||||||
| <US$ 125/month | 134 | 0.91 (0.87–0.95) | 168 | 1.07 (1.02–1.12) | ||
| >US$ 125/month | 102 | 0.90 (0.85–0.95) | 0.86 | 82 | 1.07 (1.00–1.14) | 0.94 |
| UV exposure/occupation | ||||||
| Outdoor worker | 155 | 0.90 (0.86–0.94) | 0.69 | 178 | 1.08 (1.04–1.13) | 0.30 |
| Indoor worker | 81 | 0.92 (0.87–0.97) | 72 | 1.04 (0.98–1.12) | ||
| Education | ||||||
| <High school | 28 | 0.91 (0.82–1.01) | 44 | 1.04 (0.96–1.11) | ||
| High school/college | 208 | 0.91 (0.87–0.94) | 0.91 | 206 | 1.08 (1.04–1.12) | 0.49 |
| Housing | ||||||
| Formal | 123 | 0.91 (0.87–0.96) | 149 | 1.06 1.02–1.11 | ||
| Informal | 113 | 0.90 (0.86–0.95) | 0.69 | 101 | 1.09 1.03–1.15 | 0.77 |
| Duration of smoking | ||||||
| Nil | 200 | 0.91 (0.88–0.95) | 180 | 1.08 (1.03–1.12) | ||
| <10 years | 12 | 0.93 (0.80–1.08) | 0.36 | 32 | 1.07 (0.97–1.17) | 0.92 |
| ≥10 years | 24 | 0.85 (0.77–0.95) | 38 | 1.07 (0.98–1.16) | ||
| Alcohol (amount per week) | ||||||
| Nil | 164 | 0 . 92 (0.86–0.96) | 0.18* | 141 | 1.08 (1.03–1.13) | 0.04 |
| <1 l | 48 | 0.89 (0.82–0.95) | 54 | 1.13 (1.05–1.22) | ||
| >1 l | 24 | 0.86 (0.77–0.95) | 55 | 1.00 (0.93–1.08) | ||
| BMI (kg/m2) | ||||||
| <25 | 87 | 0.88 (0.83–0.93) | 0.08 | 60 | 1.04 (0.97–1.11) | 0.35 |
| 25–29.9 | 71 | 0.96 (0.90–0.98) | 55 | 1.12 (1.04–1.20) | ||
| >30 | 78 | 0.90 (0.85–0.95) | 135 | 1.07 (0.98–1.12) | ||
| Comorbid condition | ||||||
| No | 152 | 0.91 (0.87–0.95) | 138 | 1.08 (1.04–1.14) | ||
| Yes | 84 | 0.90 (0.85–0.95) | 0.88 | 112 | 1.06 (1.00–1.11) | 0.65 |
| TB status | ||||||
| No history | 77 | 0.90 (0.85–0.96) | 0.88 | 219 | 1.07 (1.03–1.11) | 0.71 |
| Current/past | 159 | 0.91 (0.87–0.95) | 31 | 1.09 (0.99–1.20) | ||
| (b) Relative CDKN2A expression in HIV-infected participants and HIV-seronegative individuals, adjusted for age. | ||||||
| Variable | N | HIV-infected participants Mean CDKN2A | P | N | HIV-seronegative participants Mean CDKN2A | P |
| Age (years) | ||||||
| 30–39 | 113 | 0.41 (0.36–0.47) | 98 | 0.29 (0.25–0.35) | ||
| 40–49 | 74 | 0.45 (0.38–0.53) | 0.04* | 70 | 0.44 (0.37–0.54) | 0.006 |
| >50 | 40 | 0.54 (0.43–0.68) | 49 | 0.38 (0.30–0.48) | ||
| Sex | ||||||
| Male | 59 | 0.42 (0.35–0.50) | 53 | 0.37 (0.30–0.47) | ||
| Female | 168 | 0.45 (0.41–0.51) | 0.46 | 163 | 0.35 (0.31–0.40) | 0.71 |
| Income/month (US$) | ||||||
| <US$ 125/month | 129 | 0.43 (0.38–0.48) | 145 | 0.39 (0.34–0.44) | ||
| >US$ 125/month | 98 | 0.47 (0.41–0.54) | 0.44 | 72 | 0.30 (0.23–0.37) | 0.03 |
| UV exposure/occupation | ||||||
| Outdoor worker | 147 | 0.45 (0.40–0.50) | 155 | 0.36 (0.32–0.41) | ||
| Indoor worker | 80 | 0.45 (0.38–0.52) | 0.81 | 62 | 0.34 (0.28–0.42) | 0.86 |
| Education | ||||||
| <High school | 27 | 0.48 (0.36–0.64) | 39 | 0.34 (0.26–0.44) | ||
| High school/college | 200 | 0.44 (0.40–0.49) | 0.60 | 178 | 0.36 (0.32–0.41) | 0.66 |
| Housing | ||||||
| Formal | 121 | 0.44 (0.39–0.50) | 126 | 0.34 (0.30–0.40) | ||
| Informal | 106 | 0.45 (0.40–0.52) | 0.63 | 91 | 0.37 (0.31–0.44) | 0.47 |
| Duration of smoking | ||||||
| Nil | 192 | 0.45 (0.40–0.49) | 157 | 0.36 (0.31–0.40) | ||
| <10 years | 12 | 0.65 (0.43–0.93) | 0.08 | 27 | 0.43 (0.31–0.58) | 0.23 |
| >10 years | 23 | 0.36 (0.28–0.49) | 33 | 0.30 (0.22–0.43) | ||
| Alcohol (amount per week) | ||||||
| Nil | 155 | 0 . 45 (0.40–0.50) | 126 | 0.35 (0.30–0.40) | ||
| <1 l | 48 | 0.44 (0.36–0.54) | 0.72 | 42 | 0.36 (0.28–0.47) | 0.87 |
| >1 l | 24 | 0.41 (0.31–0.55) | 49 | 0.37 (0.29–0.47) | ||
| BMI (kg/m2) | ||||||
| <25 | 82 | 0.41 (0.36–0.49) | 55 | 0.33 (0.26–0.41) | ||
| 25–29.9 | 69 | 0.49 (0.41–0.57) | 0.44 | 46 | 0.38 (0.30–0.48) | 0.59 |
| >30 | 76 | 0.45 (0.38–0.52) | 116 | 0.36 (0.31–0.42) | ||
| Comorbid condition | ||||||
| No | 144 | 0.44 (0.38–0.48) | 119 | 0.39 (0.34–0.46) | ||
| Yes | 83 | 0.46 (0.41–0.55) | 0.73 | 98 | 0.32 (0.26–0.38) | 0.08 |
| TB status | ||||||
| No history | 73 | 0.45 (0.38–0.53) | 0.93 | 191 | 0.36 (0.32–0.40) | 0.80 |
| Current/past | 154 | 0.44 (0.40–0.50) | 26 | 0.34 (0.25–0.48) | ||
CI, confidence interval; TB, tuberculosis. *P value test for trend.
Discussion
Levels of two validated biomarkers of ageing (telomere length and CDKN2A expression) were found to be consistent with increased biological ageing in South African HIV-infected individuals compared with age and sex-matched HIV-seronegative controls. This important finding is supported by our previous observations in this same study population that HIV infection is associated with increased frequency of a frailty phenotype [25], and changes in retinal vessel calibre, lens density and corneal endothelial cells that are consistent with increased biological ageing [26–28]. Among patients receiving ART and in whom plasma viral load was suppressed, the biological ageing estimated by both biomarkers was greatest in those with low current CD4+ cell counts. Although long-term prospective cohort studies will be required to definitively characterize the impact of HIV on biological ageing in sub-Saharan Africa, the combination of these case–control biomarker data and the existing frailty and ocular phenotypic data provide a strong evidence base on which to justify future long-term studies.
Both biomarkers were strongly associated with chronological age in HIV-seronegative individuals, validating their use as BoAs. Reduced telomere length is associated with markers of low socioeconomic status in industrialized countries [4,24]. Lower income was associated with increased CDKN2A expression in HIV-seronegative individuals, but socioeconomic factors were not associated with either BoA in HIV-infected participants. Adjustment for socioeconomic factors did not alter estimates of BoA between HIV-infected participants and HIV-seronegative individuals, suggesting that HIV infection, rather than social deprivation, is the main driver of biological ageing in this population.
Ageing reflects an accumulation of multiple molecular deficits in varying organ systems [29]. Interindividual differences in rates of ageing have prompted the search for informative biomarkers of biological ageing. Measurement of telomere length in PBLs is the standard method of evaluating biological ageing in epidemiological studies, with changes in PBL telomere length in effective synchrony with changes in telomere length in solid organs, thus providing a suitable surrogate for biological age in the whole organism [30,31]. However, there is potential for confounding by methodological and design difference between studies [15]. The number of techniques available for telomere length measurement highlights that no one technique is entirely satisfactory [32]. Southern blotting of terminal restriction fragments, single telomere length analysis (STELA) and real-time qPCR (qPCR) may also be used to assess telomere length in PBLs. We chose to use qPCR, as it is the most suitable methodology for high-throughput analyses [15,33]. Although interassay comparisons are very good (as evidenced by our coefficient of variance of 0.6%), intra-laboratory comparisons can be poor [34]. Logistical and budgetary constraints precluded assessment of telomere length in T-cell subsets, a methodology to address telomere dynamics advocated previously for investigation in the context of HIV infection [35]. Although we acknowledge that this methodology would give a higher resolution data set, the primary aim of this study was to provide preliminary data to inform future work, and assessment of telomere length in PBLs has been the approach used by others in this field [36,37].
In view of these methodological issues, we elected to measure an additional BoA, CDKN2A. CDKN2A expression increases with increasing chronological age in PBLs and solid organs [16,19,22], and it functions as a direct marker of cellular growth arrest [16]. CDKN2A represents a superior BoA to telomere length when judged by the Baker and Sprott criterion [3]. It can be a stronger predictor of function than chronological age and displays less interindividual variation [19,22]. CDKN2A expression was substantially greater in HIV-infected adults than in age-matched HIV-seronegative individuals. As well as functioning as a tumour suppressor, CDKN2A is also a component of stress-induced premature senescence [38], which prevents T-cell replication following acute insult [39]. The decoupling of the relationship between CDKN2A expression and chronological age in HIV-infected individuals is a direct consequence of HIV-associated premature T-cell senescence [2], and lack of further T-cell replication with HIV infection. Nelson et al.[40] found that ART-naive individuals had higher levels of CDKN2A expression than HIV-seronegative individuals, and suggested that active HIV replication accounted for elevated expression. Our data show that with viral suppression and ART, high levels of CDKN2A are still detectable. Thus, high rates of HIV replication are not a prerequisite for continued, elevated expression of this biomarker.
Both biomarkers were significantly associated with current CD4+ cell count in those receiving ART in whom viral load was undetectable. Increased CDKN2A expression was associated with lower CD4+ cell counts, consistent with the finding that CDKN2A expression is inversely related to T-cell replicative capacity [39]. Telomere length was also shorter in participants on ART with lower current CD4+ cell counts. Thus, levels of both BoA suggested that lower current CD4+ cell counts were associated with greater biological ageing. Human telomerase comprises a reverse transcriptase sharing homology with the HIV reverse transcriptase [41]. It is plausible that ART may inhibit its activity, leading to differences in telomere lengths between ART-naive and treated groups. Previous studies have produced inconsistent findings regarding such an association [36,42,43]. Comparison of levels in treated and ART-naive groups in our study did not support this potential mechanism, and furthermore, neither BoA was associated with ART duration. It should, however, be noted that dynamic changes over time in the PBL composition of the blood during ART may account for some of the observed effects, reflecting a potential limitation of using mixed PBLs in this context.
Although we have demonstrated a substantial effect of HIV infection on biological ageing, estimating the magnitude of the effect is challenging. One important reason is because disease states can cause stress-induced premature senescence [38], leading to acute growth arrest (in contrast to gradual replicative senescence). Thus, the ‘rate of biological ageing’ may not be accurately predicted in HIV infection. In other words, HIV-infected individuals effectively display a biological age observed in older uninfected individuals due to disease-induced stress. Therefore, quantification in terms of an effective biological age difference between infected and uninfected individuals is problematic, as the component of stress-induced premature senescence in infected individuals will be missing in uninfected controls, whereas in turn, replicative senescence may be accelerated due to disease stress. Estimation of telomere length as a function of given chronological age has been quantified [35,44]; however telomere length as an isolated measure at a given age may be imprecise, reflecting psychosocial, genetic and epigenetic confounders. The predictive capability of CDKN2A in determining effective biological age is relatively novel [16], and as CDKN2A expression is related to cellular growth arrest, it may not be subject to similar attrition phenomena [19,22,33]. Future work should involve development of methods to establish quantification of differences in effective biological age in disease states wherein stasis is involved. Any measure of the difference in biological age between HIV-infected and HIV-seronegative individuals will also have to be viewed in a functional context between these two groups, wherein differences in biochemical parameters, such as interleukin-6, CD14 and D-dimer, are also addressed [45]. Inflammation and activation of coagulation pathways are central to the pathophysiology leading to morbidity. Immune activation is a hallmark of chronic HIV infection and may be mediated by several mechanisms: increased pathogen burden as a result of impaired immunity, chronic viral replication of HIV and other viruses (e.g. cytomegalovirus, hepatitis viruses) and microbial translocation of bacterial products across damaged mucosa (e.g. in the gut). These factors may also need to be correlated to biomarkers of ageing to fully elucidate accelerated ageing trajectories in HIV infection.
A key strength of our study is the inclusion of an age and sex-matched control group with a similar sociodemographic profile as the HIV-infected participants. The hypothesis of accelerated ageing in HIV has received criticism due to limitations in characterization of participants, particularly the possibility of differential exposure to potential risk factors between HIV-infected and uninfected populations [46]. As the HIV epidemic in South Africa is generalized and recruitment took place from the same community, the likelihood of differential exposure was minimized. The study design means that ‘survivor effects’ cannot be excluded. Individuals with poor biological ageing may die earlier; thus, participants comprise survivors with different biological characteristics to nonsurvivors. However, recruitment of individuals from similar socioeconomic backgrounds should have limited potential confounding and survivor effects. The differences in smoking and alcohol consumption between the two groups could be due to HIV-infected participants modifying their smoking and alcohol consumption behaviours in response to lifestyle counselling. However, it is also plausible that the reported differences are due to misclassification, with HIV-infected participants wanting to demonstrate ‘healthy behaviour’, possibly leading to confounding. We used location of work as a proxy measure of UV exposure, which may also have been confounded by socioeconomic status. The sex composition of participants was three-quarters female, consistent with the characteristics of the clinic cohort from where our participants were enumerated [47], and reflective of the African AIDS epidemic. Lastly, as all our study participants are of African ancestry, our results are only generalizable to the African population.
The AIDS epidemic in sub-Saharan Africa is entering a new phase wherein HIV-infected individuals are living longer and may be ageing faster [9,48]. Our data indicate increased biological ageing in HIV-infected individuals compared with HIV-seronegative individuals. The increased number of older HIV-infected individuals in this region, compounded by accelerated biological ageing, may have wide-ranging implications for HIV management. Delivery of healthcare systems integrating HIV treatment and age-related morbidity may be necessary to manage this phase of the epidemic.
In light of these findings, important research questions arise: prospective assessment of biological age in HIV-infected and HIV-seronegative individuals is needed to ascertain whether the accelerated ageing trajectory develops as soon as HIV infection is acquired. Further questions include whether biological age is dependent upon the duration of untreated disease or nadir CD4+ cell count, and if the biological age of the two groups continues to diverge during long-term ART, or rather is modified by ART. Finally, assessment of the relative contributions of HIV and ART towards biological ageing may provide mechanistic insight.
In summary, greater biological age, as determined by shorter telomere length and higher CDKN2A expression, is associated with HIV infection in South Africa. The ‘ageing of the HIV epidemic’ poses many challenges and these may be amplified by accelerated biological ageing, potentially resulting in important health and social implications for the millions of patients receiving ART in Africa.
Acknowledgements
The authors wish to thank the staff and patients at the recruitment sites, the Department of Ophthalmology, Groote Schuur Hospital, Cape Town and DHL (Z.A.) for assistance with sample logistics and transport from Cape Town to Glasgow.
S.P. conceived and designed experiments; undertook clinical data collection; statistical analysis; wrote first draft; did the critical revision and provided intellectual input to further drafts. S.D.L. designed the experiments; did the critical revision and provided intellectual input to further drafts. C.G. conceived and designed the experiments; did the critical revision and provided intellectual input to further drafts. D.M. and L.M. did the laboratory data analysis; critical revision and provided intellectual input to further drafts. H.W. did the statistical analysis; critical revision and provided intellectual input to further drafts. J.P. T.C.. and KP carried out the laboratory data analysis. L.G.B. and R.W. did the critical revision and provided intellectual input to further drafts. P.G.S. conceived and designed the experiments; did the critical revision and provided intellectual input to further drafts.
This work was funded by a Wellcome Trust grant awarded to S.P. (Grant number: 090354/Z/09/Z). S.D.L. is funded by the Wellcome Trust, London (Grant number: 088590). R.W. is supported by IEDEAA, International Epidemiologic Database to Evaluate AIDS (Grant number: 5U01AI069924-02) and CEPAC Cost-Effectiveness of Preventing AIDS Complications 5 (R01AI058736-02).
The funders played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Correspondence to Dr Sophia Pathai, International Centre for Eye Health, Department of Clinical Research, London School of Hygiene & Tropical Medicine, Keppel Street, London WC1E 7HT, UK. Tel: +44 20 7958 8343; fax: +44 20 7958 8325; e-mail: Sophia.pathai@lshtm.ac.uk
References
- 1.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2009; 17:118–123 [PubMed] [Google Scholar]
- 2.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker GT, 3rd, Sprott RL. Biomarkers of aging. Exp Gerontol 1988; 23:223–239 [DOI] [PubMed] [Google Scholar]
- 4.Shiels PG, Mcglynn LM, Macintyre A, Johnson PCD, Batty GD, Burns H, et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS One 2011; 6:e22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012; 60 Suppl 1:S1–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, et al. Persistent microbial translocation and immune activation in HIV-1–infected South Africans receiving combination antiretroviral therapy. J Infect Dis 2010; 202:723–733 [DOI] [PubMed] [Google Scholar]
- 7.Ledwaba L, Tavel JA, Khabo P, Maja P, Qin J, Sangweni P, et al. Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One 2012; 7:e24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice AC, Braithwaite RS. Lessons learned from the first wave of aging with HIV. AIDS 2012; 26:S11–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills EJ, Rammohan A, Awofeso N. Ageing faster with AIDS in Africa. Lancet 2011; 377:1131–1133 [DOI] [PubMed] [Google Scholar]
- 10.Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol 2011; 46:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci 2008; 63:979–983 [DOI] [PubMed] [Google Scholar]
- 12.Starr JM, McGurn B, Harris SE, Whalley LJ, Deary IJ, Shiels PG. Association between telomere length and heart disease in a narrow age cohort of older people. Exp Gerontol 2007; 42:571–573 [DOI] [PubMed] [Google Scholar]
- 13.Joosten SA, van Ham V, Nolan CE, Borrias MC, Jardine AG, Shiels PG, et al. Telomere shortening and cellular senescence in a model of chronic renal allograft rejection. Am J Pathol 2003; 162:1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Takayama Y, Saito M, Ishikawa F, Saneyoshi M. Telomerase-inhibitory effects of the triphosphate derivatives of some biologically active nucleosides. Nucleic Acids Res Suppl 2001; 211–212 [DOI] [PubMed] [Google Scholar]
- 15.Shiels PG. Improving precision in investigating aging: why telomeres can cause problems. J Gerontol A Biol Sci Med Sci 2010; 65:789–791 [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 2009; 8:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS 2005; 19:2141–2148 [DOI] [PubMed] [Google Scholar]
- 18.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koppelstaetter C, Schratzberger G, Perco P, Hofer J, Mark W, Ollinger R, et al. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell 2008; 7:491–497 [DOI] [PubMed] [Google Scholar]
- 20.Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, et al. The association between telomere length, physical health, cognitive ageing, and mortality in nondemented older people. Neurosci Lett 2006; 406:260–264 [DOI] [PubMed] [Google Scholar]
- 21.Carrero JJ, Stenvinkel P, Fellstrom B, Qureshi AR, Lamb K, Heimburger O, et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med 2008; 263:302–312 [DOI] [PubMed] [Google Scholar]
- 22.McGlynn LM, Stevenson K, Lamb K, Zino S, Brown M, Prina A, et al. Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell 2009; 8:45–51 [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–408 [DOI] [PubMed] [Google Scholar]
- 24.Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell 2006; 5:361–365 [DOI] [PubMed] [Google Scholar]
- 25.Pathai S, Gilbert C, Weiss HA, Cook C, Wood R, Bekker LG, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr 2013; 62:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathai S, Weiss HA, Lawn SD, Peto T, D’Costa LM, Cook C, et al. Retinal arterioles narrow with increasing duration of anti-retroviral therapy in HIV infection: a novel estimator of vascular risk in HIV?. PLoS One 2012; 7:e51405.Epub 2012 Dec 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathai S, Lawn SD, Weiss HA, Cook C, Bekker LG, Gilbert CE. Increased ocular lens density in HIV-infected individuals with low nadir CD4 counts in South Africa: evidence of accelerated aging. J Acquir Immune Defic Syndr 2013; 63:307–314 [DOI] [PubMed] [Google Scholar]
- 28.Pathai S, Lawn SD, Shiels PG, Weiss HA, Cook C, Wood R, Gilbert CE. Corneal endothelial cells provide evidence of accelerated cellular senescence associated with HIV infection: a case-control study. PLoS One 2013; 8:e57422.Epub 2013 Feb 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulop T, Larbi A, Witkowski J, McElhaney J, Loeb M, Mitnitski A, et al. Aging, frailty and age-related diseases. Biogerontology 2010; 11:547–563 [DOI] [PubMed] [Google Scholar]
- 30.Gardner JP, Kimura M, Chai W, Durrani JF, Tchakmakjian L, Cao X, et al. Telomere dynamics in macaques and humans. J Gerontol A Biol Sci Med Sci 2007; 62:367–374 [DOI] [PubMed] [Google Scholar]
- 31.Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Exp Hematol 2010; 38:854–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res 2012; 730:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiels PG. CDKN2A might be better than telomere length in determining individual health status. BMJ 2012; 344:e1415. [DOI] [PubMed] [Google Scholar]
- 34.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res 2011; 39:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol 2006; 35:1424–1429 [DOI] [PubMed] [Google Scholar]
- 36.Leeansyah E, Cameron PU, Solomon A, Tennakoon S, Velayudham P, Gouillou M, et al. Inhibition of telomerase activity by human immunodeficiency virus (HIV) nucleos(t)ide reverse transcriptase inhibitors: a potential factor contributing to HIV-associated accelerated aging. J Infect Dis 2013; 207:1157–1165 [DOI] [PubMed] [Google Scholar]
- 37.Malan-Muller S, Hemmings SM, Spies G, Kidd M, Fennema-Notestine C, Seedat S. Shorter telomere length: a potential susceptibility factor for HIV-associated neurocognitive impairments in South African woman. PLoS One 2013; 8:e58351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol 2000; 1:72–76 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Johnson SM, Fedoriw Y, Rogers AB, Yuan H, Krishnamurthy J, et al. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood 2011; 117:3257–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson JA, Krishnamurthy J, Menezes P, Liu Y, Hudgens MG, Sharpless NE, et al. Expression of p16(INK4a) as a biomarker of T-cell aging in HIV-infected patients prior to and during antiretroviral therapy. Aging Cell 2012; 11:916–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng Y, Mian IS, Lue NF. Analysis of telomerase processivity: mechanistic similarity to HIV-1 reverse transcriptase and role in telomere maintenance. Mol Cell 2001; 7:1201–1211 [DOI] [PubMed] [Google Scholar]
- 42.Ballon G, Ometto L, Righetti E, Cattelan AM, Masiero S, Zanchetta M, et al. Human immunodeficiency virus type 1 modulates telomerase activity in peripheral blood lymphocytes. J Infect Dis 2001; 183:417–424 [DOI] [PubMed] [Google Scholar]
- 43.Wolthers KC, Otto SA, Wisman GB, Fleury S, Reiss P, ten Kate RW, et al. Normal T-cell telomerase activity and upregulation in human immunodeficiency virus-1 infection. Blood 1999; 93:1011–1019 [PubMed] [Google Scholar]
- 44.Der G, Batty GD, Benzeval M, Deary IJ, Green MJ, McGlynn L, et al. Is telomere length a biomarker for aging: cross-sectional evidence from the west of Scotland?. PLoS One 2012; 7:e45166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV?. Clin Infect Dis 2012; 54:984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin J, Volberding P. HIV and premature aging: A field still in its infancy. Ann Intern Med 2010; 153:477–479 [DOI] [PubMed] [Google Scholar]
- 47.Nglazi MD, Lawn SD, Kaplan R, Kranzer K, Orrell C, Wood R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr 2011; 56:e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, et al. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS 2011; 25:1637–1646 [DOI] [PubMed] [Google Scholar]


