Abstract
BACKGROUND
Poor engraftment due to low cell doses restricts the usefulness of umbilical-cord-blood transplantation. We hypothesized that engraftment would be improved by transplanting cord blood that was expanded ex vivo with mesenchymal stromal cells.
METHODS
We studied engraftment results in 31 adults with hematologic cancers who received transplants of 2 cord-blood units, 1 of which contained cord blood that was expanded ex vivo in cocultures with allogeneic mesenchymal stromal cells. The results in these patients were compared with those in 80 historical controls who received 2 units of unmanipulated cord blood.
RESULTS
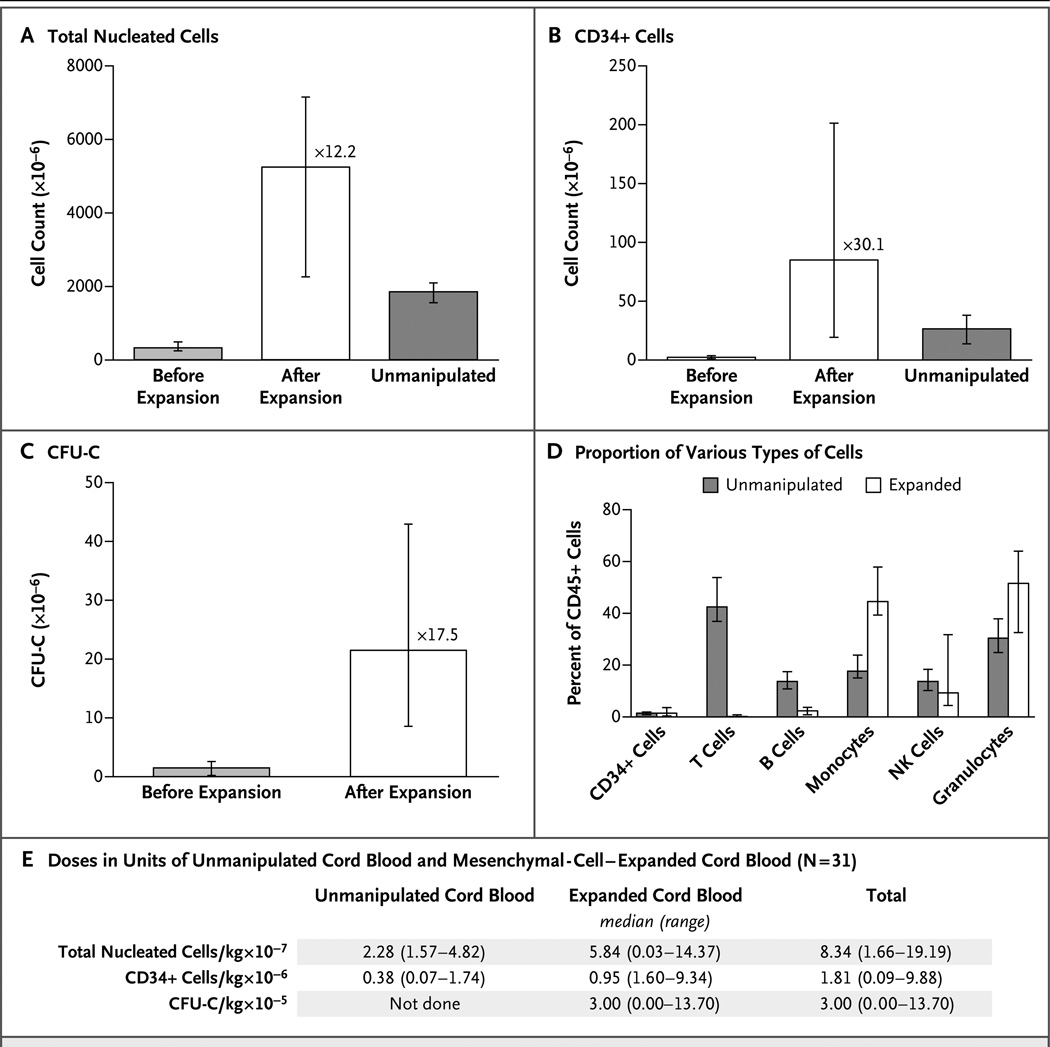
Coculture with mesenchymal stromal cells led to an expansion of total nucleated cells by a median factor of 12.2 and of CD34+ cells by a median factor of 30.1. With transplantation of 1 unit each of expanded and unmanipulated cord blood, patients received a median of 8.34×107 total nucleated cells per kilogram of body weight and 1.81×106 CD34+ cells per kilogram — doses higher than in our previous transplantations of 2 units of unmanipulated cord blood. In patients in whom engraftment occurred, the median time to neutrophil engraftment was 15 days in the recipients of expanded cord blood, as compared with 24 days in controls who received unmanipulated cord blood only (P<0.001); the median time to platelet engraftment was 42 days and 49 days, respectively (P = 0.03). On day 26, the cumulative incidence of neutrophil engraftment was 88% with expansion versus 53% without expansion (P<0.001); on day 60, the cumulative incidence of platelet engraftment was 71% and 31%, respectively (P<0.001).
CONCLUSIONS
Transplantation of cord-blood cells expanded with mesenchymal stromal cells appeared to be safe and effective. Expanded cord blood in combination with unmanipulated cord blood significantly improved engraftment, as compared with unmanipulated cord blood only. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00498316.)
Umbilical-cord blood is an attractive source of hematopoietic support for patients who lack a suitable HLA-matched donor. Despite the advantages offered by cord-blood transplantation, such as the use of a frozen, readily available allograft in patients who are members of minority groups, who often have limited access to adult donors, the clinical usefulness in adults has been restricted by the relatively low number of hematopoietic progenitors in a unit of cord blood.1–4 Delayed or failed engraftment of neutrophils and platelets with cord-blood transplantation can result in an increased risk of transplant-related complications or death and increased health care costs, as compared with the transplantation of bone marrow progenitor cells or peripheral-blood progenitor cells.5–11
Transplantation of 2 cord-blood units has extended the use of cord-blood transplantation to adults, but the engraftment remains inferior to that achieved with marrow or peripheral-blood stem cells.12–14 Thus, our group has focused on the ex vivo expansion of cord-blood cells to increase the numbers of myeloid and megakaryocyte progenitors after myeloablative treatment. Suspension cultures of cord-blood mononuclear cells without the use of CD34 selection result in minimal, if any, expansion of nucleated cells or progenitor cells. In our experience, CD34 selection of frozen cord-blood products has resulted in low purities and poor expansion.15,16 We have previously shown that expansion of both primitive and mature hematopoietic progenitors in unfractionated cord-blood cells is markedly enhanced by coculture with mesenchymal stromal cells.17 These data suggest that mesenchymal stromal cells provide vital molecular signals for ex vivo expansion that are missing in expansion systems based on suspension culture of hematopoietic progenitors in cytokines alone.
We describe a series of 31 adults with hematologic cancers who received transplants of 2 cord-blood units, 1 of which contained cord blood that was expanded ex vivo in cocultures with allogeneic mesenchymal stromal cells. Eighty patients whose data were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) and who received transplants of 2 units of unmanipulated cord blood were used as controls, as was a separate cohort of 60 controls treated at the M.D. Anderson Cancer Center (MDACC).
METHODS
STUDY DESIGN AND OVERSIGHT
The first and last authors designed the study, made the decision to submit the manuscript for publication, and vouch for the completeness and accuracy of the data and for the fidelity of the study to the protocol, which is available with the full text of this article at NEJM.org. Mesoblast supported the study and had a confidentiality agreement with the MDACC. Mesoblast provided the “off-the-shelf” mesenchymal precursor cells free of charge as well as support for research nurses and data management. No one who is not listed as an author contributed to the writing of the manuscript. This study was approved by the institutional review board and the Food and Drug Administration (FDA).
ELIGIBILITY
Patients 18 to 65 years of age with hematologic cancers who lacked an HLA-compatible donor were enrolled at the MDACC between August 2007 and February 2010, after providing written informed consent. Enrollment required the receipt of 2 cord-blood units, each containing more than 1.5×107 total nucleated cells per kilogram of body weight, that were matched at four or more HLA loci by intermediate-resolution typing for HLA class I alleles (A and B) and high-resolution typing for HLA class II DRB1 alleles.
COCULTURE OF CORD-BLOOD MONONUCLEAR CELLS AND MESENCHYMAL CELLS
For the initial cohort of seven patients, a haploidentical family member was the donor of mesenchymal stromal cells. Cells were isolated from 100 ml of marrow and cultured in flasks with 50 ml of alpha minimal essential medium supplemented with 20% fetal bovine serum (HyClone). Nonadherent cells were removed after 3 days, and adherent cells were cultured until their confluence reached 70% or higher and then subcultured further until there were confluent mesenchymal stromal cells in 10 flasks. Fourteen days before transplantation, the cord-blood unit with the lowest dose of total nucleated cells was thawed, and equal fractions were placed into each of 10 flasks containing mesenchymal stromal cells with 50 ml of Good Manufacturing Practices (GMP)–grade serum-free medium containing 100 ng per milliliter each of stem-cell factor, Flt3 ligand, throm-bopoietin (CellGenix), and granulocyte colony-stimulating factor (Amgen).
After 7 days of coculture, the nonadherent cells were transferred to 10 1-liter culture bags (American Fluoroseal) containing 450 ml of coculture medium with cytokines and cultured for an additional 7 days. On day 7 of culture, the flasks were also replenished with 50 ml of the coculture medium containing the cytokines and cultured for another 7 days. On day 14 of culture (day 0 with regard to transplantation), all the nonadherent cells in the flasks and bags were pooled, washed, and infused. The release criteria included cell viability (≥70%), a negative endotoxin test, and a negative result on Gram’s staining.
The logistics and time required to generate mesenchymal stromal cells from a family-member marrow donor for each patient limited recruitment for the trial. Thus, we evaluated a clinical-grade, off-the-shelf mesenchymal precursor cell product manufactured under GMP conditions (Mesoblast).18,19 The cells were selected with the use of the STRO-3 monoclonal antibody (specific for an alkaline phosphatase isoform expressed by mesenchymal stem cells) from bone marrow aspirates obtained from healthy donors. A master cell bank was created, and the mesenchymal cells were generated, cryopreserved, and tested comprehensively for infectious agents and tumor formation in accordance with FDA guidelines. The frozen mesenchymal cells were transported to the MDACC and maintained in liquid nitrogen until needed. We found that a single vial of STRO-3+ mesenchymal cells could routinely generate 10 flasks of cells in 4 days, with expansion results that did not differ substantially from those achieved with mesenchymal stem cells derived from a family member.20 Thus, we amended our protocol to use the STRO-3+ mesenchymal cells in the subsequent 24 treated patients.
IMMUNOPHENOTYPING AND COLONY-FORMATION ASSAY
Cord-blood cells before and after expansion were analyzed by means of flow cytometry and assays of colony-forming units in culture (CFU-C) for hematopoietic stem cells and progenitor cells, as previously described.21,22
CONDITIONING REGIMEN AND PROPHYLAXIS
The ablative conditioning regimen consisted of melphalan at a dose of 140 mg per square meter of body-surface area 8 days before transplantation (day −8), thiotepa at a dose of 10 mg per kilogram on day −7, fludarabine at a daily dose of 40 mg per square meter on days −6 through −3, and rabbit antithymocyte globulin (Genzyme) at a dose of 1.25 mg per kilogram on day −4 and 1.75 mg per kilogram on day −3. Prophylaxis against graft-versus-host disease (GVHD) consisted of tacrolimus at a daily dose of 0.03 mg per kilogram on days −2 through 180 and mycophenolate mofetil at a dose of 1 g orally twice daily on days −2 through 100.
TRANSPLANTATION PROCEDURE AND SUPPORTIVE CARE
On day 0, the unit of unmanipulated cord blood was thawed, washed, and infused intravenously, followed by infusion of the expanded cord-blood cells. All patients received subcutaneous granulocyte colony-stimulating factor (Amgen) at a daily dose of 5 μg per kilogram from day 1 until neutrophil recovery.
HEMATOPOIETIC RECOVERY
The time to neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of 0.5×109 per liter or higher, and the time to platelet engraftment as the first of 7 consecutive days with a platelet count of 20×109 per liter or higher without platelet transfusion. Chimerism in the peripheral blood or bone marrow was documented between days 20 and 30, on day 60, and every 3 months thereafter by means of a polymerase-chain-reaction assay, with primer sets flanking microsatellite repeats.
COMPARISON WITH CIBMTR AND MDACC HISTORICAL DATA
We compared engraftment results and survival in the group of patients who received cord-blood cells expanded by coculture with STRO-3+ mesenchymal cells with the outcomes among 80 patients who received 2 units of unmanipulated cord blood between 2008 and 2010 at 24 U.S. transplantation centers and whose data were reported to the CIBMTR. Controls were matched for age, diagnosis, disease stage, intensity of the myeloablative conditioning regimen, and GVHD prophylaxis with a calcineurin inhibitor and mycophenolate. The most commonly used conditioning regimen was total-body irradiation (1320 cGy) in combination with fludarabine and cyclophos-phamide, followed in frequency by a regimen of melphalan, fludarabine, and thiotepa. The median follow-up time for controls was 6 months (range, 3 to 12). We also compared the study group with institutional controls at the MDACC (consecutive adults who received 2 units of unmanipulated cord blood after myeloablative conditioning between 2001 and 2011). The most commonly used conditioning regimens were thiotepa combined with melphalan and fludarabine or with clofarabine and busulfan. The median follow-up time was 7 months (range, 1 to 72).
END POINTS
The primary end points were safety, feasibility and cumulative incidence of neutrophil and platelet engraftment, median time to neutrophil and to platelet recovery among patients who reached these end points, the proportion of patients in whom transplantation was successful (as defined below), and the cumulative incidence of acute or chronic GVHD. Acute GVHD was graded according to consensus criteria.23 Chronic GVHD was diagnosed when clinical signs were present or developed for the first time after day 100. Because this was a small, short-term study, we used an early composite definition of successful transplantation (engraftment of neutrophils by day 26, engraftment of platelets by day 60, and survival at day 100), as suggested by others,24 and compared recipients of expanded cord-blood transplants with the CIBMTR and MDACC controls.
STATISTICAL ANALYSIS
Cumulative incidence curves were constructed with the use of the approach described by Gooley et al.,25 with death considered to be a competing event. The methods of Fine and Gray were used to compare cumulative incidence curves between groups of patients.26 The percentages of patients in whom engraftment had occurred at specific time points were compared between groups with the use of a two-sample z-test, and the Wilcoxon rank-sum test was used to compare recipients of expanded cord blood and recipients of unmanipulated cord blood with respect to median values.
RESULTS
PATIENTS
Table 1 shows the characteristics of the 31 adults who received expanded cord–blood units as well as those of the CIBMTR and institutional controls who received unmanipulated cord-blood units only. Among all patients who received a unit of expanded cord blood, 58% had advanced disease (as defined by morphologic criteria for leukemias and the myelodysplastic syndrome) at the time of transplantation. Of the 24 patients who received STRO-3+ mesenchymal-cell–expanded cord blood, 12 (50%) had advanced disease; data on these patients were compared with the CIBMTR historical data. A lower proportion of the CIBMTR controls had advanced disease at the time of transplantation (31 of 80 [39%]).
Table 1.
Demographic and Clinical Characteristics of the Patients and Controls.*
| Characteristic | Patients | Controls | ||
|---|---|---|---|---|
| Haploidentical Mesenchymal Stromal Cells (N = 7) |
STRO-3+ Mesenchymal Progenitor Cells (N = 24) |
MDACC (N = 60)† |
CIBMTR (N = 80) |
|
| Weight — kg | ||||
| Median | 79 | 75 | 75 | 82 |
| Range | 53–95 | 51–118 | 48–122 | 40–170 |
| Age — yr | ||||
| Median | 31 | 39 | 32 | 36 |
| Range | 26–55 | 18–61 | 18–64 | 18–61 |
| Diagnosis — no. (%) | ||||
| AML or MDS | 5 (71) | 16 (67) | 31 (52) | 52 (65) |
| ALL | 1 (14) | 4 (17) | 15 (25) | 20 (25) |
| Non-Hodgkin’s or Hodgkin’s lymphoma | 0 | 3 (12) | 5 (8) | 7 (9) |
| CLL | 1 (14) | 1 (4) | 2 (3) | 1 (1) |
| CML or other MPD | 0 | 0 | 6 (10) | 0 |
| Myeloma | 0 | 0 | 1 (2) | 0 |
| Disease status at time of transplantation — no. (%) | ||||
| Complete remission | 1 (14) | 12 (50) | 26 (43) | 49 (61) |
| First remission | 1 (14) | 2 (8) | 8 (13) | 17 (21) |
| Second or subsequent remission | 0 | 10 (42) | 18 (30) | 32 (40) |
| Active disease | 6 (86) | 12 (50) | 34 (57) | 31 (39) |
| Donor–recipient HLA compatibility — no. (%) | ||||
| 6/6 | 0 | 1 (4) | 4 (7) | 4 (5) |
| 5/6 | 3 (43) | 3 (12) | 13 (22) | 14 (18) |
| 4/6 | 4 (57) | 20 (83) | 43 (72) | 58 (72) |
| 3/6 | 0 | 0 | 0 | 2 (2) |
| Not reported | 0 | 0 | 0 | 2 (2) |
ALL denotes acute lymphocytic leukemia, AML acute myeloid leukemia, CIBMTR Center for International Blood and Marrow Transplant Research, CLL chronic lymphocytic leukemia, CML chronic myeloid leukemia, MDS myelodysplastic syndrome, and MPD myeloproliferative disorder.
Two controls from the M.D. Anderson Cancer Center (MDACC) were excluded owing to lack of engraftment and chimerism documentation.
CORD-BLOOD EXPANSION AND INFUSION
After 14 days of culture, total nucleated cells were expanded by a median factor of 12.2, CD34+ cells were expanded by a factor of 30.1, and CFU-C had expanded by a factor of 17.5 (Fig. 1A, 1B, and 1C). The units of mesenchymal-cell–expanded cord blood had increased proportions of monocytes and granulocytes but decreased proportions of T and B cells, as compared with the units of unmanipulated cord blood (Fig. 1D). Megakaryocyte and platelet progenitors were evaluated in a cohort of patients. In the units of unmanipulated cord blood (received by 15 patients), a median of 202×106 CD41a+CD61+ cells (range, 94×106 to 308×106) were present in the platelet gate on flow cytometry. In the units of expanded cord blood (received by 11 patients), the median number of CD41a+CD61+ cells was 406×106 (range, 50×106 to 1350×106). Patients received a median of 5.08×106 CD41a+CD61+ cells per kilogram from the units of mesenchymal-cell–expanded cord blood (data not shown).
Figure 1. Expansion of Cord Blood with Mesenchymal Stromal Cells.
Panels A and B show the median numbers of total nucleated cells and CD34+ cells, respectively, in the cord-blood grafts before and after ex vivo expansion, as compared with the numbers in units of unmanipulated cord blood. I bars represent the interquartile range. The numbers of nucleated cells and CD34+ cells were higher in the unit of expanded cord blood than in the unit of unmanipulated cord blood (P<0.001 and P = 0.01, respectively). The median numbers of colony-forming units in culture (CFU-C) before and after ex vivo expansion are shown in Panel C. There were significant increases in nucleated cells, CD34+ cells, and CFU-C in the unit of expanded cord blood as compared with the values before expansion (P<0.001 for all comparisons). Nucleated cells were expanded by a median factor of 12.2 (range, 1.0 to 29.8), the CD34+ cells by a median factor of 30.1 (range, 0 to 137.8), and CFU-C by a median factor of 17.5 (range, 0 to 435.0). The percentages of various types of cells in the cord-blood unit before and after ex vivo expansion in mesenchymal-cell cocultures are shown in Panel D. The units of mesenchymal-cell–expanded cord blood had increased proportions of monocytes (P<0.001) and granulocytes (P = 0.003), decreased proportions of T lymphocytes (P<0.001) and B lymphocytes (P<0.001), and similar proportions of CD34+ cells (P = 0.99) and natural killer (NK) cells (P = 0.85). Percent of CD45+ cells refers to the percent of the various cells in this gate on flow cytometry. The doses of nucleated cells and CD34+ cells in each unit and the total doses of nucleated cells, CD34+ cells, and CFU-C infused in the transplant recipients are shown in Panel E.
There were no significant differences in nucleated cells, CD34+ cells, or CFU-C between the family-member–derived and STRO-3+ mesenchymal-cell–expanded units of cord blood (Fig. S1 in the Supplementary Appendix, available at NEJM.org). The numbers of CD34+CD38− cells and CD133+CD33+ cells did differ significantly according to the cord-blood source, with more of these cell types in the STRO-3+ mesenchymal-cell–expanded cord blood (P = 0.02 and P = 0.03, respectively). Expansion yielded a median of 5.84×107 nucleated cells per kilogram, 0.95×106 CD34+ cells per kilogram, and 3.00×105 CFU-C per kilogram (Fig. 1E). These doses were higher than in our previous transplantations of 2 units of unmanipulated cord blood. For all cord-blood units, the cell viability was greater than 70%, and Gram’s staining and endotoxin tests were negative before infusion. There were no serious adverse events related to the infusions of cord blood.
NEUTROPHIL AND PLATELET RECOVERY
One patient died from fungal sepsis on day 30 without engraftment of neutrophils or platelets. In patients in whom engraftment occurred, the median time to neutrophil recovery was 15 days (range, 9 to 42) and the median time to platelet recovery was 42 days (range, 15 to 62). There were no significant differences in the time to neutrophil or platelet engraftment between patients who received cord blood expanded in cocultures with family-member–derived mesenchymal stromal cells and those who received cord blood expanded with STRO-3+ mesenchymal cells (Fig. S2 and Table S1 in the Supplementary Appendix). In addition, within the limits of the small sample, the degree of donor–recipient HLA matching did not correlate with the speed or frequency of engraftment.
A decision was made to move forward with STRO-3+ mesenchymal cells rather than family-member–derived cells. Thus, we restricted our comparative analysis with the CIBMTR controls to recipients of cord blood expanded with the STRO-3+ mesenchymal cells. The median time to neutrophil engraftment was 15 days in the recipients of expanded cord blood versus 24 days in the CIBMTR controls who received unmanipulated cord blood only (P<0.001) (Table 2). The median time to platelet engraftment was 42 days in the recipients of expanded cord blood versus 49 days in controls (P = 0.03).
Table 2.
Engraftment in Recipients of Ex Vivo Expanded Cells and MDACC and CIBMTR Controls.
| Engraftment | Recipients of Ex Vivo Expanded Cells (N = 24) |
MDACC Controls (N = 60) |
P Value* | CIBMTR Controls (N = 80) |
P Value† |
|---|---|---|---|---|---|
| Neutrophil engraftment | |||||
| No. of patients | 23 | 51 | 67 | ||
| Time to engraftment — days | |||||
| Median | 15 | 21 | 0.08 | 24 | <0.001 |
| Range | 9–42 | 6–45 | 12–52 | ||
| Cumulative incidence — % (95% CI) | |||||
| By 26 days | 88 (66–96) | 62 (48–73) | 0.006 | 53 (41–63) | <0.001 |
| By 42 days | 96 (74–99) | 83 (71–91) | 0.05 | 78 (67–86) | 0.005 |
| Platelet engraftment | |||||
| No. of patients | 18 | 38 | 37 | ||
| Time to engraftment — days | |||||
| Median | 42 | 41 | 0.33 | 49 | 0.03 |
| Range | 15–62 | 26–126 | 18–264 | ||
| Cumulative incidence — % (95% CI) | |||||
| By 60 days | 71 (48–85) | 52 (38–63) | 0.10 | 31 (21–41) | <0.001 |
| By 180 days | 75 (53–88) | 63 (50–74) | 0.28 | 46 (35–58) | 0.01 |
P values are for the comparison between recipients of STRO-3+ mesenchymal precursor cells and MDACC controls.
P values are for the comparison between recipients of STRO-3+ mesenchymal precursor cells and CIBMTR controls.
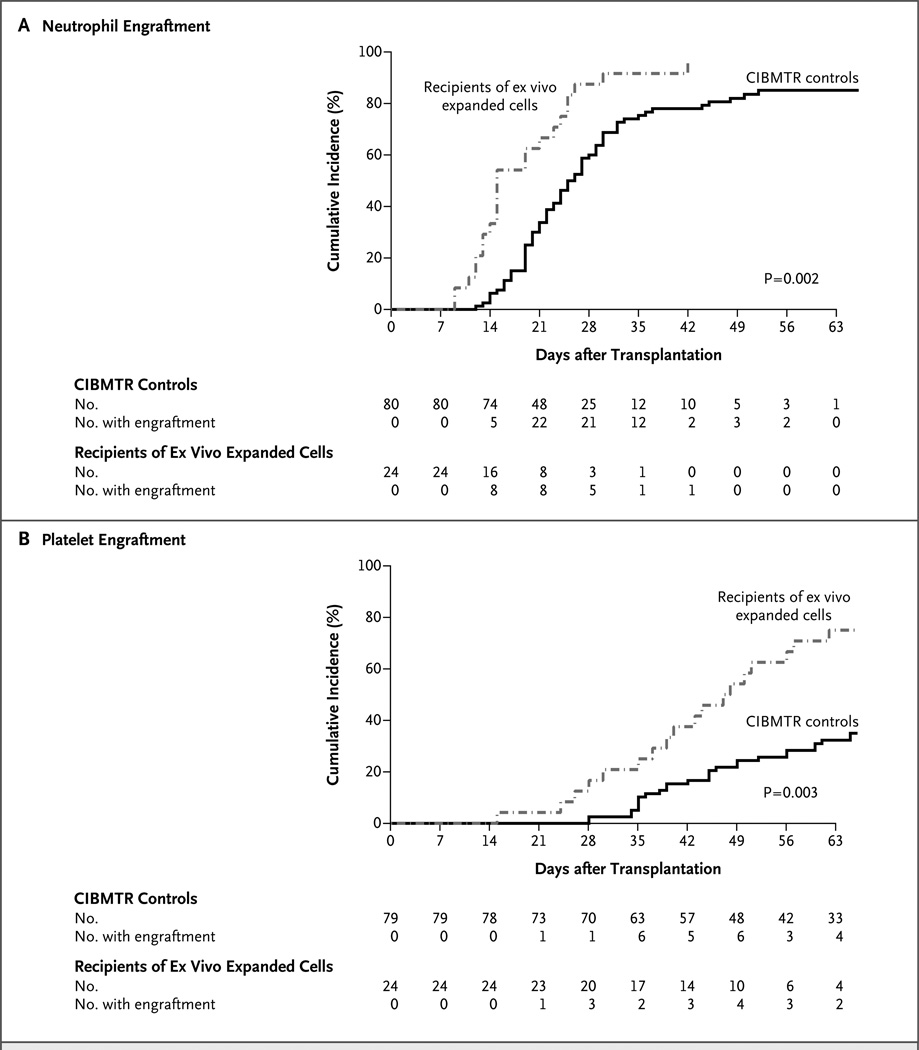
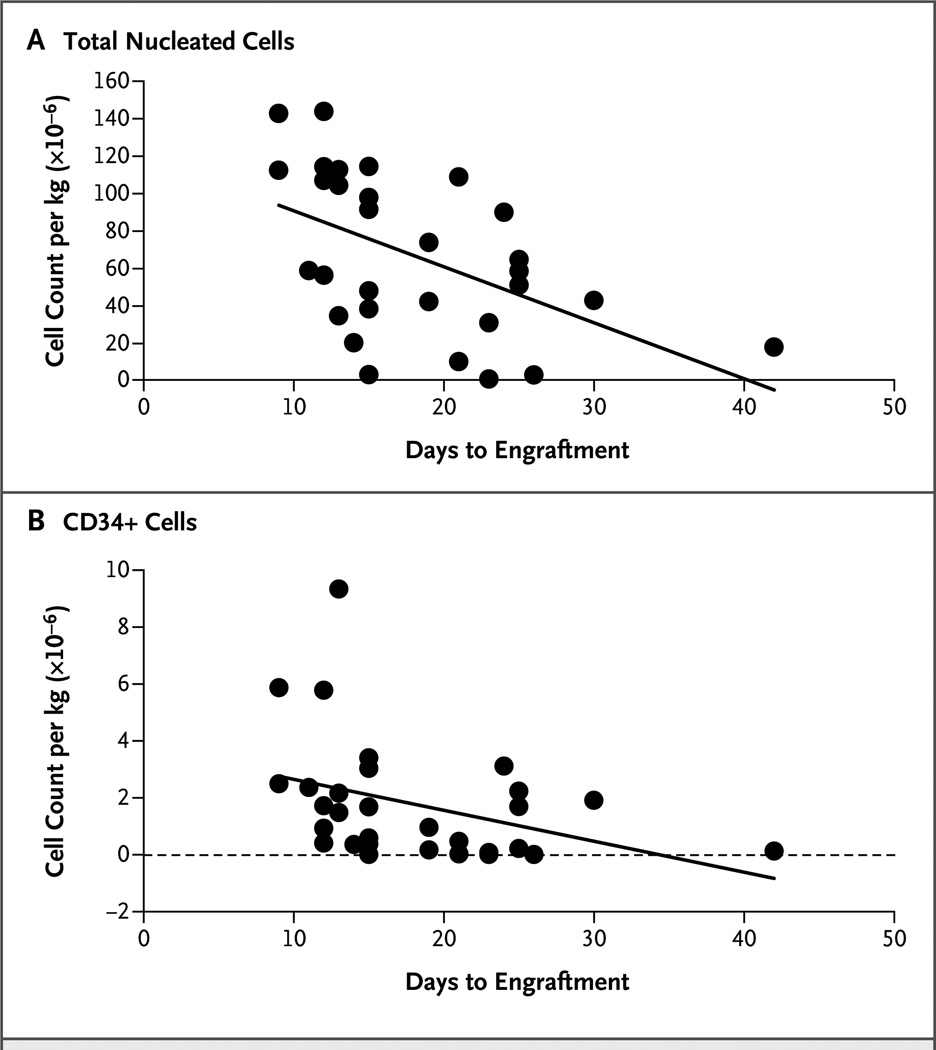
On day 26, the cumulative incidence of neutrophil engraftment was 88% among recipients of expanded cord blood as compared with 53% among CIBMTR controls (P<0.001) (Table 2 and Fig. 2A). On day 60, the cumulative incidence of platelet engraftment was 71% among recipients of expanded cord blood as compared with 31% in the CIBMTR cohort (P<0.001) (Table 2 and Fig. 2B). The comparison of the STRO-3+ group with the institutional controls is also shown in Table 2. The dose of nucleated cells per kilogram in the recipients of expanded cord blood correlated with the time to neutrophil recovery (P = 0.004) (Fig. 3A). The dose of CD34+ cells per kilogram in the recipients of expanded cord blood also correlated with the time to neutrophil engraftment (P = 0.006) (Fig. 3B). For the composite end point of neutrophil engraftment by day 26, platelet engraftment by day 60, and survival at 100 days, success rates were 63% among recipients of expanded cord blood versus 24% among CIBMTR controls (P<0.001) and 35% among MDACC controls (P = 0.03).
Figure 2. Cumulative Incidences of Neutrophil Engraftment and Platelet Engraftment.
A total of 24 patients who received 2 units of cord blood, 1 of which contained cord blood that was expanded ex vivo in cocultures with STRO-3+ mesenchymal cells, were compared with 80 control patients who received 2 units of unmanipulated cord blood and whose data were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). Controls were matched according to age, diagnosis, intensity of the preparative regimen, and prophylaxis against graft-versus-host disease. Panel A shows the cumulative incidence of neutrophil recovery. At 26 days, the cumulative incidence was 88% among recipients of expanded cord blood and 53% among CIBMTR controls (P<0.001). Panel B shows the cumulative incidence of platelet recovery. At 60 days, the cumulative incidence was 71% among recipients of expanded cord blood and 31% among CIBMTR controls (P<0.001). Data on platelet engraftment were not available for one CIBMTR control. Ex vivo expansion led to more rapid neutrophil and platelet engraftment and to a higher proportion of patients with engraftment of both cell types.
Figure 3. Correlation of Total Nucleated Cells and CD34+ Cells with Neutrophil Engraftment.
In the units of expanded cord blood, the number of total nucleated cells per kilogram of body weight (Panel A) correlated with the speed of neutrophil engraftment (Spearman correlation coefficient, −0.51; P = 0.004), and the number of −CD34+ cells per kilogram (Panel B) also correlated with the speed of neutrophil engraftment (Spearman correlation coefficient, −0.48; P = 0.006).
CHIMERISM
Twenty-eight patients who could be evaluated had complete (100%) donor chimerism with 1 or both cord-blood units in the T-cell and myeloid-cell compartments between days 21 and 30 after transplantation. Fifteen of the 28 patients (54%) had evidence of hematopoiesis solely from the unit of unmanipulated cord blood, and 13 (46%) had hematopoiesis derived from both units (the unit of unmanipulated cord blood predominated in 9 patients, and the unit of expanded cord blood predominated in 4). At 6 months after transplantation, the expanded cord blood was present in 13% of the patients, although the unmanipulated cord blood predominated. Long-term engraftment (>1 year) was produced primarily by the unit of unmanipulated cord blood in all patients.
ACUTE AND CHRONIC GVHD
The cumulative incidence of grade II to IV acute GVHD was 42% and the cumulative incidence of grade III or IV disease was 13%; chronic GVHD was observed in 45% of the patients. There were no significant differences between the recipients of STRO-3+ mesenchymal-cell–expanded cord blood and the CIBMTR or institutional controls with regard to grade II to IV acute GVHD or grade III or IV disease.
SURVIVAL AND CAUSES OF DEATH
At a median follow-up of 12 months (range, 6 to 20), 10 of these 31 high-risk patients remained alive. Causes of death among the recipients of expanded cord blood included relapse (4 patients), GVHD (4), chemotherapy-induced toxicity (2), and infections (11). Infections were bacterial in 3 patients, viral in 1, and fungal in 5; the source of infection was unknown in 2 patients.
DISCUSSION
We sought to improve hematopoiesis and engraftment by propagating hematopoietic progenitors derived from cord blood in allogeneic mesenchymal-cell cocultures before transplantation. Brunstein et al. reported that in 536 adult recipients of 2 units of unmanipulated cord blood, the median time to engraftment of neutrophils was 26 days, and the median time to engraftment of platelets was 53 days — intervals that were significantly longer than those in recipients of bone marrow or peripheral-blood stem cells in their series.24 In this case series, we found that patients who received myeloablative therapy followed by 2 units of cord blood, with 1 unit containing cord blood expanded ex vivo in mesenchymal-cell cocultures, had a shorter time to engraftment and higher cumulative incidences of neutrophil and platelet engraftment, as compared with historical controls and controls at other sites who received unmanipulated cord blood only.
We attribute the positive engraftment results to the increased numbers of committed myeloid and megakaryocytic progenitors in the expanded cord-blood graft that were capable of rapid engraftment after transplantation. Indeed, for approximately half the patients evaluated, engraftment of expanded cells was associated with early neutrophil recovery. Our data suggest that mesenchymal cells, by recapitulating some of the physiological cues of the stem-cell niche that are absent in suspension cultures, provide conditions that enhance the survival and proliferation of cord-blood progenitor cells responsible for early marrow repopulation.16,17 Another potential advantage of transplanting 1 unit of expanded cord blood and 1 unit of unmanipulated cord blood is that this approach guarantees early hematopoietic recovery with the unit of expanded cord blood and ultimate engraftment of the unit of nonexpanded cord blood with better HLA-matching to the patient. The expansion process appears to favor an increase in cells capable of early repopulation; however, because long-term engraftment was uniformly from the unit of unmanipulated cord blood, it must also be acknowledged that the culture process appears to deplete the cells capable of long-term repopulation.
The incidence and severity of GVHD were similar to those in other trials of cord-blood transplantation with expansion27,28 or without expansion. 4,6,14,28 During the past decade, several strategies to expand cord-blood progenitors ex vivo have been investigated, with little tangible improvement in the time to engraftment.27,29,30 Recently, however, Delaney et al. investigated the use of CD34-selected cord-blood progenitors cultured with growth factors in the presence of Notch ligand.31 They reported an improvement in the time to neutrophil engraftment that was similar to the improvement we observed. Other promising strategies to enhance engraftment include the intraosseous administration of a single cord-blood unit,32 the addition of haploidentical CD34+ peripheral-blood progenitor cells to a single unit of unmanipulated cord blood,33 and the expansion of a fraction of a single cord-blood unit with growth factors and a copper chelator.34 Another potentially effective approach is to enhance the homing of cord blood to the marrow by fucosylating the cord-blood unit35 or treating it with prostaglandin E236 before transplantation. Our group chose to investigate the expansion of the entire cord-blood mononuclear-cell fraction, because in our prior clinical studies of CD34+ cord-blood expansion, 27,28 we observed prohibitive cell losses with immunomagnetic enrichment.
Our findings support the hypothesis that transplantation of mesenchymal-cell–expanded cord blood shortens the time to neutrophil and platelet recovery after transplantation in adults. The transition from family-member–derived mesenchymal cells to unrelated STRO-3+ mesenchymal cells significantly increased the clinical feasibility of our strategy without compromising cord-blood expansion.20 Whether the differences that we observed will hold up remains to be proven in direct head-to-head comparisons.
Supplementary Material
Acknowledgments
Supported in part by Mesoblast, grants from the National Cancer Institute (RO1 CA061508-18, to Drs. Shpall, Simmons, and Robinson; and P01 CA148600-02, to Drs. Shpall, Simmons, Robinson, Molldrem, Cooper, and Bollard and Mr. Munsell) and the Cancer Prevention Research Institute of Texas (RO1 RP100469, to Drs. Shpall, Simmons, and Robinson and Mr. Munsell), and a Cancer Center Core Grant (CA016672, to the M.D. Anderson Cancer Center investigators).
We thank the patients who agreed to participate in this study; our Cell Therapy Laboratory staff for their support; the teams of nurses, pharmacists, midlevel practitioners, and physicians for their patient care; and John Gilbert for editorial assistance with an earlier draft of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 2.Bradstock K, Hertzberg M, Kerridge I, et al. Single versus double unrelated umbilical cord blood units for allogeneic transplantation in adults with advanced haematological malignancies: a retrospective comparison of outcomes. Intern Med J. 2009;39:744–751. doi: 10.1111/j.1445-5994.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 3.Barker JN. Umbilical cord blood (UCB) transplantation: an alternative to the use of unrelated volunteer donors? Hematology (Am Soc Hematol Educ Program) 2007;2007:55–61. doi: 10.1182/asheducation-2007.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Rocha V, Gluckman E. Outcomes of transplantation in children with acute leukaemia. Lancet. 2007;369:1906–1908. doi: 10.1016/S0140-6736(07)60892-7. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V. History of the clinical use of umbilical cord blood hematopoietic cells. Cytotherapy. 2005;7:219–227. doi: 10.1080/14653240510027136. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman E. Hematopoietic stem-cell transplants using umbilical-cord blood. N Engl J Med. 2001;344:1860–1861. doi: 10.1056/NEJM200106143442410. [DOI] [PubMed] [Google Scholar]
- 7.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 9.Bashir Q, Robinson SN, de Lima MJ, Parmar S, Shpall E. Umbilical cord blood transplantation. Clin Adv Hematol Oncol. 2010;8:786–801. [PubMed] [Google Scholar]
- 10.Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15:564–573. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Körbling M, Freireich EJ. Twenty-five years of peripheral blood stem cell transplantation. Blood. 2011;117:6411–6416. doi: 10.1182/blood-2010-12-322214. [DOI] [PubMed] [Google Scholar]
- 12.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1871. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 13.De Lima M, St John LS, Wieder ED, et al. Double-chimaerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol. 2002;119:773–776. doi: 10.1046/j.1365-2141.2002.03893.x. [DOI] [PubMed] [Google Scholar]
- 14.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 15.Briddell RA, Kern BP, Zilm KL, Stoney GB, McNiece IK. Purification of CD34+ cells is essential for optimal ex vivo expansion of umbilical cord blood cells. J Hematother. 1997;6:145–150. doi: 10.1089/scd.1.1997.6.145. [DOI] [PubMed] [Google Scholar]
- 16.McNiece I, Harrington J, Turney J, Kellner J, Shpall EJ. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–317. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 17.Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 19.Idem. CD34 expression by stromal precursors in normal human adult bone marrow. Blood. 1991;78:2848–2853. [PubMed] [Google Scholar]
- 20.Robinson SN, Simmons PJ, Brouard N, et al. Efficacy of “off-the-shelf,” commercially- available, third-party mesenchymal stem cells (MSC) in ex vivo cord blood (CB) co-culture expansion. Presented at the 49th annual meeting of the American Society of Hematology; Atlanta. December 8-11, 2007; abstract. [Google Scholar]
- 21.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 22.Miller CL, Lai B. Human and mouse hematopoietic colony-forming cell assays. Methods Mol Biol. 2005;290:71–89. doi: 10.1385/1-59259-838-2:071. [DOI] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–888. [PubMed] [Google Scholar]
- 24.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 27.Shpall EJ, Quinones R, Giller R, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 28.de Lima M, McMannis J, Komanduri K, et al. Randomized study of double cord blood transplantation (CBT) with versus without ex-vivo expansion (EXP). Presented at the 49th annual meeting of the American Society of Hematology; Atlanta. December 8-11, 2007; abstract. [Google Scholar]
- 29.Kraus M, Gunter K, Laning J, et al. Phase I clinical results using selectively amplified hematopoietic stem/progenitor cells (HPC) meets primary endpoint for safety in 10 patient trial. Proceedings of the International Society of Cell Therapy Annual Meeting; Sydney. June 24-27, 2007; abstract. [Google Scholar]
- 30.Madlambayan GJ, Rogers I, Kirouac DC, et al. Dynamic changes in cellular and microenvironmental composition can be controlled to elicit in vitro human hematopoietic stem cell expansion. Exp Hematol. 2005;33:1229–1239. doi: 10.1016/j.exphem.2005.05.018. [Erratum, Exp Hematol 2006;34:122.] [DOI] [PubMed] [Google Scholar]
- 31.Delaney C, Heimfeld S, Brashem- Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frassoni F, Gualandi F, Podesta M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9:831–839. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 33.Fernández MN, Regidor C, Cabrera R, et al. Unrelated umbilical cord blood transplants in adults: early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol. 2003;31:535–544. doi: 10.1016/s0301-472x(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 34.de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson SN, Simmons PJ, Thomas MW, et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rgamma(null) mice. Exp Hematol. 2012;40:445–456. doi: 10.1016/j.exphem.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.