Abstract
Background
Despite a national organ donor shortage and a growing population of patients with end-stage heart disease, the acceptance rate of donor hearts for transplantation is low. We sought to identify donor predictors of allograft non-utilization, and to determine whether these predictors are in fact associated with adverse recipient post-transplant outcomes.
Methods and Results
We studied a cohort of 1,872 potential organ donors managed by the California Transplant Donor Network from 2001–2008. Forty five percent of available allografts were accepted for heart transplantation. Donor predictors of allograft non-utilization included age>50 years, female sex, death due to cerebrovascular accident, hypertension, diabetes, a positive troponin assay, left ventricular dysfunction and regional wall motion abnormalities, and left ventricular hypertrophy. For hearts that were transplanted, only donor cause of death was associated with prolonged recipient hospitalization post-transplant, and only donor diabetes was predictive of increased recipient mortality.
Conclusions
While there are many donor predictors of allograft discard in the current era, these characteristics appear to have little effect on recipient outcomes when the hearts are transplanted. Our results suggest that more liberal use of cardiac allografts with relative contraindications may be warranted.
Keywords: Organ donor, Heart transplantation, Transplant recipients, Transplant outcomes
INTRODUCTION
Despite the availability of successful medical therapies for end-stage heart failure, and now of mechanical circulatory support,1 heart transplantation remains the best option for appropriate candidates with end-stage heart disease.2 The severe and persistent shortage of donor organs, however, considerably limits the availability of heart transplantation. While it is estimated that more than 20,000 patients could benefit from this life-saving procedure each year,3 only 1,853 heart transplants were performed in the United States in 2009, with a concurrent waiting list mortality of 13.7% (OPTN/SRTR Annual Data Report 2010). These alarming statistics have motivated the search for ways to increase the size of the donor pool and the use of available organs.4
In 2003, Sheehy et al estimated the annual number of brain-dead potential organ donors in the United States to be between 10,500 and 13,800; of these potential donors, only 42% donated one or more organs for transplantation.5 While public health efforts to increase donor identification and consent rates address a major limiting factor in donor availability, the transplant community must also focus on ways to increase the utilization of suitable grafts from available donors. At this time, approximately 60% of currently available cardiac allografts are discarded due to stringent acceptance criteria that have not been rigorously tested in clinical and research settings. In fact, single-center experience using marginal or high-risk donor hearts for transplantation has demonstrated excellent clinical results.6–9
Given the discrepancy between the pressing need for donor organs and the low utilization rate of available grafts, we sought to identify current predictors of cardiac allograft non-utilization, and to determine whether these predictors are in fact associated with adverse recipient outcomes.
METHODS
Subjects
We studied a contemporary cohort of 1,872 organ donors between the ages of 14 and 69 years who were managed by the California Transplant Donor Network (CTDN, Oakland, CA) from 2001–2008. This age range was chosen for study based on the observation that allografts from donors less than 14 and greater than 69 years of age were not accepted for transplantation into adult recipients during this time period. CTDN is the largest organ procurement agency in Northern California and supplies donor organs mainly to transplant centers in northern and central California, and occasionally to neighboring regions. Potential brain dead organ donors were identified by treating physicians at hospitals throughout the region, and consent for organ donation was obtained from family members or next-of-kin. Management of the organ donor was subsequently assumed by CTDN staff, and consent was obtained from the donor’s family to collect patient data and biological samples. This study was approved by CTDN and by the Stanford University Institutional Review Board.
Donor Management
During the eight year time period studied, all brain dead organ donors at CTDN were managed according to a standardized protocol that included: methylprednisolone administered at the onset of donor management and until organ procurement (15mg/kg every 12 hours); dopamine as the first-line inotropic agent (maximum 20 mcg/kg/min); phenylephrine as the second-line vasoactive agent (maximum 300 mcg/min); intravenous fluid and/or loop diuretic administration to obtain a goal central venous pressure of 5–8 mmHg and a urine output of > 30ml/hour; electrolyte repletion to achieve normalization of potassium, phosphorous, magnesium and calcium levels; empiric antimicrobial therapy with vancomycin and levofloxacin; and inhaled, nebulized albuterol (2.5 mg every four hours). Vasoactive and inotropic medications were titrated according to pulmonary artery catheter readings to achieve a target systemic vascular resistance of 800–1200 dynes-seconds/cm5 and cardiac index >2 l/min/m2. Esmolol infusions were initiated for tachycardia that was deemed unrelated to beta-agonist infusion and were discontinued upon initiation of organ procurement. Thyroid hormone (levothyroxine) was administered when requested by the accepting transplant centers.
Clinical Data
Upon assumption of donor management, comprehensive data on donor-level variables were recorded by CTDN staff in a standardized fashion, including demographic variables, cause of death, health-related behaviors, and past medical history. Data on comprehensive laboratory testing were also recorded, including serologies; hematologic, hepatic, and renal function indices; and cardiac enzyme assays. Standard testing for potential donors who were not immediately ruled-out for cardiac graft donation (due to known coronary artery disease treated with percutaneous stents or bypass surgery, prior cardiac valve surgery, lack of consent for donation, or coroner exclusion) included an electrocardiogram; one or more echocardiograms; and a coronary angiogram for male donors over 40 years and female donors over 45 years. All cardiac testing was performed and interpreted at the donor hospital and results were recorded by CTDN personnel. Data on vital signs, invasive hemodynamics, and medications were also recorded.
Donor data were extracted from the medical records and were entered into the research study database by study personnel. A subsequent quality-assessment review of 5% of the medical records was performed, reviewing 177 fields per donor chart, and demonstrated >95% accuracy of data collection.
Allograft Utilization
All donor hearts transplanted in the United States were considered “utilized” and followed for recipient outcomes. Data on heart transplant recipient characteristics and post-transplant outcomes were obtained from the United Network for Organ Sharing (UNOS) provided by way of Standard Transplant Analysis and Research (STAR) files. Any hearts that were part of a multi-organ transplant were excluded from further follow up.
Donor Predictors of Cardiac Allograft Non-Utilization
Eleven donor “risk factors” for allograft non-utilization were selected a priori, based on prior literature. These included (1) donor age>50 years,2, 10–12 (2) female sex,13–17 (3) cerebrovascular accident/stroke as the cause of death,12, 18–20 (4) hypertension,11, 12 (5) diabetes,10, 11 (6) history of cocaine or methamphetamine use,21–23 (7) high inotrope requirement (dopamine>10 mcg/kg/min) during donor management,10–12, 24 (8) cardiac troponin I >1.0 mcg/L,25–27 (9) left ventricular dysfunction (defined as left ventricular ejection fraction<50%),12, 28 (10) left ventricular regional wall motion abnormalities,10 and (11) left ventricular hypertrophy (defined as septal or posterior wall thickness>1.1 cm).6, 12, 29, 30 A cut-off of 1.0 mcg/L was used to define an elevated troponin level based on the knowledge that donor hospitals used a variety of assays from multiple manufacturers to perform this test, and reference values for a positive troponin level varied from 0.04–1.0 mcg/L depending on the specific assay used. We therefore selected the upper boundary to ensure specificity in capturing abnormal troponin values, albeit with a recognized loss of sensitivity.
Recipient Outcomes
Based on the assumption that donor characteristics would preferentially influence short-term post-transplant outcomes (rather than long-term outcomes such as overall survival), we examined the following three recipient outcomes: delayed hospital discharge (hospital discharge after 21 days post-transplant), 30-day graft survival, and 1-year graft survival. A cut-off of 21 days for recipient post-transplant hospitalization was chosen prior to data analysis based on the authors’ clinical experience. Recipients were classified as having a length of stay less than 21 days if they were discharged within 21 days after the heart transplant procedure and did not die (or were not retransplanted) prior to discharge. Graft failure was defined as death or retransplantation within the specified time period.
Recipient covariates included age, sex, etiology of heart disease, serum creatinine and total bilirubin at transplant, presence of diabetes, most recent panel of reactive antibodies, pulmonary artery systolic pressure, use of inotropic support, medical condition at time of transplantation, wait list priority status (1A, 1B, or 2), requirement for mechanical ventilation, and allograft cold ischemic time.
Statistical Analysis
Allograft Utilization
Time trends of rates of graft utilization and prevalence of donor risk factors were calculated. Odds ratios were calculated to study associations between these eleven donor risk factors and cardiac allograft non-utilization.
An allograft utilization score was then derived using all available donor predictors in our research database. These included demographic factors (race, weight, height), clinical factors (use of cardiopulmonary resuscitation, history of coronary artery disease, diabetes, hypertension), laboratory values (hemoglobin, creatinine), hemodynamics (heart rate, central venous pressure, systolic and diastolic blood pressure), use of vasoactive medications (phenylephrine, norepinephrine, and esmolol), and hormonal therapy (corticosteroids and levothyroxine). To combine these donor characteristics into a single utilization score, the predicted probability of allograft utilization was calculated. To predict allograft utilization we used the Random Forest algorithm31—a robust extension of decision trees that has been used extensively in other biomedical studies. Two notable features of the Random Forest model are that it can handle missing values and it validates internally, alleviating the need for cross-validation. To compare the utility of these scores in predicting actual allograft utilization, ROC curves and the associated c-statistics were calculated.
Recipient Outcomes
Logistic regression models were used to examine associations between donor predictors and recipient outcomes. We first examined the 11 donor risk factors identified a priori, and then assessed the relationship between the total number of donor risk factors present and recipient outcomes. To examine the utilization choice hearts were dichotomized based on the predicted probability of utilization (<50% vs >50%) from the Random Forest scores and were then compared. Finally, Random Forest models were then generated for the three recipient outcomes of interest, using all available donor predictors. All reported p-values are two-tailed. Analyses were performed using R version 2.15 with the Random Forest package
RESULTS
From 2001–2008, 1,872 potential organ donors were managed by CTDN. Allografts from 808 (43%) of these donors were accepted for heart transplantation. Demographic characteristics of the donors whose hearts were and were not transplanted are presented in Table 1. Donors whose hearts were not accepted for transplantation were more likely to be older, female, and had cerebrovascular accident/stroke as a cause of death. These donors had a higher incidence of smoking, hypertension, diabetes, and coronary artery disease, and were more likely to have a positive cardiac troponin assay. These donors had a slightly higher dopamine requirement during the donor management period and were less likely to have received corticosteroids and thyroxine supplementation. Finally, non-utilized grafts were less likely to have had an echocardiogram, had a lower mean left ventricular ejection fraction and a higher incidence of left ventricular regional wall motion abnormalities and left ventricular hypertrophy. These results confirmed our initial assumption that the donor “risk factors” selected a priori for analytic purposes were significant predictors of cardiac allograft non-utilization, except for high donor dopamine requirement (>10 mcg/kg/min) during the donor management period and donor history of cocaine or methamphetamine use.
Table 1.
Donor characteristics, stratified by cardiac allograft acceptance for transplantation
| Not Transplanted | Transplanted | p-value | |
|---|---|---|---|
| N=1064 (57%) | N=808 (43%) | ||
| Demographics | |||
| Age (years) | 49 (40, 56) | 30 (20, 43) | < 1 × 10−8 |
| Sex (Male) | 572 (54%) | 581 (72%) | < 1 × 10−8 |
| Cause of death | < 1 × 10−8 | ||
| Anoxia | 151 (14%) | 70 (9%) | |
| Cerebrovascular accident/stroke | 665 (63%) | 242 (30%) | |
| Head Trauma | 237 (22%) | 489 (61%) | |
| Central nervous system tumor | 4 (< 1%) | 4 (< 1%) | |
| Other | 5 (< 1%) | 3 (< 1%) | |
| Race | < 1 × 10−8 | ||
| African American | 105 (10%) | 815 (10%) | |
| Asian | 118 (11%) | 38 (5%) | |
| Caucasian | 621 (58%) | 427 (53%) | |
| Hispanic | 200 (19%) | 246 (30%) | |
| Other | 20 (2%) | 16 (2%) | |
| Body-mass index (kg/m2) | 26.3 (23.0, 30.9) | 25.6 (22.7, 29.0) | 2.08 × 10−3 |
| Blood type | < 1 × 10−8 | ||
| A | 374 (36%) | 265 (33%) | |
| B | 147 (14%) | 81 (10%) | |
| AB | 63 (6%) | 11 (1%) | |
| O | 468 (44%) | 447 (56%) | |
| Clinical | |||
| Cardiopulmonary resuscitation | 241 (57%) | 168 (53%) | 0.360 |
| Smoking | 596 (57%) | 418 (53%) | 0.069 |
| Cocaine/Methamphetamines | 281 (27%) | 236 (21%) | 0.225 |
| Hypertension | 447 (38%) | 106 (29%) | < 1 × 10−8 |
| Diabetes | 142 (14%) | 24 (3%) | < 1 × 10−8 |
| Coronary artery disease | 91 (9%) | 9 (1%) | < 1 × 10−8 |
| Laboratory Values | |||
| Troponin > 1.0 mgcl/L | 346 (38%) | 211 (29%) | 1.36 × 10−4 |
| CPK-MB (IU/L) | 6.9 (2.9, 20.0) | 7.7 (3.2, 22.0) | 0.204 |
| Vasoactive medications | |||
| Dopamine | 822 (77%) | 658 (81%) | 0.035 |
| Peak dopamine dose (mcg/kg/min) | 6.0 (4.0,10.0) | 5.3 (4.0, 9.6) | 0.183 |
| Final dopamine dose (mcg/kg/min) | 2.4 (0, 4.0) | 2.0 (0, 3.5) | 0.056 |
| Neosynephrine | 766 (72%) | 668 (83%) | 1.11 × 10−7 |
| Peak neosynephrine dose (mcg/min) | 100 (50, 190) | 100 (50, 160) | 0.100 |
| Final neosynephrine dose (mcg/min) | 20 (0, 50) | 15 (0, 40) | 8.98 × 10−4 |
| Epinephrine | 40 (4%) | 32 (4%) | 0.947 |
| Norepinephrine | 37 (6%) | 43 (8%) | 0.208 |
| Esmolol | 169 (17%) | 143 19%) | 0.355 |
| Hormones | |||
| Corticosteriods | 1010 (95%) | 808 (100%) | < 1 × 10−8 |
| Methylprednisolone dose (g/24 hours) | 2.0 (1.5, 3.0) | 2.0 (1.7, 3.0) | 0.758 |
| Thyroxine | 222 (22%) | 207 (27%) | 0.022 |
| Echocardiogram | |||
| Echocardiogram performed | 573 (54%) | 785 (97%) | < 1 × 10−8 |
| Left ventricular ejection fraction (%) | 60% (50, 70) | 65% (60, 70) | < 1 × 10−8 |
| Regional wall motion abnormalities | 173 (29%) | 107 (13%) | < 1 × 10−8 |
| Left ventricular hypertrophy | 246 (48%) | 166 (23%) | < 1 × 10−8 |
Results presented as percentages or median values with interquartile range
CPK-MB: creatine phosphokinase, muscle band
During the study period, cardiac allograft utilization decreased by an average of 4.2% per year (95% CI 1.9%–6.4%), from a high of 56% utilization in 2002 to a low of 37% utilization in 2007. This decrease in utilization was independent of changes in donor risk factors with a 4.3% (2.3%–6.3%) annual decrease in allograft acceptance for transplantation after adjusting for other covariates. Of the 11 pre-identified donor risk factors, only the incidence of diabetes increased during this time period (p<0.0054). In fact, the following donor risk factors decreased in incidence: donor age>50 years (p<0.03), death due to cerebrovascular accident (p<0.0004), and high dopamine requirement (p<1×10−8).
We then studied associations between the eleven donor risk factors selected a priori and cardiac allograft acceptance for transplantation. Figure 1 demonstrates the distribution of these donor risk factors in grafts that were and were not accepted. All of the risk factors were highly associated with allograft utilization (p<0.001) except for the amount of dopamine administered during donor management and donor history of cocaine or methamphetamine use. The total number of donor risk factors was also strongly associated with allograft utilization (p< 1×10−8) with accepted grafts having a median of 2 (IQR 1, 3) risk factors and discarded grafts having a median of 3 (IQR 2, 5) risk factors.
Figure 1.
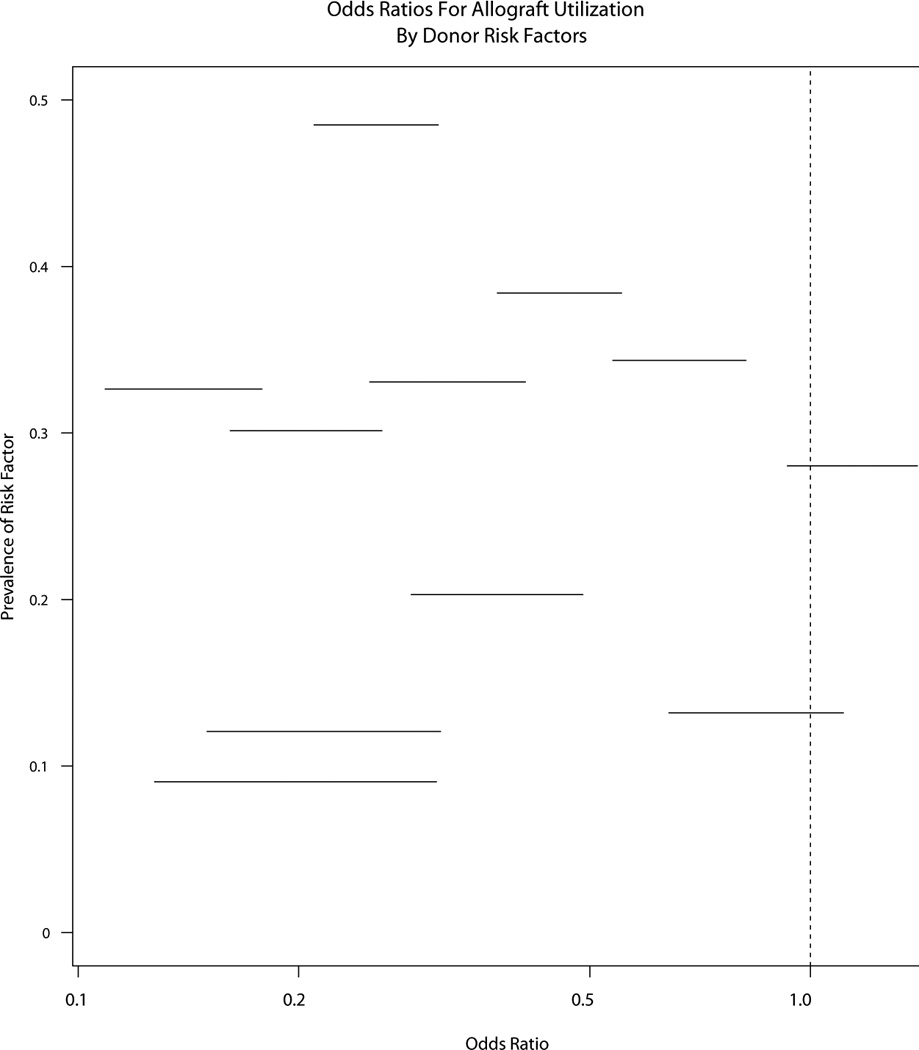
Odds ratios for cardiac allograft acceptance for transplantation, by donor risk factors
Finally, all available donor variables were used to predict allograft utilization using the Random Forest algorithm. A total of 59 covariates were used and 2000 trees were grown. The most important predictors in the Random Forest models were donor age, cause of death, left ventricular ejection fraction, and history of hypertension. The Random Forest model demonstrated excellent predictive ability with an overall c-statistic of 0.86.
Donor Predictors of Recipient Outcomes
Recipient outcomes data were available for 806 of the 808 cardiac allografts accepted for transplantation. Of the recipients, 29 were excluded from analysis as they had received multi-organ transplants. Table 2 demonstrates recipient characteristics at the time of transplantation, stratified by those who received an allograft with greater than 50% probability of acceptance, based on the Random Forest predictions. Not surprisingly, 64% of the allografts were those that had a greater than 50% probability of utilization. The recipients who received the “less desirable” grafts were more likely to be female, had better clinical status and were less likely to be on life support; no other significant clinical differences were identified.
Table 2.
Heart transplant recipient characteristics, stratified by probability of allograft utilization
| Predicted probability of utilization | |||
|---|---|---|---|
| < 50% | > 50% | p-value | |
| N=277 (36%) | N=502 (64%) | ||
| Demographics | |||
| Age (years) | 53 (42, 60) | 52 (38, 60) | 0.109 |
| Sex (male) | 174 (63%) | 395 (79%) | 2.68 × 10−6 |
| Diagnosis | 0.932 | ||
| Dilated cardiomyopathy | 125 (45%) | 233 (46%) | |
| Ischemic cardiomyopathy | 91 (33%) | 163 (32%) | |
| Other | 61 (22%) | 106 (21%) | |
| Medical Condition | 0.222 | ||
| Not hospitalized | 144 (52%) | 229 (46%) | |
| Hospitalized, not in ICU | 33 (12%) | 72 (14%) | |
| Hospitalized, in ICU | 100 (36%) | 201 (40%) | |
| Waiting list status | 0.018 | ||
| 1A | 74 (27%) | 148 (29%) | |
| 1B | 86 (31%) | 192 (38%) | |
| 2 | 117 (42%) | 162 (32%) | |
| Cardiopulmonary support | |||
| Inotropic support | 108 (39%) | 228 (45%) | 0.097 |
| Life support | 136 (49%) | 287 (57%) | 0.037 |
| Mechanical ventilation | 5 (2%) | 8 (2%) | 0.942 |
| Clinical variables | |||
| Serum creatinine (mg/dl) | 1.2 (1.0, 1.5) | 1.2 (1.0, 1.6) | 0.195 |
| Mean pulmonary artery systolic pressure (mmHg) | 41 (31, 52) | 42 (35, 50) | 0.372 |
| Diabetes | 37 (14%) | 74 (15%) | 0.932 |
| Total bilirubin (mg/dl) | 1.0 (0.7, 1.6) | 1.0 (0.7, 1.6) | 0.685 |
| Allograft cold ischemic time (hours) | 3.7 (3.3, 4.3) | 3.7 (3.1, 4.2) | 0.971 |
Probability of allograft utilization based on Random Forest models.
Results presented as percentages or median values with interquartile range.
ICU: intensive care unit
The primary outcomes examined were time to hospital discharge and recipient 30-day and 1-year survival. Overall, the outcomes were generally positive and consistent with national heart transplant statistics2 with 75% of recipients discharged within 21 days, and only 3.9% and 11.4% dying within 30 days and one year, respectively. Kaplan-Meier curves stratified by the probability of graft acceptance (based on the Random Forest predictions) are presented in Figure 2. Overall, recipients of allografts that were more likely to be accepted were discharged earlier from the hospital (log rank p<0.035). However, there was minimal difference in overall survival (p=0.067).
Figure 2.
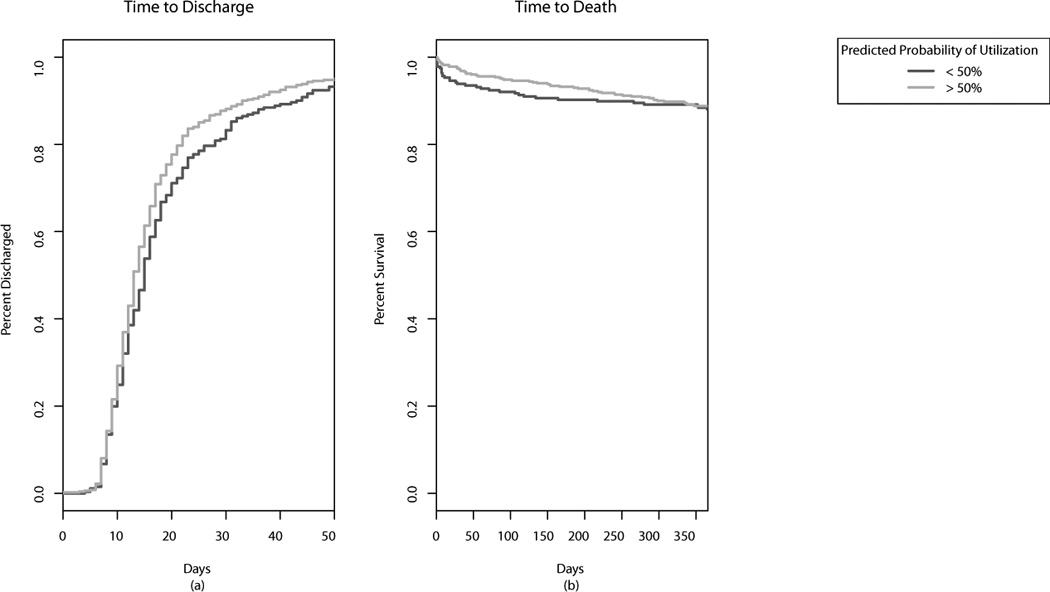
Effect of allograft predicted probability of utilization (< or >50%) on (a) time to hospital discharge after heart transplantation, and (b) 1 year graft survival.
Associations between the eleven donor risk factors identified a priori, and the recipient outcomes of interest were then examined and the odds ratios are presented in Table 3. While most donor risk factors appeared associated with prolonged hospital stay post-transplant, only CVA/stroke as the donor cause of death was marginally significantly associated [OR 1.41 (95% CI 1.00, 2.00)] with prolonged recipient hospitalization. Similarly, while many donor risk factors appeared to increase recipient risk of death, left ventricular hypertrophy and history of diabetes were the only donor characteristics significantly associated with recipient 30-day and 1-year mortality. After adjustment for recipient characteristics, diabetes remained the only donor predictor of recipient mortality [OR 3.58 (95% CI 1.18–10.84) for 1-year mortality]. A notable finding was that the presence of multiple donor risk factors did not increase the occurrence of adverse recipient outcomes (Figure 3).
Table 3.
Unadjusted odds ratios for associations between donor risk factors and recipient post-transplant outcomes
| Donor characteristic | Not discharged within 21 days |
p- value |
30-day mortality | p- value |
1-year mortality | p-value |
|---|---|---|---|---|---|---|
| Age > 50 years | 1.43 (0.88, 2.31) | 0.145 | 1.97 (0.78, 4.96) | 0.150 | 1.23 (0.64, 2.36) | 0.540 |
| Sex (female) | 1.25 (0.87, 1.78) | 0.224 | 1.28 (0.59, 2.79) | 0.527 | 1.07 (0.66, 1.74) | 0.783 |
| Cause of death (CVA/Stroke) | 1.41 (1.00, 2.00) | 0.050 | 1.17 (0.54, 2.53) | 0.699 | 1.02 (0.63, 1.64) | 0.950 |
| Hypertension | 1.26 (0.79, 2.03) | 0.330 | 1.42 (0.53, 3.81) | 0.488 | 0.76 (0.37, 1.56) | 0.449 |
| Diabetes | 1.56 (0.62, 3.92) | 0.346 | 6.35 (2.00, 20.13) | 0.002 | 3.07 (1.17, 8.07) | 0.023 |
| Cocaine/methamphetime use | 0.85 (0.59, 1.22) | 0.375 | 0.91 (0.40, 2.09) | 0.825 | 0.85 (0.51, 1.40) | 0.513 |
| Peak dopamine dose>10 mcg/kg/min | 1.12 (0.68, 1.82) | 0.662 | 0.79 (0.24, 2.66) | 0.707 | 0.82 (0.40, 1.69) | 0.586 |
| Troponin I > 1.0 mcg/L | 0.97 (0.66, 1.41) | 0.855 | 0.68 (0.27, 1.71) | 0.414 | 0.63 (0.36, 1.11) | 0.109 |
| Left Ventricular Hypertrophy | 1.05 (0.70, 1.59) | 0.798 | 2.23 (1.02, 4.86) | 0.044 | 0.96 (0.56, 1.65) | 0.884 |
| Left ventricular ejection fraction < 50% | 1.69 (0.86, 3.29) | 0.126 | 1.41 (0.32, 6.17) | 0.647 | 0.60 (0.18, 2.00) | 0.409 |
| Regional wall motion abnormalities | 1.09 (0.68, 1.75) | 0.722 | 1.02 (0.35, 3.00) | 0.967 | 1.36 (0.75, 2.47) | 0.318 |
CVA, cerebrovascular accident
Figure 3.
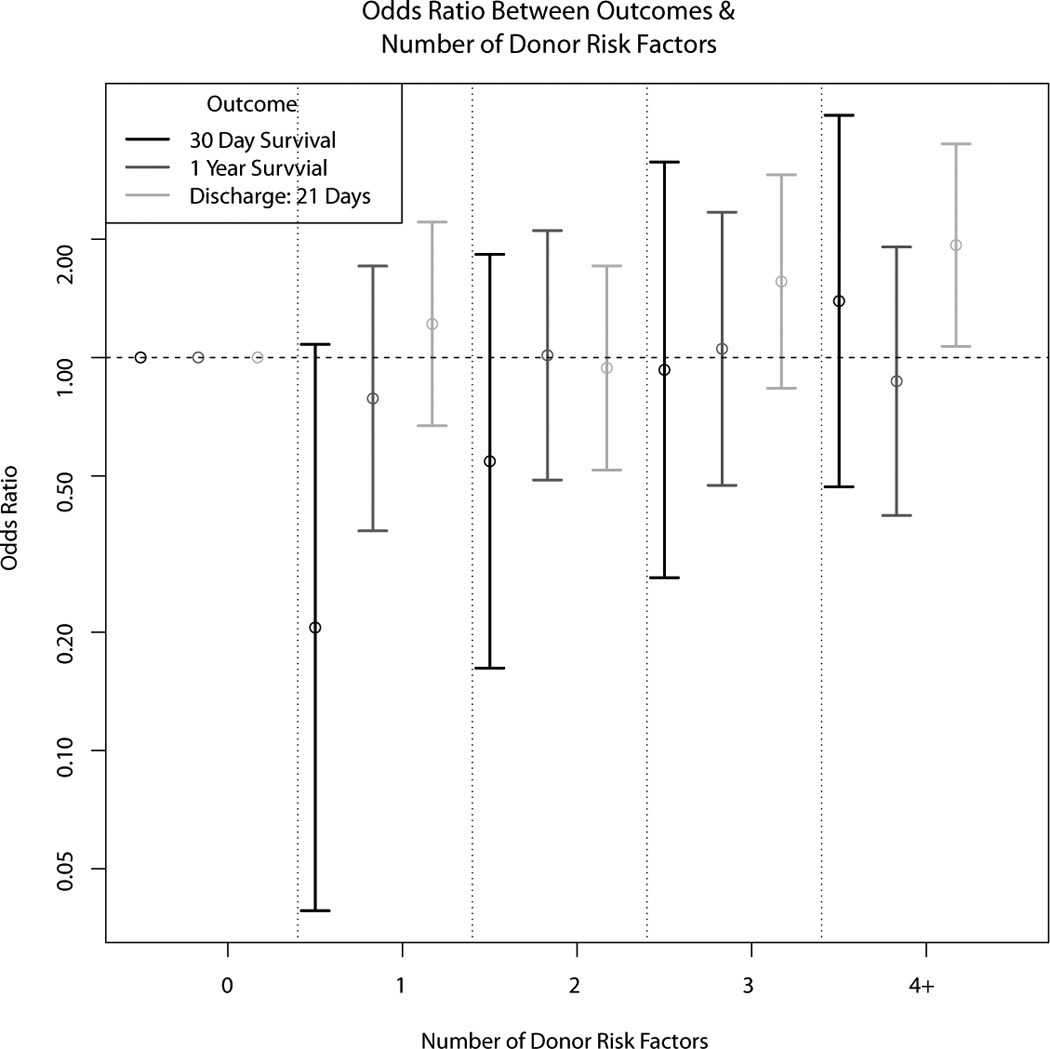
Associations between number of donor risk factors and heart transplant recipient outcomes (discharge within 21 days post-transplant, 30 day graft survival, and 1 year graft survival)
Finally, we used all of the available donor characteristics to predict the three outcomes of interest using Random Forest models. The ROC curves, along with the ROC curve for allograft utilization, are shown in Figure 4. While the donor characteristics were highly predictive of graft utilization, those same characteristics were not predictive of recipient outcomes.
Figure 4.
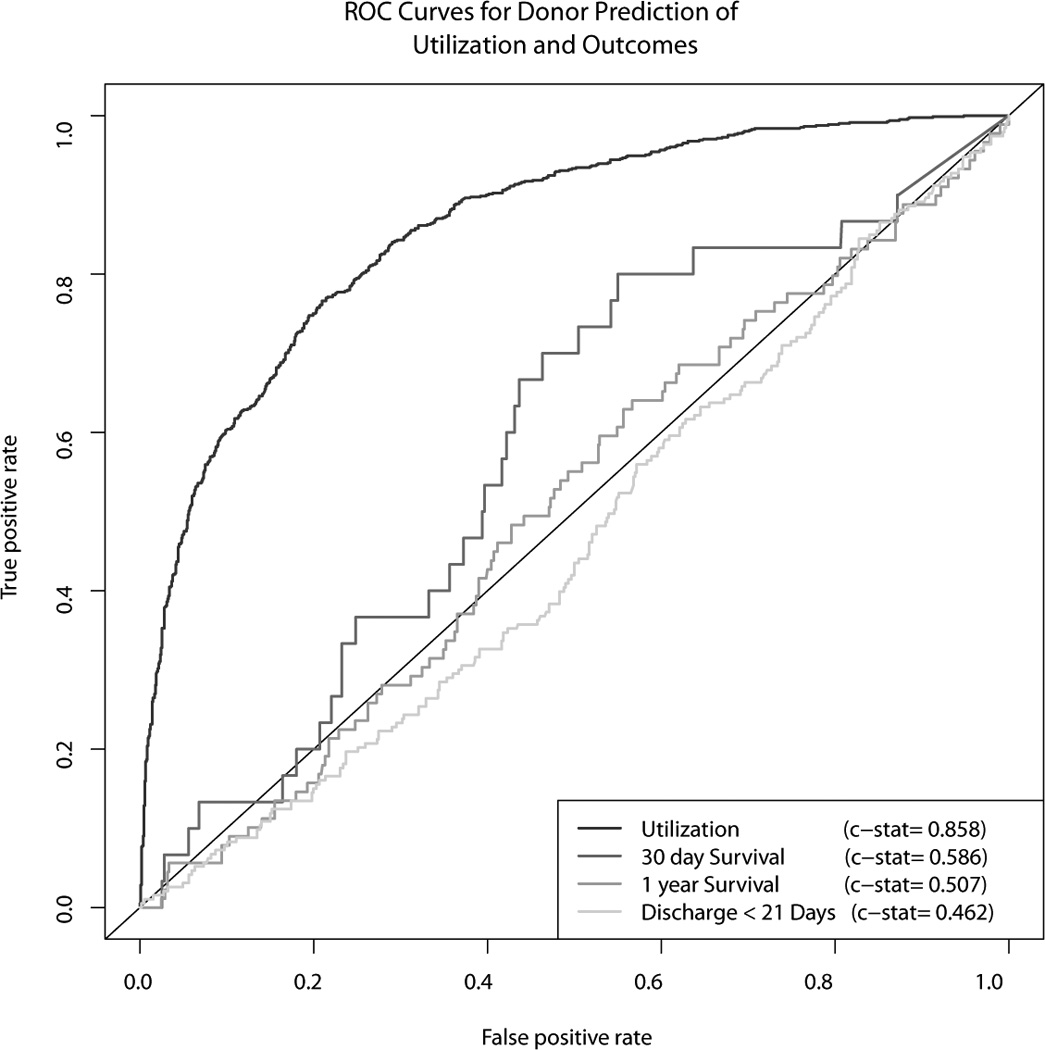
Receiver operating characteristic curves, based on Random Forest models, for donor prediction of (1) cardiac allograft utilization for transplantation, (2) discharge within 21 days post-transplant, (3) 30 day graft survival, (4) 1 year graft survival.
DISCUSSION
National transplant data collected by the Association of Organ Procurement Organizations (www.aopo.org, accessed June 21, 2012) reveals a cardiac allograft utilization rate (number of hearts accepted for transplantation/total number of donors) of 28.2%-30.1% from 2009–2011. Many reasons exist for discarding donor hearts, including older donor age, small size, co-morbidities, logistical issues, left ventricular hypertrophy, and donor left ventricular dysfunction. Unfortunately, the current criteria for acceptance of donor hearts are poorly standardized and are often based on retrospective single-center studies and anecdotal experience. Indeed, large registry analyses have shown that very few donor characteristics have significant impact on recipient outcomes,32, 33 suggesting that recipient factors figure more prominently toward the risk of death after heart transplantation.
Our aim in conducting this study was to closely examine current practices with respect to cardiac allograft acceptance in a contemporary cohort of potential organ donors. During the eight year time period examined, we found that utilization rates decreased by an average of 4.2% per year, from a high of 56% in 2002 to a low of 37% in 2007. This decline in allograft acceptance occurred despite a decreased incidence of donor risk factors for non-utilization, such as death due to cerebrovascular accident and older age. While utilization rates in our donation service area are higher than the national average, the significant decline over time suggests increasing avoidance of risk—a practice that could potentially lead to longer waiting list times and increasing waiting list mortality. One explanation for the trend towards avoidance of “high risk” donors could be related to advances in mechanical circulatory support (particularly ventricular assist devices, VADs) as a bridge to transplantation. Improvements in VAD design and a reduction in size of the devices has allowed for predictable clinical stabilization of a growing population of end-stage heart failure patients,1, 34 even those who are critically ill. The concern with adopting this approach indiscriminately is that bridging devices are expensive and can double the cost of an already expensive procedure. VAD implantation also exposes patients to the risk of additional surgical procedures, infections, and development of HLA antibodies,35 which may contribute to poorer outcomes after heart transplantation.36, 37
We first examined the associations between the eleven donor risk factors identified a priori, based on our review of the literature, and allograft utilization. Almost all of these risk factors, except for high dopamine requirement and cocaine/methamphetamine use, significantly predicted non-utilization. The total number of donor risk factors was also strongly associated with non-utilization.
Recognizing the fact that other donor characteristics may influence allograft acceptance decisions, we used all available donor covariate data to construct a Random Forest algorithm. Random Forest models confer several advantages: they can handle a large number of input variables, they can give an estimate of which variables are important in the classification, and they can produce a highly accurate classifier. In addition, they internally validate, thereby obviating the need for cross-validation. This model had excellent discrimination, with a c-statistic of 0.86.
After identifying donor predictors of allograft non-utilization, we examined the associations between these predictors and recipient outcomes. Only cerebrovascular accident as the donor cause of death marginally predicted prolonged recipient post-transplant hospitalization, and diabetes was the only donor predictor of increased recipient mortality. These findings concur with prior studies demonstrating the relatively small contribution of donor characteristics to post-transplant adverse events.4, 38, 39
One criticism of studies examining the influence of donor characteristics on recipient outcomes is that of selection bias. Allografts with many undesirable features are rarely accepted for transplantation, making it difficult to determine whether the recipient outcomes would have been acceptable. On the other hand, grafts with one or two unfavorable characteristics (such as reduced left ventricular systolic function) may be accepted if the graft is otherwise favorable (e.g. from a young and healthy male donor). Thus, it is difficult, if not impossible, to determine the relative contributions of unfavorable characteristics to recipient events. In other words, the grafts with undesirable characteristics are very carefully chosen to minimize the risk of adverse events. This phenomenon, combined with the relatively low mortality within 30-days to 1-year post-transplant, may paradoxically make it seem as though unfavorable characteristics are associated with improved recipient outcomes.
We used the excellent discriminative ability of our Random Forest model to predict which allografts in our cohort “would” and “would not” have been accepted for transplantation, based on a threshold of >50% predicted probability of utilization. Based on this model, 36% of the allografts accepted for transplantation had a <50% probability of utilization, based on donor characteristics. We once again examined recipient post-transplant outcomes, this time based on the predicted probability of allograft acceptance, and were unable to identify any significant differences in recipient survival. Thus, receipt of an allograft that was unlikely to be used for transplantation, based on combinations of unfavorable donor characteristics, did not result in adverse recipient events. Finally, donor characteristics in aggregate did not prove to be a reliable predictor of recipient outcomes.
This study has significant strengths and limitations. First and foremost, this represents the largest existing research database of detailed, rigorously adjudicated clinical data on over 1,800 potential organ donors managed in a standardized fashion in the current era. The recipient outcomes selected for analysis are robust, as all heart transplant centers in the United States are required to report metrics such as length of post-transplant hospitalization and recipient survival to UNOS. The primary limitation is the observational nature of the data. We are reluctant to make any causal statements about the relationship between donor characteristics and recipient outcomes. There are likely selection effects that may explain the relatively positive outcomes among recipients of the less desirable allografts. Also, reasons for allograft discard were not documented in the donor medical record. Thus, it is possible that reasons other than donor characteristics (lack of a suitable recipient, time considerations, donor family preference) could have accounted for some cases of non-utilization. Nonetheless, we were still able to identify a number of strong donor predictors of allograft non-utilization. Several important predictors were based on echocardiography: reduced left ventricular systolic function, left ventricular regional wall motion abnormalities, and left ventricular hypertrophy. However, donor echocardiograms were interpreted at local hospitals and were not centrally reviewed; we therefore cannot verify the accuracy of echocardiogram interpretation and measurements. Another limitation lies in the fact that relatively few recipients died within the first year post-transplant; this study may therefore have been underpowered to detect subtle influences of donor characteristics on post-transplant outcomes. Finally, this study was limited to donors managed by CTDN and may not be generalizable to heart transplant procedures performed throughout the United States or worldwide.
In conclusion, we have demonstrated the persistent low utilization of available cardiac allografts for transplantation in the face of a national organ donor shortage and a growing number of patients with end-stage heart disease. We identified predictors of allograft non-utilization and demonstrated that the anticipated relationship between these donor predictors and adverse recipient outcomes was not seen in our heart transplant cohort. These findings support the following statement recently put forth by the National Heart, Lung, and Blood Institute regarding the next decade of heart transplantation research: “Without clear evidence about the outcomes associated with different donor characteristics informing the donor selection process, it is probable that many potentially useful organs are currently being discarded. Because an important rate limiting factor in [heart transplantation] is the number of available donor organs, studies that define how to optimize donor use and develop biomarkers to define organ utility might increase the donor pool by providing evidence that would support the use of those organs deemed to be less than perfect.”40 The field of heart transplantation would therefore benefit greatly from prospective, multi-center trials studying the effects of liberalizing allograft acceptance criteria.
ACKNOWLEDGMENTS
We thank the California Transplant Donor Network staff for access to the donor data required for this study.
FUNDING
This work was supported in part by the Health Resources and Services Administration contract 234-2005-370011C, by the American Heart Association (0865249F, KKK), and by the National Heart, Lung, and Blood Institute (K23HL091143, KKK).
Footnotes
DISCLOSURES
The content of this study is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, not does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The authors have no further conflicts of interest to disclose.
REFERENCES
- 1.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Costanzo MR. Selection and treatment of candidates for heart transplantation. Semin Thorac Cardiovasc Surg. 1996;8:113–125. [PubMed] [Google Scholar]
- 4.Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D'Alessandro A, Dec GW, Edwards NM, Higgins RS, Jeevanandum V, Kauffman M, Kirklin JK, Large SR, Marelli D, Peterson TS, Ring WS, Robbins RC, Russell SD, Taylor DO, Van Bakel A, Wallwork J, Young JB. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28–29, 2001, Crystal City, Va. Circulation. 2002;106:836–841. doi: 10.1161/01.cir.0000025587.40373.75. [DOI] [PubMed] [Google Scholar]
- 5.Sheehy E, Conrad SL, Brigham LE, Luskin R, Weber P, Eakin M, Schkade L, Hunsicker L. Estimating the number of potential organ donors in the United States. N Engl J Med. 2003;349:667–674. doi: 10.1056/NEJMsa021271. [DOI] [PubMed] [Google Scholar]
- 6.Goland S, Czer LS, Kass RM, Siegel RJ, Mirocha J, De Robertis MA, Lee J, Raissi S, Cheng W, Fontana G, Trento A. Use of cardiac allografts with mild and moderate left ventricular hypertrophy can be safely used in heart transplantation to expand the donor pool. J Am Coll Cardiol. 2008;51:1214–1220. doi: 10.1016/j.jacc.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 7.Laks H, Marelli D, Fonarow GC, Hamilton MA, Ardehali A, Moriguchi JD, Bresson J, Gjertson D, Kobashigawa JA. Use of two recipient lists for adults requiring heart transplantation. J Thorac Cardiovasc Surg. 2003;125:49–59. doi: 10.1067/mtc.2003.62. [DOI] [PubMed] [Google Scholar]
- 8.Lima B, Rajagopal K, Petersen RP, Shah AS, Soule B, Felker GM, Rogers JG, Lodge AJ, Milano CA. Marginal cardiac allografts do not have increased primary graft dysfunction in alternate list transplantation. Circulation. 2006;114:I27–I32. doi: 10.1161/CIRCULATIONAHA.105.000737. [DOI] [PubMed] [Google Scholar]
- 9.Ott GY, Herschberger RE, Ratkovec RR, Norman D, Hosenpud JD, Cobanoglu A. Cardiac allografts from high-risk donors: excellent clinical results. Ann Thorac Surg. 1994;57:76–81. doi: 10.1016/0003-4975(94)90368-9. discussion 81-72. [DOI] [PubMed] [Google Scholar]
- 10.Young JB, Naftel DC, Bourge RC, Kirklin JK, Clemson BS, Porter CB, Rodeheffer RJ, Kenzora JL. Matching the heart donor and heart transplant recipient. Clues for successful expansion of the donor pool: a multivariable, multiinstitutional report. The Cardiac Transplant Research Database Group. J Heart Lung Transplant. 1994;13:353–364. [PubMed] [Google Scholar]
- 11.Stehlik J, Feldman DS, Brown RN, VanBakel AB, Russel SD, Ewald GA, Hagan ME, Folsom J, Kirklin JK. Interactions among donor characteristics influence post-transplant survival: a multi-institutional analysis. J Heart Lung Transplant. 2010;29:291–298. doi: 10.1016/j.healun.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Smits JM, De Pauw M, de Vries E, Rahmel A, Meiser B, Laufer G, Zuckermann A. Donor scoring system for heart transplantation and the impact on patient survival. J Heart Lung Transplant. 2012;31:387–397. doi: 10.1016/j.healun.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Al-Khaldi A, Oyer PE, Robbins RC. Outcome analysis of donor gender in heart transplantation. J Heart Lung Transplant. 2006;25:461–468. doi: 10.1016/j.healun.2005.11.456. [DOI] [PubMed] [Google Scholar]
- 14.Zeier M, Dohler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13:2570–2576. doi: 10.1097/01.asn.0000030078.74889.69. [DOI] [PubMed] [Google Scholar]
- 15.Tsai FC, Marelli D, Bresson J, Gjertson D, Kermani R, Ardehali A, Esmailian F, Hamilton M, Fonarow GC, Moriguchi J, Plunkett M, Hage A, Tran J, Kobashigawa JA, Laks H. Recent trends in early outcome of adult patients after heart transplantation: a single-institution review of 251 transplants using standard donor organs. Am J Transplant. 2002;2:539–545. doi: 10.1034/j.1600-6143.2002.20608.x. [DOI] [PubMed] [Google Scholar]
- 16.Bryan CF, Mitchell SI, Borkon AM, Curtis J, Demmy T, Estep TH, Moran J. Influence of donor gender on patient mortality after heart transplantation. Transplant Proc. 1996;28:149–151. [PubMed] [Google Scholar]
- 17.Sharples LD, Caine N, Mullins P, Scott JP, Solis E, English TA, Large SR, Schofield PM, Wallwork J. Risk factor analysis for the major hazards following heart transplantation--rejection, infection, and coronary occlusive disease. Transplantation. 1991;52:244–252. doi: 10.1097/00007890-199108000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Singhal AK, Sheng X, Drakos SG, Stehlik J. Impact of donor cause of death on transplant outcomes: UNOS registry analysis. Transplant Proc. 2009;41:3539–3544. doi: 10.1016/j.transproceed.2009.06.192. [DOI] [PubMed] [Google Scholar]
- 19.Cohen O, De La Zerda DJ, Beygui R, Hekmat D, Laks H. Donor brain death mechanisms and outcomes after heart transplantation. Transplant Proc. 2007;39:2964–2969. doi: 10.1016/j.transproceed.2007.08.102. [DOI] [PubMed] [Google Scholar]
- 20.Sobieszczanska-Malek M, Zielinski T, Korewicki J. The influence of donor-related factors on the frequency of acute cellular rejection by the recipient in the first year following heart transplantation. Ann Transplant. 2007;12:38–43. [PubMed] [Google Scholar]
- 21.Houser SL, MacGillivray T, Aretz HT. The impact of cocaine on the donor heart: a case report. J Heart Lung Transplant. 2000;19:609–611. doi: 10.1016/s1053-2498(00)00082-6. [DOI] [PubMed] [Google Scholar]
- 22.Freimark D, Czer LS, Admon D, Aleksic I, Valenza M, Barath P, Harasty D, Queral C, Azen CG, Blanche C, Trento A. Donors with a history of cocaine use: effect on survival and rejection frequency after heart transplantation. J Heart Lung Transplant. 1994;13:1138–1144. [PubMed] [Google Scholar]
- 23.Brieke A, Krishnamani R, Rocha MJ, Li W, Patten RD, Konstam MA, Patel AR, Udelson JE, Denofrio D. Influence of donor cocaine use on outcome after cardiac transplantation: analysis of the United Network for Organ Sharing Thoracic Registry. J Heart Lung Transplant. 2008;27:1350–1352. doi: 10.1016/j.healun.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Tsao CI, Chen RJ, Chou NK, Ko WJ, Chi NH, Yu HY, Chen YS, Chen SC, Wang SS. The influence of donor characteristics on survival after heart transplantation. Transplant Proc. 2008;40:2636–2637. doi: 10.1016/j.transproceed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Potapov EV, Ivanitskaia EA, Loebe M, Mockel M, Muller C, Sodian R, Meyer R, Hetzer R. Value of cardiac troponin I and T for selection of heart donors and as predictors of early graft failure. Transplantation. 2001;71:1394–1400. doi: 10.1097/00007890-200105270-00007. [DOI] [PubMed] [Google Scholar]
- 26.Potapov EV, Wagner FD, Loebe M, Ivanitskaia EA, Muller C, Sodian R, Jonitz B, Hetzer R. Elevated donor cardiac troponin T and procalcitonin indicate two independent mechanisms of early graft failure after heart transplantation. Int J Cardiol. 2003;92:163–167. doi: 10.1016/s0167-5273(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 27.Khush KK, Menza RL, Babcock WD, Zaroff JG. Donor cardiac troponin I levels do not predict recipient survival after cardiac transplantation. J Heart Lung Transplant. 2007;26:1048–1053. doi: 10.1016/j.healun.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Zaroff JG, Babcock WD, Shiboski SC. The impact of left ventricular dysfunction on cardiac donor transplant rates. J Heart Lung Transplant. 2003;22:334–337. doi: 10.1016/s1053-2498(02)00554-5. [DOI] [PubMed] [Google Scholar]
- 29.Aziz S, Soine LA, Lewis SL, Kruse AP, Levy WC, Wehe KM, Fishbien DP, Allen MD. Donor left ventricular hypertrophy increases risk for early graft failure. Transpl Int. 1997;10:446–450. doi: 10.1007/s001470050084. [DOI] [PubMed] [Google Scholar]
- 30.Marelli D, Laks H, Fazio D, Moore S, Moriguchi J, Kobashigawa J. The use of donor hearts with left ventricular hypertrophy. J Heart Lung Transplant. 2000;19:496–503. doi: 10.1016/s1053-2498(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 31.Brieman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 32.Chen JM, Sinha P, Rajasinghe HA, Suratwala SJ, McCue JD, McCarty MJ, Caliste X, Hauff HM, John R, Edwards NM. Do donor characteristics really matter? Short- and long-term impact of donor characteristics on recipient survival, 1995–1999. J Heart Lung Transplant. 2002;21:608–610. doi: 10.1016/s1053-2498(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 33.Weiss ES, Allen JG, Kilic A, Russell SD, Baumgartner WA, Conte JV, Shah AS. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. J Heart Lung Transplant. 2012;31:266–273. doi: 10.1016/j.healun.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 35.Drakos SG, Kfoury AG, Kotter JR, Reid BB, Clayson SE, Selzman CH, Stehlik J, Fisher PW, Merida M, 3rd, Eckels DD, Brunisholz K, Horne BD, Stoker S, Li DY, Renlund DG. Prior human leukocyte antigen-allosensitization and left ventricular assist device type affect degree of post-implantation human leukocyte antigen-allosensitization. J Heart Lung Transplant. 2009;28:838–842. doi: 10.1016/j.healun.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnaoutakis GJ, George TJ, Kilic A, Weiss ES, Russell SD, Conte JV, Shah AS. Effect of sensitization in US heart transplant recipients bridged with a ventricular assist device: update in a modern cohort. J Thorac Cardiovasc Surg. 2011;142:1236–1245. doi: 10.1016/j.jtcvs.2011.07.019. 1245 e1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bull DA, Reid BB, Selzman CH, Mesley R, Drakos S, Clayson S, Stoddard G, Gilbert E, Stehlik J, Bader F, Kfoury A, Budge D, Eckels DD, Fuller A, Renlund D, Patel AN. The impact of bridge-to-transplant ventricular assist device support on survival after cardiac transplantation. J Thorac Cardiovasc Surg. 2010;140:169–173. doi: 10.1016/j.jtcvs.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Jeevanandam V, Furukawa S, Prendergast TW, Todd BA, Eisen HJ, McClurken JB. Standard criteria for an acceptable donor heart are restricting heart transplantation. Ann Thorac Surg. 1996;62:1268–1275. doi: 10.1016/0003-4975(96)00626-1. [DOI] [PubMed] [Google Scholar]
- 39.Wittwer T, Wahlers T. Marginal donor grafts in heart transplantation: lessons learned from 25 years of experience. Transpl Int. 2008;21:113–125. doi: 10.1111/j.1432-2277.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 40.Shah MR, Starling RC, Schwartz Longacre L, Mehra MR. Heart transplantation research in the next decade--a goal to achieving evidence-based outcomes: National Heart, Lung, And Blood Institute Working Group. J Am Coll Cardiol. 2012;59:1263–1269. doi: 10.1016/j.jacc.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


