Abstract
The high sensitivity but low specificity of breast MRI has prompted exploration of breast 1H MRS for breast cancer detection. However, several obstacles still prevent the routine application of in vivo breast 1H MRS, including poor spatial resolution, long acquisition time associated with conventional multi-voxel MRS imaging (MRSI) techniques, and the difficulty of “extra” lipid suppression in a magnetic field with relatively poor achievable homogeneity compared to the brain. Using a combination of a recently developed echo-filter (EF) suppression technique and an elliptical sampling scheme, we demonstrate the feasibility of overcoming these difficulties. It is robust (the suppression technique is insensitive to magnetic field inhomogeneity), fast (acquisition time of about 12 min) and offers high spatial resolution (up to 0.6 cm3 per voxel at 1.5 T with a TE of only 60 ms). This approach should be even better at 3 T with higher resolution and/or shorter TE.
Keywords: Breast cancer, Magnetic resonance spectroscopy, Echo-filter, Short TE, High spatial resolution
1. Introduction
Dynamic contrast-enhanced MRI (DCE-MRI) is currently used as an adjunct to mammography in women at high risk, or those with extremely dense breasts, breast implants, irradiated breasts, post-surgical breasts or highly invasive breast cancers because of its greater sensitivity than mammography [1–4]. However, this high sensitivity (88–100%) [5–13] comes with low specificity such that 53–80% of biopsies prompted by MRI prove benign [14–16]. Reducing the large number of benign biopsies performed today is an important goal of breast imaging research. In vivo proton (1H) magnetic resonance spectroscopy (MRS) has the potential to achieve this goal. Complementary to MRI, 1H MRS provides an independent biochemical profile of the tissue and can be easily integrated into a breast MRI protocol. It is well known that tumor, necrotic tissue and healthy tissue have different biochemical profiles. Furthermore, because certain 1H metabolites are directly or indirectly involved in tumor metabolism such as rapid cell proliferation, 1H MRS has the potential to detect cancer even earlier than clinical MRI. This has been demonstrated by abnormal 1H metabolite levels appearing in regions beyond those shown in contrast-enhanced MRI areas in brain tumors [17].
Breast cancer can be distinguished from benign lesions and from normal breast tissues by a dramatic increase (from undetectable to clearly visible at 1.5 T) of the choline (Cho) signal in 1H MRS [18–29]. Cho, consisting of free, phospho-, glycerophospho- and phospholipid- choline, is a classic in vivo biomarker for cellular proliferation. Virtually all tumors cause Cho elevation. More specifically, a large increase in the cellular concentration of phosphocholine is one of the earliest responses of tumor cells to growth factor proteins. Breast cancer cells contain at least 10 times more phosphocholine than do normal mammary epithelial cells [30]. In vitro MRS of breast cell lines confirms that Cho levels increase with progression from normal to immortalized to oncogene-transformed to tumor-derived cells [31]. Analysis of early pooled clinical experience with in vivo 1H MRS yielded a sensitivity and specificity for detection of breast cancer of 83% and 85%, respectively, with near 100% for both in a subgroup of young women [32]. A reduction or disappearance of the Cho signal has been associated with response to neoadjuvant chemotherapy of locally advanced breast cancer [33].
Several obstacles have limited the routine application of in vivo 1H MRS for human breast cancer, including poor spatial resolution, long acquisition time associated with conventional multi-voxel MRS imaging (MRSI) techniques and the difficulty of “extra” lipid suppression in a magnetic field with relatively poor achievable homogeneity compared to the brain. Despite the fact that multiple MRI-enhanced lesions are often observed in one breast, most 1H MRS studies of human breast cancer are performed using single-voxel techniques with low spatial resolution (typically >1.5 cm3). Due to the increasing difficulty of lipid suppression at a short echo-time (TE), all current existing 1H MRSI studies of the breast have used a long echo time (TE=270 ms) [34–36]. A long TE results in signal loss and thus poor spatial resolution. Using inversion-recovery methods to suppress strong lipid signals, and a TE of 272 ms, investigators acquired 2D 1H MRSI of the breast with spatial resolution of about 1 cm3 in 12 min at 1.5 T [34]. Despite this significant achievement, such spatial resolution remains inadequate for assessing the majority of suspicious lesions (typically <1 cm in largest diameter). Another challenge in high spatial resolution metabolite imaging for routine clinical examinations is keeping acquisition time short enough to maintain tolerable examination time for patients.
Recently, a powerful suppression technique, the echo-filter (EF) suppression technique, has been proposed with the capability of eliminating all undesired large signals in the human brain [37]. The modified version has been successfully applied for in vivo high spatial resolution 1H MRSI of human muscles, where lipid signal is also high [38]. In order to overcome the long acquisition time in conventional MRSI methods, several techniques have been proposed, including elliptical weighted k-space phase encoding with Hamming filtering sampling [39–41]. In contrast to most other fast k-space schemes where the signal-to-noise ratio (SNR) per unit time is, at best, the same as a conventional k-space sampling method, the elliptical sampling scheme can improve SNR per unit time by approximately 20% as demonstrated in the literature [39–41]. Thus, the goal of this study was to investigate whether the combination of the echo-filter suppression technique and the elliptical sampling scheme can address the key obstacles for in vivo 1H MRSI of human breast lesions: lipid suppression, high spatial resolution and a clinically acceptable acquisition time. We report here our preliminary studies.
2. Materials and methods
2.1. Pulse sequence
Fig. 1 shows the proposed technique, consisting of three parts: inversion recovery [short tau inversion recovery (STIR)], outer volume pre-saturation (OVP) and an echo-filter MRSI with an elliptically weighted k-space sampling scheme. The simplest echo-filter pulse sequence consists of a 90° RF pulse (or series of pulses) to define the volume of interest (VOI), a delay TE/2, a frequency-selective (FS) 180° pulse and another delay TE/2 with equally strong crusher gradients (G+G1) on each side of the FS pulse [37]. In this study, two SINC pulses and OVP were used to define the VOI, and a Gaussian pulse with a 60-Hz bandwidth and its frequency setting at 3.2 ppm was used as the FS pulse (Fig. 1). Undesired water and lipid signals were suppressed in two ways: (1) passively, by virtue of their position outside the frequency range of the 180°FS pulse, and (2) actively, by the gradients that destroy the phase coherence of any residual undesired signals due to the imperfections of the FS pulse. This “double” suppression mechanism ensures sufficient suppression of undesired signal frequencies with properly designed FS pulse and gradient strengths. The EF technique has been shown to suppress water and lipid signal-to-noise level [37]. We take advantage of its suppression efficiency to use two (instead of three) SINC pulses and OVP to define VOI for a short echo. Of course, three SINC pulses could be used to reduce the potential contamination from the OVP direction, particularly when an even shorter echo time such as 30 ms is used at 3 T. Despite its efficiency for strong water and lipid, however, the EF suppression technique does not work well for undesired signal that overlaps with desired signal (such as the Cho signal at 3.2 ppm). This is a problem for the “small” lipid signal around 2.9 ppm from methylene protons of CH=CH–CH2–CH=CH at a short TE. Therefore, in our study, this lipid signal is suppressed using an inversion recovery pulse (STIR) with a delay of 200 ms. The OVP is determined by applying slice-excitation pulses to select the area outside the VOI followed by spoiling gradients to destroy them and can be turned on or off as needed. To improve sampling efficiency, a weighted k-space sampling scheme is used to acquire MRSI data sets [39–41]. The weighted k-space samples only the points located on or within the k-space ellipse. When the number of averages (NA) is greater than 1, the central points of k-space are measured NA times and points on the boundary of the ellipse at least once. For intermediate points, the sampling frequency is determined by their radial distance from the center of k-space. This incorporates the Hamming filter during the measurement, resulting in an improved SNR per unit time of approximately 20% on a phantom test [39–41].
Fig. 1.
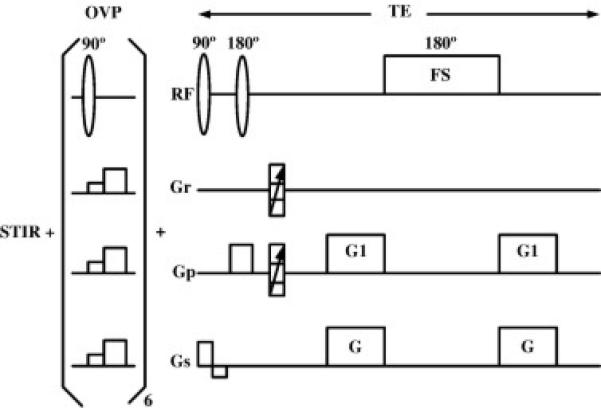
The proposed pulse sequence consists of three parts: STIR for lipid suppression around 2.9 ppm, OVP for outer-volume pre-saturation and an echo-filter MRSI with an elliptically weighted k-space sampling scheme.
2.2. Patients
Ten patients (age range, 22 to 87; mean, 67) with pathologically known or suspected breast cancer were studied with the new EF MRSI technique. DCE-MRI studies revealed contrast enhanced lesions in all patients. Consent was obtained from each patient for the MRSI study approved by the Institutional Human Investigation Committee.
2.3. Experimental procedure
All patient studies were performed with a Siemens 1.5-T whole-body imager (Sonata, Erlangen, Germany). A standard phased array breast coil (two channels for each breast coil; MRI Devices, Waukesha, WI, USA) was used for both MRI and 1H MRS. Patients lay prone in the magnet and, whenever possible, gentle breast compression was applied to minimize motion. After global shimming, standard DCE-MRI was performed, which defined the volume of interest for MRSI. The MRSI spectra were then acquired with our new method. Typical measurement parameters were field-of-view (FOV)=100×100 mm2, phasing-encoding steps=16×16, TR=1500 ms, TE=60 ms, slice thickness=10–20 mm, NA=8 and acquisition time=11 min 40 s. Data processing consisted of line broadening (Gaussian function with 256 ms), standard Fast Fourier Transformation and phasing. No baseline correction was applied. The metabolite images were formed from spectral fitting and zero-filled to 256×256 points using the Siemens spectroscopy software package.
3. Results
Nine of the 10 studies were technically successful with excellent lipid and water suppression; one study failed before adding the inversion recovery option. No choline was detected after one or two courses of chemotherapy from two patients previously diagnosed with invasive ductal carcinoma. Additionally, no choline was seen in two patients with fibroadenoma. Illustrative examples are described below.
Fig. 2A and B shows the spectra from two different areas in biopsy-proven invasive tubular carcinoma, respectively. Fig. 2C shows the spectrum from a control voxel. The spectra were acquired with a TE of only 60 ms and a spatial resolution of only 0.6 cm3, the shortest TE and highest spatial resolution for in vivo 1H breast MRS at 1.5 T to date. Other parameters are TR=1600 ms, NA=8, acquisition time=12.1 min and a 60-Hz Gaussian FS pulse setting at Cho resonance (3.2 ppm). As expected, spectra from breast cancer lesions (Fig. 2A and B) demonstrate a distinct Cho peak (SNR >3), consistent with malignancy, while the spectrum from the control area (Fig. 2C) shows no detectable Cho signal. Moreover, Spectra 2A and 2B demonstrate, for the first time, the heterogeneity of human breast cancer detected by in vivo 1H MRS, as evident by two new peaks between 3.2 and 4.2 ppm in Fig. 2A. Excellent lipid suppression (almost to the noise level) and excellent water suppression were achieved here, even for a relatively inhomogeneous magnetic field in the control voxel, as indicated by a much broader residual water peak in Fig. 2C than in Fig. 2A or B. Lipid and water suppression was not a problem for the EF technique in all studies so far. Sufficient lipid and water suppression was achieved without any operator interference, and even with poor shimming (the width of the water peak at half height larger than 30 Hz). Aside from an 8-Hz filter in the time domain and fast Fourier transform (FFT), no other processing was used to generate the spectra.
Fig. 2.
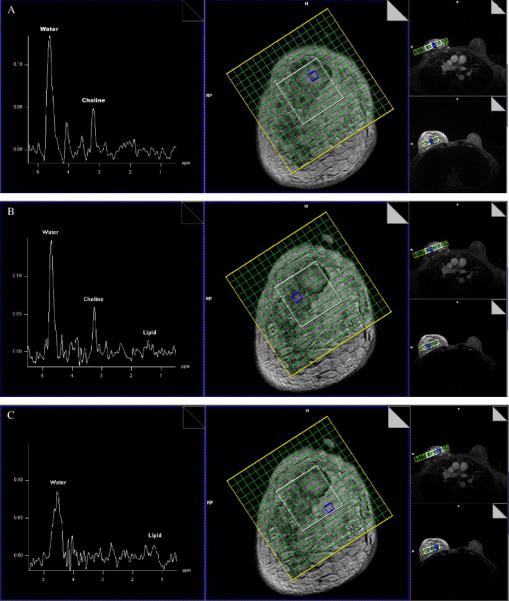
Three 1H spectra from (A) a breast lesion, (B) another breast lesion and (C) a control area with a TE of only 60 ms and spatial resolution of 0.6 cm3. The corresponding sampled volume is indicated on the grid overlying a coronal CE-MRI image. This is the shortest TE and highest spatial resolution for 1H breast MRS to date. It also demonstrates the heterogeneity in breast cancer by in vivo 1H MRS.
Fig. 3A is a coronal DCE MRI of a patient with malignant morphologic and kinetic features. Fourteen-gauge biopsy yielded poorly differentiated invasive ductal carcinoma. Fig. 3B is a color-coded Cho image overlaid on the corresponding coronal MRI. Fig. 3C and D are 1H spectra from tumor area (labeled as 1 in Fig. 3A) and control area (labeled as 2 in Fig. 3A), respectively. Choline concentration is clearly varied across the DCE-MRI defined tumor, as illustrated in Fig. 3B. Unlike the case in Fig. 2, there is a “hot area” (high concentration of Cho content in low right portion of the lesion) as well as “cold area”(low or barely detectable Cho signals in the rest of the enhanced area). This is consistent with the presence of biopsy-proven tumor, necrosis and sclerosis in the DCE-MRI defined lesion. To the best of our knowledge, this is the first time that a distinctive “hot area” is observed in breast lesions by in vivo 1H MRS.
Fig. 3.
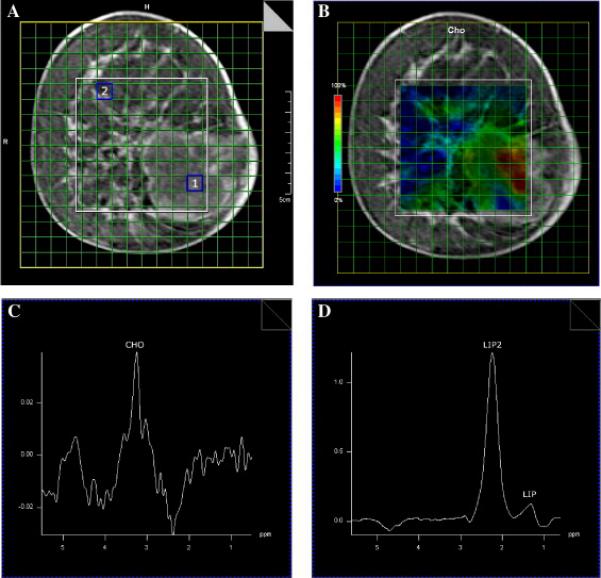
Illustration of a “hot” area in a breast cancer lesion. (A) Coronal DCE-MRI of a patient with invasive breast cancer. (B) A color-coded Cho image overlaid on the corresponding MRI. (C) 1H spectra from tumor area [labeled as 1 in (A)]. (D) 1H Spectrum from control area [labeled as 2 in (A)].
4. Discussion
The challenge for the routine use of breast 1H MRSI is the need for robust lipid suppression, high spatial resolution and a clinically acceptable acquisition time. Our preliminary results demonstrate that the proposed technique could be a solution to this challenge. First, the EF suppression technique offers powerful suppression capability; it can even suppress undesired strong lipid/water signal-to-noise level with properly designed frequency-selective pulse and gradients [37]. Second, it is robust; the EF suppression technique is insensitive to magnetic field inhomogeneity as long as the desired Cho signal is spectrally separated from undesired signal frequencies as demonstrated by excellent lipid and water suppression in all cases. Third, it is fast, adding only about 15 min to an existing breast MRI protocol. Thus, it is practical for breast 1H MRSI to be routinely integrated into a breast MRI protocol. Fourth, spectral separation increases linearly with strength of the magnetic field, and therefore the EF technique should have no difficulty in achieving adequate lipid suppression at very short TE (~30 ms) at 3 T. Fifth, spatial resolution can be further improved at higher field strengths, increasing by a factor of 2 at 3 T over 1.5 T. Sixth, the desired Cho signal will be modulated by less than 2.5% if the FS excitation profile is designed to vary within 5% of the desired 180° excitation angle over the range of frequency of interest (i.e., 171°<α<189°). Assuming the crusher gradients (G+G1 in Fig. 1) are sufficiently high to crush all nonrefocused signal frequencies, the effective amplitude of magnetization for a spin echo-like sequence after a 90° rf pulse and an α° rf pulse is given by M0[1–cos(2α)]/2 [43,44]. Thus, the signal loss due to imperfect 180° excitation is [1–cos(2α)]/2, which gives about 2.4% loss at 171° or 189° excitation. This is the theoretical basis for the reliable detection of undistorted Cho signals in this study, and it has been already demonstrated by the lack of noticeable distortion in previous brain studies at 4 T [37].
The importance of high spatial resolution breast 1H MRSI cannot be overemphasized. The spatial resolution of existing 1H MRS techniques does not permit the study of the majority of suspicious breast lesions. It is known that small lesions are the most likely to be ambiguous by MRI and, if malignant, have the best chance for cure. High spatial resolution 1H MRSI also facilitates the study of tumor heterogeneity, as demonstrated in Fig 2. Recent in vitro 1H MRS spectra of breast fine-needle aspiration biopsies at 8.5 T have demonstrated that there are distinctive spectral characteristics among malignant, DCIS with microinvasion, DCIS without microinvasion and benign tissues [42]. In addition, high spatial resolution breast 1H MRSI can help identify a “hot spot” in a DCE-MRI defined lesion, as illustrated in Fig. 3 This result suggests the potential of high spatial resolution breast 1H MRSI in the selection of the optimal biopsy site and treatment planning for a targeted area. Moreover, 1H MRSI offers the opportunity to detect “new” cancer or lesions beyond MRI-detectable areas, as has been well documented in brain tumors where high spatial resolution 2D or 3D MRSI is commonly applied.
Several caveats should be recognized before routine implementation of this technique for in vivo breast 1H MRSI. First, the type of FS pulse has a great effect on the appearance of the spectra, offering both the advantage of flexibility and the disadvantage of changing spectral appearance. For example, if Cho is the only metabolite of interest, a Gaussian FS pulse might be used because of its high selectivity and powerful suppression capability. The resulting spectral pattern, however, could be unfamiliar at first sight because of dramatic and uneven reduction, or even disappearance of water and lipid signals. If spectral heterogeneity of breast tumors is the focus of a study, a FS pulse with a flat excitation profile over a given range of frequencies should be considered. Second, sometimes it can be difficult to determine the exact position of an internal reference peak, such as water, due to the elimination of that signal or change in its chemical shift. That is, the EF technique can be too powerful sometimes. In such a case, the bandwidth and shape of the FS pulse have to be chosen so that enough residual signal for those internal reference peaks remains for their identification or reconstruction back to their original pattern in post-processing. Third, as with any new technique, the proposed method has to be fully tested before its routine use. It is extremely difficult to build a phantom that can satisfactorily mimic human breast lesions due to complex lipid components in human breasts. Thorough testing in a clinical setting is particularly important for in vivo 1H breast MRS because Cho is usually undetectable in a healthy breast.
In conclusion, we have developed a technique for short-TE in vivo 1H MRSI of human breast cancer and demonstrated several potential advantages over single-voxel techniques. This technique uses a combination of echo-filter suppression and elliptical sampling to overcome existing challenges for the routine application of 1H breast MRSI. It is a robust, fast and potentially useful tool with high spatial resolution for in vivo 1H MRS examinations of human breast cancers.
Acknowledgment
This work was supported in part by The Susan G. Komen Breast Cancer Foundation (IMG0402881), NIH (R21 CA118569-01A1) and MTTC grant (#085P5200251).
References
- 1.Kneeshaw P, Turnbull L, Drew P. Current applications and future direction of MR mammography. Br J Cancer. 2003;88:4–10. doi: 10.1038/sj.bjc.6600713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rieber A, Schramm K, Helms G, von Puckler S, Nuessle K, Kreienberg R, et al. Breast-conserving surgery and autogenous tissue reconstruction in patients with breast cancer: efficacy of MRI of the breast in the detection of recurrent disease. Eur Radiol. 2003;13:780–7. doi: 10.1007/s00330-002-1538-4. [DOI] [PubMed] [Google Scholar]
- 3.Partridge S, Gibbs J, Lu Y, Esserman L, Sudilovsky D, Hylton N. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193–9. doi: 10.2214/ajr.179.5.1791193. [DOI] [PubMed] [Google Scholar]
- 4.Liberman L, Morris E, Dershaw D, Abramson A, Tan L. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol. 2002;180:901–10. doi: 10.2214/ajr.180.4.1800901. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser W, Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Radiology. 1989;170:681–6. doi: 10.1148/radiology.170.3.2916021. [DOI] [PubMed] [Google Scholar]
- 6.Gilles R, Guinebretiere J, Lucidarme O, Cluzel P, Janaud G, Finet J, et al. Nonpalpable breast tumors: diagnosis with contrast-enhanced subtraction dynamic MR imaging. Radiology. 1994;191:625–31. doi: 10.1148/radiology.191.3.8184038. [DOI] [PubMed] [Google Scholar]
- 7.Boetes C, Barentsz J, Mus R, van der Sluis R, van Erning L, Hendriks J, et al. MR characterization of suspicious breast lesions with a Gd-enhanced TurboFlash subtraction technique. Radiology. 1994;193:777–81. doi: 10.1148/radiology.193.3.7972823. [DOI] [PubMed] [Google Scholar]
- 8.Chenevert T, Helvie M, Aisen A, Francis I, Adler D, Roubidoux M, et al. Dynamic three-dimensional imaging with partial k-space sampling: initial application for Gd-enhanced rate of characterization of breast lesions. Radiology. 1995;196:135–42. doi: 10.1148/radiology.196.1.7784556. [DOI] [PubMed] [Google Scholar]
- 9.Kelcz F, Santyr G, Cron G, Mongin S. Application of a quantitative model to differentiate benign from malignant breast lesions detected by dynamic, Gd-enhanced MRI. J Magn Reson Imaging. 1996;6:743–52. doi: 10.1002/jmri.1880060507. [DOI] [PubMed] [Google Scholar]
- 10.Muller-Schimpfle M, Ohmenhauser K, Sand J, Stoll P, Claussen C. Dynamic 3D-MR mammography: is there a benefit of sophisticated evaluation of enhancement curves for clinical routine? J Magn Reson Imaging. 1997;7:236–40. doi: 10.1002/jmri.1880070137. [DOI] [PubMed] [Google Scholar]
- 11.Wiberg M, Bone B, Bronge L, Aspelin P. Comparison of the contrast enhancement pattern in two different T1-weighted 3D sequences in MR imaging of the breast. Acta Radiol. 1998;39:680–5. doi: 10.3109/02841859809175496. [DOI] [PubMed] [Google Scholar]
- 12.Nunes L, Schnall M, Orel S, Hochman M, Lauglotz C, Reynolds C, et al. Correlation of lesion appearance and histological findings for the nodes of a breast MR imaging interpretation model. Radiographics. 1999;19:79–92. doi: 10.1148/radiographics.19.1.g99ja0379. [DOI] [PubMed] [Google Scholar]
- 13.Yen Y, Han K, Daniel B, Heiss S, Birdwell R, Herfkens R, et al. Dynamic breast MRI with spiral trajectories: 3D versus 2D. J Magn Reson Imaging. 2000;11:351–9. doi: 10.1002/(sici)1522-2586(200004)11:4<351::aid-jmri2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Kuhl C, Morakkabati N, Leutner C, Schmiedel A, Wardelmann E, Schild H. MR imaging-guided large-core(14 gauge) needle biopsy of small lesions visible at breast MR imaging alone. Radiology. 2001;220:31–9. doi: 10.1148/radiology.220.1.r01jl0731. [DOI] [PubMed] [Google Scholar]
- 15.Lee G, Orel S, Woo I, Cruz-Jove E, Putt M, Solin L, et al. MR imaging screening of the contralateral breast in patients with newly diagnosed breast cancer: preliminary results. Radiology. 2003;226:773–8. doi: 10.1148/radiol.2263020041. [DOI] [PubMed] [Google Scholar]
- 16.Liberman L, Morris E, Kim C, Kaplan J, Abramson A, Menell J, et al. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol. 2003;180:333–41. doi: 10.2214/ajr.180.2.1800333. [DOI] [PubMed] [Google Scholar]
- 17.Nelson S. Magnetic resonance spectroscopic imaging: evaluating response to therapy for gliomas. IEEE Eng Med Biol Mag. 2004:30–9. doi: 10.1109/memb.2004.1360406. [DOI] [PubMed] [Google Scholar]
- 18.Yeung DK, Cheung HS, Tse GM. Human breast lesions: characterization with contrast-enhanced in vivo proton MR spectroscopy — initial results [comment]. Radiology. 2001;220(1):40–6. doi: 10.1148/radiology.220.1.r01jl0240. [DOI] [PubMed] [Google Scholar]
- 19.Thomas M, Binesh N, Yue K, DeBruhl N. Volume-localized two-dimensional correlated magnetic resonance spectroscopy of human breast cancer. J Magn Reson Imaging. 2001;14:181–6. doi: 10.1002/jmri.1170. [DOI] [PubMed] [Google Scholar]
- 20.Cecil K, Schnall M, ES S, Lenkinski R. The evaluation of human breast lesions with magnetic resonance imaging and proton magnetic resonance spectroscopy. Breast Cancer Res Treat. 2001;68:45–54. doi: 10.1023/a:1017911211090. [DOI] [PubMed] [Google Scholar]
- 21.Gribbestad I, Sitter B, Lundgren S, Krane J, Axelson D. Metabolite composition in breast tumors examined by proton nuclear magnetic resonance spectroscopy. Anticancer Res. 1999;19:1737–46. [PubMed] [Google Scholar]
- 22.Gribbestad IS, Singstad TE, Nilsen G, Fjosne HE, Engan T, Haugen OA, et al. In vivo 1H MRS of normal breast and breast tumors using a dedicated double breast coil. J Magn Reson Imaging. 1998;8(6):1191–7. doi: 10.1002/jmri.1880080602. [DOI] [PubMed] [Google Scholar]
- 23.Roebuck JR, Cecil KM, Schnall MD, Lenkinski RE. Human breast lesions: characterization with proton MR spectroscopy. Radiology. 1998;209(1):269–75. doi: 10.1148/radiology.209.1.9769842. [DOI] [PubMed] [Google Scholar]
- 24.Kvistad KA, Bakken IJ, Gribbestad IS, Ehrnholm B, Lundgren S, Fjosne HE, et al. Characterization of neoplastic and normal human breast tissues with in vivo (1)H MR spectroscopy. J Magn Reson Imaging. 1999;10(2):159–64. doi: 10.1002/(sici)1522-2586(199908)10:2<159::aid-jmri8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Park S, Lee H, et al. In vivo 1H-MRS evaluation of malignant and benign breast diseases. Breast. 2003;12:179–82. doi: 10.1016/s0960-9776(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 26.Meisamy S, Bolan P, Baker EP, M G, Le C, Kelcz F, et al. Adding in vivo quantitative 1H MR spectroscopy to improve diagnostic accuracy of breast MR imaging: preliminary results of observer performance study at 4.0T. Radiology. 2005;236:465–75. doi: 10.1148/radiol.2362040836. [DOI] [PubMed] [Google Scholar]
- 27.Bolan P, Meisamy S, Baker E, Lin J, Emory T, Nelson M, et al. In vivo quantification of choline compounds in the breast with 1H MR spectroscopy. Magn Reson Med. 2004;50:1134–43. doi: 10.1002/mrm.10654. [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Fisher P, Dulaimy K, Tudorica L, Hea B, Button T. Detection of breast malignancy: diagnostic MR protocol for improved specificity. Radiology. 2004;232:585–91. doi: 10.1148/radiol.2322030547. [DOI] [PubMed] [Google Scholar]
- 29.Baik H, Su M, Yu H, Mehta R, Nalcioglu O. Quantification of choline-containing compounds in malignant breast tumors by 1H MR spectroscopy using water as an internal reference at 1.5T. MAGMA. 2006;19:96–104. doi: 10.1007/s10334-006-0032-4. [DOI] [PubMed] [Google Scholar]
- 30.Gluch L. Magnetic resonance in surgical oncology: II — Literature review. ANZ J Surg. 2005;75:464–70. doi: 10.1111/j.1445-2197.2005.03386.x. [DOI] [PubMed] [Google Scholar]
- 31.Aboagye E, Bhujwalla Z. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59:80–4. [PubMed] [Google Scholar]
- 32.Katz-Brull R, Lavin P, Lenkinski RE. Clinical utility of proton magnetic resonance spectroscopy in characterizing breast lesions. J Natl Cancer Inst. 2002;94:1197–203. doi: 10.1093/jnci/94.16.1197. [DOI] [PubMed] [Google Scholar]
- 33.Jagannathan NR, Kumar M, Seenu V, Coshic O, Dwivedi SN, Julka PK, et al. Evaluation of total choline from in-vivo volume localized proton MR spectroscopy and its response to neoadjuvant chemotherapy in locally advanced breast cancer. Br J Cancer. 2001;84(8):1016–22. doi: 10.1054/bjoc.2000.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs M, Barker P, Bottomley P, Bhujwalla Z, Bluemke D. Proton MR spectroscopic imaging of human breast cancer: a preliminary study. J Magn Reson Imaging. 2004;2004(19):68–75. doi: 10.1002/jmri.10427. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs M, Barker P, Argani P, Ouwerkerk R, Bhujwalla Z, Bluemke D. Combined dynamic contrast enhanced breast MR and proton spectroscopic imaging: a feasibility study. J Magn Reson Imaging. 2005;21:23–8. doi: 10.1002/jmri.20239. [DOI] [PubMed] [Google Scholar]
- 36.Hu J, Vartanian S, Xuan Y, Latif Z, Soulen R. An improved 1H magnetic resonance spectroscopic imaging technique for the human breast: preliminary results. Magn Reson Imaging. 2005;23:571–6. doi: 10.1016/j.mri.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Hu J. Mapping brain metabolites using the double echo-filter metabolic imaging (DEFMI) technique. J Magn Reson. 1999;140:363–70. doi: 10.1006/jmre.1999.1842. [DOI] [PubMed] [Google Scholar]
- 38.Hu J, Jiang Q, Xia Y, Zuo C. High spatial resolution in vivo 1H magnetic resonance spectroscopic imaging of human muscles with band-selective techniques. Magn Reson Imaging. 2001;19:1091–6. doi: 10.1016/s0730-725x(01)00438-6. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn B, Dreher W, Norris D, Leibfritz D. Fast proton spectroscopic imaging employing k-space weighting achieved by variable repetition times. Magn Reson Med. 1996;35:457–64. doi: 10.1002/mrm.1910350403. [DOI] [PubMed] [Google Scholar]
- 40.Maudsley A, Matson G, Hugg J, Weiner M. Reduced phase encoding in spectroscopic imaging. Magn Reson Med. 1994;31:645–51. doi: 10.1002/mrm.1910310610. [DOI] [PubMed] [Google Scholar]
- 41.Scheenen T, Klomp D, Roll S, Futterer J, Barentsz J, Heerschap A. Fast acquisition-weighted three-dimension proton MR spectroscopic imaging of the human prostate. Magn Reson Med. 2004;52:80–8. doi: 10.1002/mrm.20103. [DOI] [PubMed] [Google Scholar]
- 42.Mountford C, Somorjai R, Malycha P, Gluch L, Lean C, Russell P, et al. Diagnosis and prognosis of breast cancer by magnetic resonance spectroscopy of fine-needle aspirates analyzed using a statistical classification strategy. Br J Surg. 2000;88:1234–40. doi: 10.1046/j.0007-1323.2001.01864.x. [DOI] [PubMed] [Google Scholar]
- 43.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic resonance imaging: Physical principles and sequence design. John Wiley & Sons; New York: 1999. pp. 741–81. (Chapter 725) [Google Scholar]
- 44.Griffin J, Lehtimaki K, Valonen P, Grohn O, Kettunen M, Yla-Herttuala S, et al. Assignment of 1H nuclear magnetic resonance visible polyunsaturated fatty acids in BT4C gliomas undergoing gabciclovir-thymidine kinase gene therapy-induced programmed cell death. Cancer Res. 2003;63:3195–201. [PubMed] [Google Scholar]


