Abstract
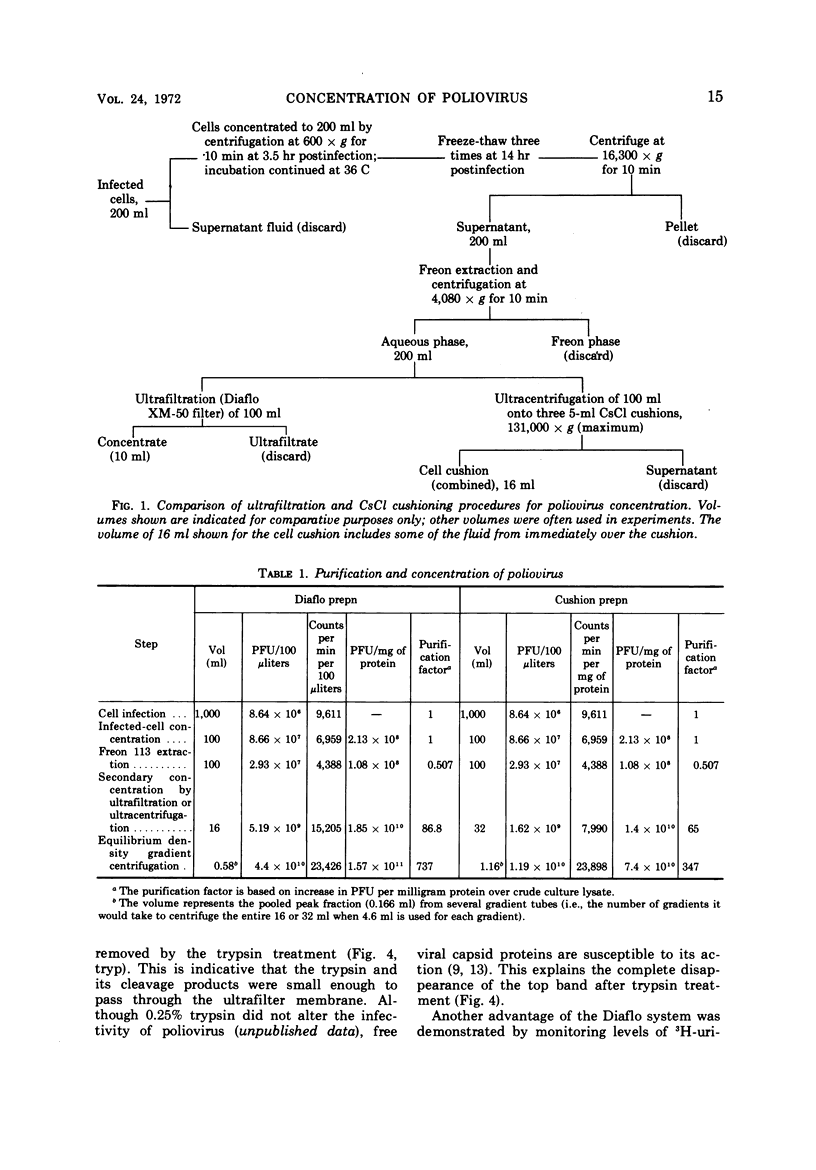
A method is described by which poliovirus can be rapidly and simply concentrated by the use of a Diaflo XM-50 ultrafilter membrane. Freon-extracted ultrafilter concentrates banded in CsCl provided a 1,724-fold volumetric concentration of poliovirus. During concentration, trypsin-digested cellular material can pass through the ultrafilter membrane, thus providing a versatile means of degrading and eliminating extraneous contaminating proteins. The ultrafilter concentration system is compared with the CsCl cushion system of poliovirus concentration, and both systems are further compared by banding virus and virus capsid material in CsCl by use of isopycnic centrifugation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agol V. I., Lipskaya G. Y., Tolskaya E. A., Voroshilova M. K., Romanova L. I. Defect in poliovirus maturation under hypotonic conditions. Virology. 1970 Jul;41(3):533–540. doi: 10.1016/0042-6822(70)90173-x. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S. Isopycnic separation of subcellular components from poliovirus-infected and normal HeLa cells. Science. 1968 Nov 1;162(3853):572–574. doi: 10.1126/science.162.3853.572. [DOI] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Guskey L. E., Smith P. C., Wolff D. A. Patterns of cytopathology and lysosomal enzyme release in poliovirus-infected HEp-2 cells treated with either 2-(alpha-hydroxybenzyl)-benzimidazole or guanidine HCl. J Gen Virol. 1970 Jan;6(1):151–161. doi: 10.1099/0022-1317-6-1-151. [DOI] [PubMed] [Google Scholar]
- Jamison R. M., Mayor H. D. Comparative study of seven picornaviruses of man. J Bacteriol. 1966 May;91(5):1971–1976. doi: 10.1128/jb.91.5.1971-1976.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PORTER C. A. Evaluation of a fluorocarbon technique for the isolation of plant viruses. Trans N Y Acad Sci. 1956 Jun;18(8):704–706. doi: 10.1111/j.2164-0947.1956.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of polioviruses. I. Kinetics of the assembly of empty capsids and the role of extracts from infected cells. Virology. 1969 Dec;39(4):811–821. doi: 10.1016/0042-6822(69)90018-x. [DOI] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Isolation and properties of poliovirus minus strand ribonucleic acid. J Virol. 1970 Nov;6(5):604–609. doi: 10.1128/jvi.6.5.604-609.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFFER F. L., SCHWERDT C. E. Purification and properties of poliovirus. Adv Virus Res. 1959;6:159–204. doi: 10.1016/s0065-3527(08)60491-1. [DOI] [PubMed] [Google Scholar]
- Van Elsen A., Boeyé A., Teuchy H. Formation of fibrillar structures from poliovirus by alkaline disruption and other treatments. Virology. 1968 Nov;36(3):511–514. doi: 10.1016/0042-6822(68)90179-7. [DOI] [PubMed] [Google Scholar]