Abstract
Pregnane glycosides appear to modulate food intake by possibly affecting the hypothalamic feeding circuits; however, the mechanisms of the appetite-regulating effect of pregnane glycosides remain obscure. Here, we show that pregnane glycoside-enriched extracts from swamp milkweed Asclepias incarnata at 25–100 mg/kg daily attenuated food intake (up to 47.1 ± 8.5% less than controls) and body weight gain in rats (10% for males and 9% for females, respectively) by activating melanocortin signaling and inhibiting gastric emptying. The major milkweed pregnane glycoside, ikemagenin, exerted its appetite-regulating effect by decreasing levels of agouti-related protein (0.6-fold) but not NPY satiety peptides. Ikemagenin treatment also increased secretion of brain-derived neurotropic factor (BDNF) downstream of melanocortin receptors in the hypothalamus (1.4-fold) and in the C6 rat glioma cell culture in vitro (up to 6-fold). These results support the multimodal effects of pregnane glycosides on feeding regulation, which depends on the activity of the melanocortin signaling pathway and BDNF.
Keywords: appetite, satiety, body weight, milkweed, Asclepias
INTRODUCTION
Body weight is maintained through an intricate balance between energy intake (food consumption) and expenditure.1 Meals are initiated, maintained, and terminated by specific sets of central and peripheral signals several times a day separated by intermeal intervals without food intake. These signals include patterns of neural afferent traffic, metabolites (glucose), energy flux (fatty acid oxidation, ATP), and hormones including hypothalamic neuropeptides and neurotransmitters that interact and provide the central and peripheral integration necessary to regulate food intake.2 Integration of peripheral and central signals related to energy homeostasis takes place in the arcuate nucleus of the hypothalamus where orexigenic peptides, neuropeptide Y (NPY), and agouti-related protein (AgRP) colocalize.3 These neurons inhibit the anorectic peptides cocaine- and amphetamine-regulated transcript (CART) and pro-opiomelanocortin (POMC) through γ-aminobutyric acid (GABA)-ergic interneuronal connections.4 Another central peptide that reduces food intake and decreases body weight gain is brain-derived neurotrophic factor (BDNF), which functions directly downstream of the melanocortin receptors as shown in the agouti Ay genetic mouse model.5 Therefore, direct or indirect targeting of the central signaling network underlying hunger, satiety, and metabolic status with novel pharmacological interventions appears to be an attractive strategy in the treatment or prevention of metabolic disorders.6,7
Milkweeds are an abundant source of pregnane glycosides.8 Slow-growing milkweed succulents Hoodia gordonii and Caralluma fimbriata have been used in traditional medicine as natural appetite suppressants.9,10 P57AS3 pregnane glycoside is the only reported active constituent from Hoodia that acts as an appetite suppressant,11 by possibly increasing the ATP content of hypothalamic neurons.12 In a single double-blind, placebo-controlled trial with 50 healthy volunteers receiving 1 g of Caralluma extract per day for 60 days, waist circumference and hunger showed a significant decline in the experimental group when compared to the placebo after 2 months of treatment.10 Limited natural range and slow growth of these succulent plants have considerably restricted their availability for research and therapeutic applications.
Species of milkweed Asclepias are adapted to a wide range of environments in nearly all states of the United States and are known for vigorous growth that allows for their successful establishment as an alternative crop for the production of fiber, latex, seed floss, and oils.13 The young shoots, stems, flower buds, immature fruits, and roots of swamp milkweed (Asclepias incarnata L.) have been boiled and eaten as a vegetable by various indigenous groups of eastern and midwestern America.14 In connection with a study on the phytochemical constituents of swamp milkweed, Warashina identified a natural library of pregnane glycosides analogous to those from Hoodia.15,16 Such diversity provides a unique and abundant source of pregnane glycosides for investigating their physiological and pharmacological effects.
To address the potential of pregnane glycosides to reduce food intake and to identify their cellular and molecular targets, this study evaluated appetite-suppressing capacities of the pregnane glycoside-enriched extract from swamp milkweed roots (incarnatin) and its major pregnane glycoside constituent, 12β-cinnamoyl-3,8,12,14β-tetrahydroxypregn-5-en-20-one glycoside (ikemagenin).
MATERIALS AND METHODS
Chemicals
The solvents used in this study were of high-performance liquid chromatography (HPLC) grade; all other chemicals were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise stated.
Plant Source, Extraction, and Compound Identification
A. incarnata (swamp milkweed) plants were grown from seeds hydroponically for 4 months and harvested. The freeze-dried root powder (100 g) was defatted with hexane (3 × 100 mL) on a shaker at room temperature for 8 h. The organic layers were discarded, and the pellet was freed from residual solvent in a fume hood overnight. The residual defatted material was then extracted with methanol (2 × 150 mL) on a shaker for 8 h at room temperature. The supernatant was then evaporated under reduced pressure and lyophilized, yielding a crude milkweed root extract designated as incarnatin (8 g). Incarnatin contained 30% (w:w) total pregnane glycosides as estimated by a xanthydrol-based colorimetric assay17 and no cardiac glycosides as confirmed by a negative Kedde reaction.18 Chromatographic separation of incarnatin into three subfraction pools of high (A), middle (B), and low (C) polarity was performed on preparatory scale HPLC using Phenomenex Luna C-8 reverse phase column, size 150 mm × 2 mm, particle size 3 μm, equipped with a Phenomenex SecurityGuard precolumn. The mobile phase consisted of two components, solvent A (0.5% acetic acid in deionized water, pH 3–3.5) and solvent B (100% acetonitrile), and was run in the gradient mode.
For isolation of individual pregnane glycosides, incarnatin was suspended in 50% methanol and sequentially extracted with hexane and ethyl acetate. The ethyl acetate fraction was separated by column chromatography using silica gel (230–400 mesh, Fisher Scientific) and eluted with dichloromethane–methanol system (99:1, 98:2, 97:3, 96:4, 95:5, and 90:10) to give 18 fractions. Fractions 8 and 9 were combined (240 mg) and further purified using semipreparative HPLC columns (Waters RP-8, C-8, 300 mm × 19 mm, 7 μm particle size and Synergy Hydro-RP 80A, C-18, 250 mm × 21.2 mm, 4 μm particle size) eluted with a step gradient of 20–95% acetonitrile in water to afford ike magenin 3,8,12,1 4-tetrahydroxypregn-5-en-20-one, (3β,12β,14β,17α)-form, 12-cinnamoyl, 3-O-[β-D-oleandropyranosyl-(1→4)-D-digitoxopyranosyl-(1→4)-β-digitoxopyranosyl-(1→4)-β-D-cymaropyranoside, which was confirmed by HPLC and liquid chromatography–mass spectrometry (LC-MS) analysis using an authentic sample from Analyticon Discovery (Potsdam, Germany). Chromatography was performed on a Waters LC-MS Integrity system (Milford, MA) consisting of a W616 pump, W600S controller, W717plus autosampler, W996 PDA detector, and Waters TMD Thermabeam electron impact mass detector. Data were collected and analyzed with the Waters Millennium 3.2 software linked with the sixth edition of the Wiley Registry of Mass Spectral Data (Figure 1A).
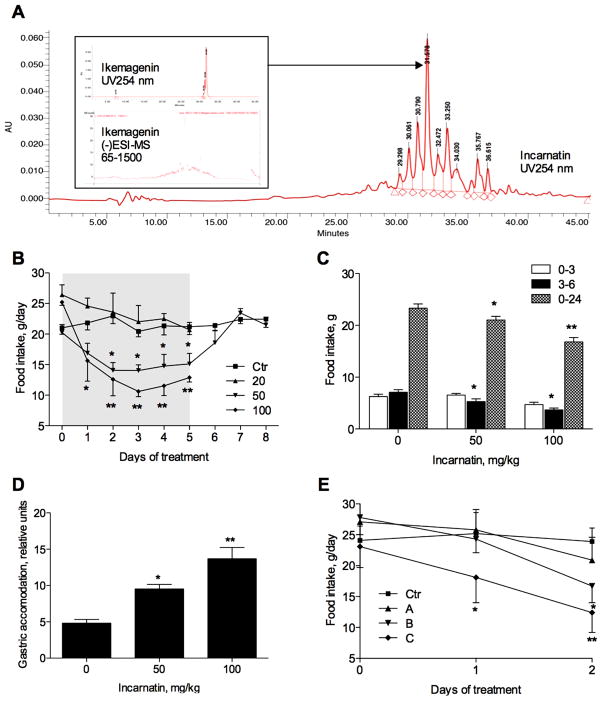
Figure 1.
Effect of A. incarnata root extract enriched with pregnane glycosides (incarnatin) on food intake in rats. (A) Chromatogram of incarnatin extract at 254 nm with (−)ESI LC-MS fragmentation of ikemagenin shown in the inset. (B) Daily food intake of male rats (n = 6) orally administered with vehicle (Ctr) or 20, 50, and 100 mg/kg/day of incarnatin during five dosing days (gray box) and three recovery days. (C) Fasting-induced food intake of male rats (n = 6) after a single dose of 50 or 100 mg/kg incarnatin during 0–3, 3–6, and 0–24 h time periods following the dosing. (D) Gastric accommodation of male rats (n = 6) 1 h after receiving a single dose of 50 or 100 mg/kg incarnatin. (E) Daily food intake of male rats (n = 4) orally administered with vehicle (Ctr) or incarnatin fractions of high (A), middle (B), and low (C) polarity. All values are means ± SEMs (n = 4–6). Asterisks indicate a significant difference (*p < 0.05, and **p < 0.01) from respective controls as determined by one-way ANOVA and Dunnett’s post-test.
Animals
Male Wistar rats (180–200 g) were obtained from Charles River Laboratories (Wilmington, MA). Rats were housed individually under controlled temperature (24 ± 2 °C) and light (12 h light–dark cycle, lights off at 1400 h) with access to AIN-93 standard diet (Research Diets, New Brunwick, NJ) and tap water ad libitum. Following an adaptation period of 1 week, animals were introduced to daily 4 h food deprivation (beginning at 1000 h) and a daily gavage at 1400 h until it was determined that the process of gavaging did not significantly affect the daily food uptake (1 week). The 4 h fasting time prior to gavage was introduced to eliminate variation in feeding patterns among different animals by ensuring that all animals did not consume food immediately before the treatment. For all studies, animals were randomized into groups (n = 6) with approximately equal mean body weight (within 2 g). Body weight and food consumed by each animal were determined daily by differential weighing after correcting for spillage on balances accurate to 0.01 g. All animal experimental procedures were carried out at the AAALAC-accredited Cook Animal Facility in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by Rutgers IACUC.
Dose Range Study
Four groups of rats (n = 6) fasted for 4 h received daily gavage for 5 consecutive days of control solution [10% dimethyl sulfoxide (DMSO) in corn oil] or 20, 50, and 100 mg/kg incarnatin suspended in a total volume of 2 mL of control solution at the beginning of the feeding cycle (lights off, 1400 h). This dose range was chosen based on the pharmacological activity of P57AS3 pregnane glycoside in rats.11 Food intake was measured daily for the duration of the experiment and corrected for spillage. Animals receiving 50 mg/kg of incarnatin were allowed to recover for 3 days following the termination of the treatment. All animals were sacrificed on day 5 of the treatment with the exception of the control (n = 3) and 50 mg/kg groups (n = 3) that were sacrificed on the day 8 of the experiment following completion of the recovery phase.
Fasting-Induced Feeding
Three groups of rats (n = 6) fasted for 24 h received a single per os dose of control solution (10% DMSO in corn oil) or 50 and 100 mg/kg incarnatin suspended in a total volume of 2 mL of control solution at the beginning of the feeding cycle (lights off, 1400 h), at which point animals were provided with food and water ad libitum. Food intake was measured 3, 6, and 24 h after the gavage treatment and corrected for spillage (day 1). Stomachs and stomach contents were collected and recorded 3 h after gavage with a respective dose of the incarnatin extract on day 2 of the experiment.
Gastric Emptying and Intestinal Transit
Three groups of rats (n = 6) fasted for 4 h received a single per os dose of control solution (10% DMSO in corn oil) or 50 and 100 mg/kg incarnatin suspended in a total volume of 2 mL of control solution at the beginning of the feeding cycle (lights off, 1400 h). Gastric emptying was determined by a modification of the phenol red method.19 Following a 30 min exposure to incarnatin, a solution of 20% peptone containing 0.05% phenol red as a marker was given intragastrically (1 mL/rat). Sixty minutes later, animals were sacrificed, the abdominal cavity was opened, the gastresophageal junction and the pylorus were clamped, and the stomach was removed, weighed, and placed in 14 mL of 0.1 N NaOH and homogenized. The suspension was allowed to settle for 1 h at room temperature, and then, 5 mL of the supernatant was added to 0.5 mL of 20% trichloroacetic acid (w/v) and centrifuged at 3000 rpm for 20 min. The supernatant was mixed with 4 mL of 0.5 N NaOH, and the amount of phenol red in the sample was determined from the absorbance at 560 nm on Synergy HT microplate reader (Biotek, Winooski, VT). At the same time, the small intestine was clamped on both ends and fully extended, and the distance from the pylorus to the geometric center of migrating dye was recorded.
Long-Term Feeding Study
Incarnatin was gavaged daily for 90 days to healthy rats with a 28 day recovery and paired feeding assessment by Covance Laboratories (Vienna, VA) (study no. 6731-133). Male and female Sprague–Dawley rats were assigned to five study groups (15/sex/group in groups 1, 4, and 5; 10/sex/group in groups 2 and 3). Groups 1 (control) and 5 (pair-fed control) received the vehicle, corn oil. Groups 2, 3, and 4 received incarnatin at dose levels of 20, 40, and 50 mg/kg/day, respectively. Dose preparations were administered at a dose volume of 5 mL/kg via oral gavage once daily to rats for at least 90 days. During the dosing phase, groups 4 and 5 were pair-fed (mean food consumption of the group 4 was used to determine the quantity of food provided to the group 5). Additional animals designated for recovery phase (5/sex/group in groups 1, 4, and 5) underwent at least 28 days of recovery following dose administration. Mortality, clinical observations, body weight, and food and water consumption were assessed.
Ikemagenin Feeding Study
When animals were treated with ikemagenin, two groups of rats (n = 3) fasted for 24 h received a single per os dose of control solution (10% DMSO in corn oil) or 10 mg/kg ikemagenin suspended in a total volume of 2 mL of control solution at the beginning of the feeding cycle (lights off, 1400 h). The food intake was measured 3 h after the gavage treatment, at which point animals were sacrificed, and the hypothalamus was dissected and processed for RNA and protein extraction.
Cell Culture
The rat glioma C6 cell line that expresses BDNF was obtained from ATCC (Manassas, VA). Cells were routinely passaged every 3–4 days and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 0.1% penicillin–streptomycin at 37 °C and 5% CO2. Cells were subcultured into 24-well dishes and, once subconfluent, exposed to fresh DMEM medium containing vehicle (0.1% ethanol) or various concentrations of ikemagenin (0.1–10 μM) added to a set of three wells per dose. This dose range was chosen based on the pharmacological activity of ikemagenin in H295R cells.20 Valproic acid (2 mM) was used as a positive control. The cell viability was estimated using the MTT assay21 by absorbance read at 550 nm on a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA).
Gene Expression Analysis
Total RNA was isolated from the brain samples or scraped cell culture using Trizol reagent (Invitrogen, Carlsbad, CA) and quantified by absorption measurements at 260 and 280 nm using the NanoDrop system (NanoDrop Technologies Inc., Wilmington, DE). The quality of RNA was assessed by gel electrophoresis. RNA was then treated with DnaseI (Invitrogen) to remove traces of DNA contamination, and the cDNAs were synthesized with 2.5 μg of RNA using Stratascript reverse transcriptase (Stratagene) according to the manufacturer’s protocols. Quantitative polymerase chain reaction (PCR) was performed in duplicate essentially as described22 using the following gene-specific primers (IDT, Coralville, IA) selected using the Primer Express 2.0 software (Applied Biosystems, Foster City, CA): GAPDH: forward primer, 5′-CAG TGC CAG CCT CGT CTC AT-3′; reverse primer, 5′-AGG GGC CAT CCA CAG TCT TC-3′; POMC: forward primer, 5′-TGC CTT TCC GCG ACA GA-3′; reverse primer, 5′-CCT GAG CGA CTG TAG CAG AAT CT-3′; AGRP: forward primer, 5′-AAG CTT TGG CAG AGG TGC TAG AT-3′; reverse primer, 5′-AGG ACT CGT GCA GCC TTA CAC-3′; NPY: forward primer, 5′-CAC AGA AAA TGC CCC CAG AA-3′; reverse primer, 5′-TCA GGA GAG CAA GTT TCA TTT CC-3′; BDNF: forward primer, 5′-GGC CCA ACG AAG AAA ACC AT-3′; reverse primer, 5′-AGG CAC TTG ACT GCT GAG CAT-3′; CART: forward primer, 5′-GCC AAG TCC CCA TGT GTG AC-3′; reverse primer, 5′-CAC CCC TTC ACA AGC ACT TCA-3′. All primers were designed with the target hybridization temperature of 55–60 °C and amplicon length of 100–250 bp. Samples were subjected to a melting curve analysis to confirm the amplification specificity. The relative change in the target gene with respect to the endogenous control gene was determined using the 2ΔΔCT method.23
Western Blot Analysis
Whole cell or tissue extracts were prepared in ice-cold lysis buffer (62.5 mM Tris·HCl pH 6.8, 2% w/v SDS, 10% glycerol, 50 mM DTT, and 0.01% w/v bromophenol blue) and centrifuged at 12000g for 20 min at 4 °C. Equal amounts of protein (50 μg) from the supernatants were separated on SDS 10% polyacrylamide gels and blotted onto the nitrocellulose membrane. Western blot analysis was performed with monoclonal antibodies for BDNF (Abcam, Cambridge, MA) and AGRP (Santa Cruz Biotechnology, Santa Cruz, CA) according to the manufacturer’s instructions. After being washed, the blots were incubated with an antirabbit peroxidase-labeled secondary antibody and visualized using ECL Western Blotting Detection Reagent (GE Healthcare, Piscataway, NJ). After being stripped, the blots were probed with β-actin antibodies (Santa Cruz) to serve as a loading control.
Statistics
Data are represented as mean ± standard errors of the mean (SEMs). Statistical analyses were performed using GraphPad Prism 4.0 (San Diego, CA) using Student’s t test or one-way analysis of variance (ANOVA) (as appropriate). Body weight was analyzed with one-way repeated-measures ANOVA with posthoc testing to localize significant differences at each time point. P values of less than 0.05 were considered significant.
RESULTS
Pregnane Glycosides from Swamp Milkweed Roots Reduce Food Intake
To assess the appetite-suppressing properties of swamp milkweed pregnane glycosides15,16 in healthy rodents, we orally administered different doses of incarnatin extract (30% pregnane glycosides, Figure 1A) in the beginning of the dark phase for 5 consecutive days (Figure 1B). A significant reduction in food intake was seen in animals receiving 50 and 100 mg/kg per day of incarnatin (p < 0.05). Once incarnatin treatment was terminated on day 5 of the study, half of the animals in the control and 50 mg/kg groups were allowed to recover for an additional 3 days. Food intake in these animals returned to normal within 24–48 h. Behavioral observation 0–4 h postgavage revealed no significant changes in grooming and activity as compared to animals receiving vehicle solution.
Next, we tested incarnatin’s ability to suppress fasting-induced feeding using 50 and 100 mg/kg doses based on the results of the dose ranging study. In the first 3 postprandial hours, incarnatin treatment was associated with reduced food intake that did not reach significance (Figure 1C). In the next 3 postprandial hours (time period between 3 and 6 h following the administration of the extract), fasting-induced feeding was reduced by 25.4 ± 6.2 and 51.7 ± 8.1% with 50 and 100 mg/kg incarnatin treatment, respectively (p < 0.05). The effect lasted at least 24 h, since both doses resulted in significant decreases of food intake of 9.4 ± 3.7 (p < 0.05) and 28.3 ± 6.1% (p < 0.01) at the end of the experiment. A significant and dose-dependent increase in gastric accommodation was seen with incarnatin treatment (Figure 1D).
Effect of Incarnatin Subfractions on Food Intake
Three fractions of different polarity obtained from incarnatin by reverse phase HPLC were then tested for appetite suppression at 100 mg/kg dose. Food intake was reduced after 1 day of dosing in both middle (B) and low (C) polarity fractions; however, the decrease reached significance only in animals receiving fraction C (Figure 1E). After 2 days of treatment, animals receiving fraction B reduced their daily food intake by 30.1 ± 4.8% (p < 0.05), while animals receiving fraction C consumed 47.1 ± 8.5% less food as compared to the control group (p < 0.01). No appetite-suppressing activity was observed with the polar fraction A. Using LC-MS analysis, we determined that both fractions B and C contained predominantly pregnane glycosides, while polar fraction A was essentially free of pregnanes (not shown). These data suggest that enhanced satiety observed in rats treated with incarnatin may be attributed to pregnane glycosides.
Effect of Pregnane Glycosides on Gastrointestinal Transit
The dose-ranging and fasting-induced feeding studies indicated that administration of the incarnatin extract enriched with pregnane glycosides induced postprandial relaxation of the stomach, suggesting that incarnatin affects food intake in part by modulating gastric emptying. Indeed, administration of a single dose of 50 or 100 mg/kg incarnatin inhibited gastric emptying of the test meal (Figure 2A). The inhibition was greater at a dose of 100 mg/kg. At the same time, the value of the mean geometric center, an indirect measure of intestinal transit, was significantly higher in rats receiving 50 or 100 mg/kg of incarnatin (Figure 2B). Colonic motor function, as measured by the number of fecal pellets expelled by each animal in 1 h following administration of incarnatin to rats, increased dose dependently (Figure 2C).
Figure 2.
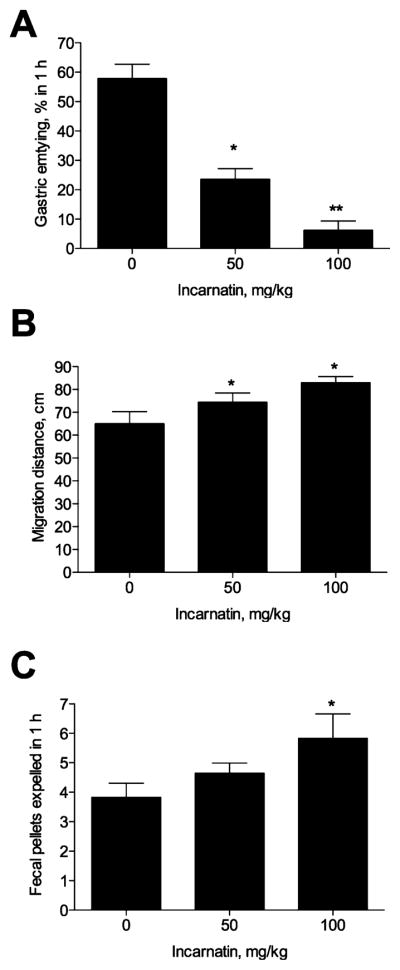
Effect of A. incarnata root extract enriched with pregnane glycosides (incarnatin) on gastrointestinal food transit. (A) Gastric emptying of male rats (n = 6) 1 h after receiving a single dose of 50 or 100 mg/kg incarnatin. (B) Intestinal transit of male rats (n = 6) as measured by the location of the mean geometric center of migrating dye after receiving a single dose of 50 or 100 mg/kg incarnatin. (C) Colonic motor function of male rats (n = 6) measured by the number of fecal pellets expelled by each animal in 1 h following the administration of 50 or 100 mg/kg incarnatin. Asterisks indicate a significant difference (*p < 0.05, and **p < 0.01) from respective controls as determined by one-way ANOVA and Dunnett’s post-test.
Effect of Ikemagenin on Food Intake and Central Satiety Peptides
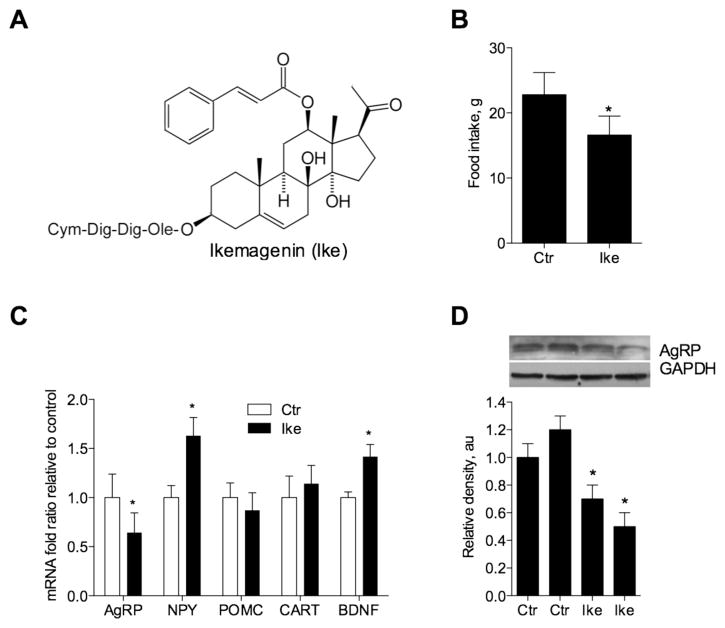
To confirm that appetite-suppressing properties of swamp milkweed can be attributed to pregnane glycosides, we purified the most abundant pregnane glycoside found in milkweed root extract and identified it as ikemagenin, 12β-cinnamoyl-3,8,12,14β-tetrahydroxypregn-5-en-20-one-Ole-Dig-Dig-Cym (Figure 1A). Ikemagenin is a pregnane glycoside with a typical steroidal core, a cinnamoyl moiety attached at C12 position, and four saccharide moieties attached at the C3 position (Figure 3A).
Figure 3.
Effect of a pregnane glycoside (ikemagenin) on food intake and levels of central satiety peptides in the hypothalamus. (A) Chemical structure of ikemagenin showing four saccharide moieties attached at the C3 position including oleandrose (Ole), digitoxose (Dig), and cymarose (Cym). (B) Daily food intake of male rats (n = 4) receiving a single oral dose of 10 mg/kg ikemagenin. (C) Hypothalamic mRNA expression of anorexic (POMC, CART, and BDNF) and orexigenic (AgRP and NPY) central satiety peptides in male rats (n = 4) treated with vehicle (Ctr) or a single oral dose of 10 mg/kg ikemagenin (Ike). (D) The hypothalamic AgRP protein level decreased following a single oral dose of 10 mg/kg ikemagenin as measured by Western blot analysis and was quantified densitometrically using GAPDH as a loading control. Two representative animals from vehicle (Ctr) or ikemagenin (Ike)-treated groups are shown. Asterisks indicate a significant difference (*p < 0.05) from respective controls as determined by Student’s t test.
Oral administration of ikemagenin at a single dose of 10 mg/kg reduced food intake in rats by 27.2 ± 6.4% relative to the vehicle-treated control group (p < 0.05) (Figure 3B). This effect was associated with the favorable changes in the mRNA levels of the central satiety peptides in the hypothalamus of the treated animals. The mean AgRP mRNA fold difference normalized to GAPDH expression fell from 1.00 ± 0.23 in vehicle-treated rats to 0.64 ± 0.20 in ikemagenin-treated animals (p < 0.05). The mean BDNF mRNA fold difference increased from 1.00 ± 0.06 in vehicle-treated rats to 1.42 ± 0.12 in ikemagenin-treated animals (p < 0.05). No changes in mean mRNA levels were observed for both POMC and CART. The mean NPY mRNA fold difference increased from 1.00 ± 0.12 in vehicle-treated rats to 1.63 ± 0.18 in ikemagenin-treated animals (Figure 3C). A decrease of AgRP protein in vivo following the treatment with ikemagenin was confirmed by Western blot analysis (Figure 3D).
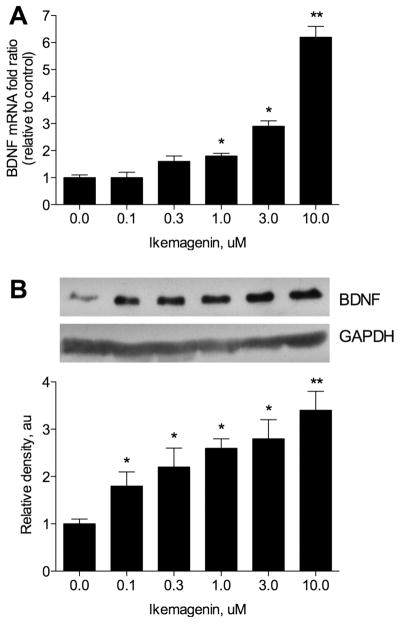
Effect of Ikemagenin on BDNF Levels in Cell Culture
BDNF is a central anorectic peptide that functions downstream of melanocortin receptors.5 To confirm a direct effect of ikemagenin on the BDNF expression, we tested the BDNF-stimulatory activity of ikemagenin in the C6 rat glioma cells (Figure 4A). Ikemagenin dose dependently enhanced BDNF mRNA levels up to 6-fold relative to vehicle-treated cells. Valporic acid served as a positive control in this assay (2 mM) and increased BDNF mRNA levels 2.1-fold under identical conditions. An increase of BDNF protein in vitro following the treatment with ikemagenin was confirmed by Western blot analysis (Figure 4B).
Figure 4.
Direct effect of ikemagenin on BDNF levels in C6 glioma cells. (A) Dose-dependent increase of BDNF mRNA levels in response to 8 h of treatment with 0–10 μM ikemagenin. (B) Western blot analysis of BDNF levels following 24 h of treatment with 0–10 μM ikemagenin following densitometric quantification using GAPDH as a loading control. Asterisks indicate a significant difference (*p < 0.05, and **p < 0.01) from respective controls as determined by one-way ANOVA and Dunnett’s post-test.
Long-Term Effect of Incarnatin on Feeding
This experiment evaluated the long-term efficacy of incarnatin (20, 40, and 50 mg/kg/day) when administered daily via oral gavage to rats for at least 90 days as compared with paired feeding assessment (this group was pair-fed to the 50 mg/kg/d incarnatin group). We also assessed the reversibility, persistence, or delayed occurrence of feeding behavior after at least 28 days of recovery. Incarnatin treatment (50 mg/kg/day) was associated with 5% reduction in daily food intake in male rats and 14% in female rats throughout the 90 day dosing phase of the study (Table 1). A residual nonsignificant 2% decrease in food intake was also observed during the recovery phase in males, while female rats overcompensated food intake. Body weight and body weight gain decreased with incarnatin administration by 6 and 10%, respectively, in males and by 3 and 9% in females but did not reach significance for any days of treatment (Figure 5). These values were also in close agreement with those from the pair-fed group, suggesting that the change in food intake alone is sufficient to explain the observed reduction in body weight and body weight gain associated with incarnatin administration. The mean water intake was increased in both sexes during the dosing phase of the experiment but did not rich significance and was not observed during the recovery phase.
Table 1.
Results of the Feeding Study Comparing the Effects of 50 mg/kg/day Incarnatin Treatment (50) vs Vehicle-Treated (Ctr) and Pair-Fed Groups (50P) during the 90 Day Dosing and 28 Day Recovery Phases Showing Significant Changes in Food Intake and Body Weight
| parameter | male
|
female
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dosing
|
recovery
|
dosing
|
recovery | |||||||||
| Ctr | 50 | 50P | Ctr | 50 | 50P | Ctr | 50 | 50P | Ctr | 50 | 50P | |
| body weight (g) | 541 ± 57 | 507 ± 34a | 495 ± 31a | 562 ± 44 | 543 ± 64 | 531 ± 27 | 286 ± 30 | 277 ± 25 | 275 ± 21 | 293 ± 36 | 320 ± 15a,b | 295 ± 16 |
| body weight gain (g) | 330 ± 31 | 297 ± 25a | 284 ± 29a | 12 ± 12 | 16 ± 14 | 26 ± 8 | 106 ± 28 | 96 ± 23 | 92 ± 19 | 8 ± 7 | 25 ± 13a,b | 8 ± 7 |
| food intake (g/day) | 20.0 ± 2.8 | 19.0 ± 1.7 | 27.0 ± 9.7 | 26.4 ± 3.2 | 27.3 ± 1.9 | 15.0 ± 2.4 | 13.0 ± 1.4a | 20.6 ± 2.7 | 24.1 ± 0.8a,b | 20.0 ± 0.5 | ||
| water intake (g/day) | 32.8 ± 8.2 | 35.0 ± 8.0 | 34.3 ± 7.6 | 33.3 ± 2.4 | 33.0 ± 3.1 | 34.0 ± 3.3 | 24.5 ± 9.3 | 30.3 ± 5.7 | 32.3 ± 9.8 | 37.0 ± 9.8 | 43.7 ± 4.9 | 39.3 ± 9.7 |
Significantly different from the control value.
Significantly different from the pair-fed value (p < 0.05).
Figure 5.
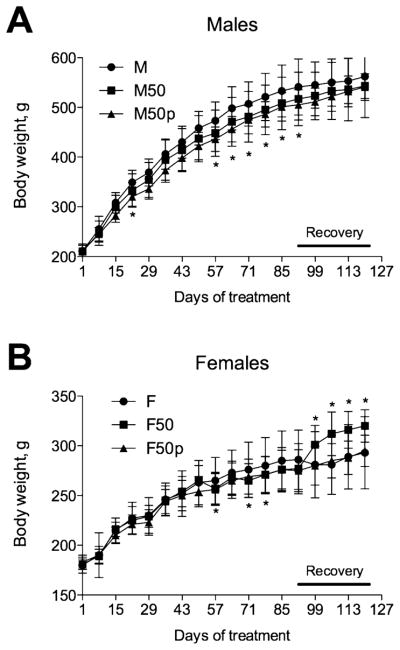
Dose–response curve of body weight changes to incarnatin administration during 90 days of dosing and 28 days of recovery phase of the subchronic toxicity study. (A) Male or (B) female rats (n = 15) were orally administered with vehicle (Ctr) or 50 mg/kg per day of incarnatin. Ten animals from each group were sacrificed following a 90 day dosing phase, while five animals from control and 50 mg/kg incarnatin groups were allowed to recover for an additional 28 days. All values are means ± SEMs (n = 5–10). Asterisks indicate a significant difference (*p < 0.05) from control animals.
DISCUSSION
The present study shows that feeding incarnatin extract enriched with pregnane glycosides from roots of swamp milkweed A. incarnata (50 and 100 mg/kg/day) dose dependently reduced both spontaneous and fasting-induced food intake in rats. Bioactivity-guided fractionation of the extract narrowed down the appetite-suppressing activity to fractions containing predominantly pregnane glycosides (Figure 1). Therefore, this study demonstrated for the first time swamp milkweed as an alternative abundant source of biologically active pregnane glycosides that suppress food intake in mammals. Previous studies identified pregnane glycoside P57AS3 as the active constituent of the slow-growing succulent plant H. gordonii11,12 that acts as an appetite suppressant. Chemical fingerprint, bioavailability, and pharmacokinetics of the P57AS3 pregnane glycoside have also been reported.24 However, no studies identified cellular and molecular targets of pregnane glycosides or examined the specific mechanism by which they affect appetite.
Reduced food intake associated with oral administration of incarnatin was observed together with a profound increase in gastric accommodation and delay of gastric emptying (Figure 2). The proximal stomach produces low-frequency, sustained contractions that generate a pressure gradient from the stomach to the small intestine and is thus responsible for gastric emptying. This pattern of gastric motility results from smooth muscle cells integrating a large number of chemical, hormonal, and neural signals, the latter ones originating predominantly from the enteric nervous system.25 A similar set of symptoms consisting of delayed liquid gastric emptying, relaxation of the proximal stomach, and accelerated small intestinal transit has been described previously in humans for serotonin receptor agonist MKC-73326 and metformin.27 The speed of gastric emptying is important in the regulation of food intake and glucose homeostasis, especially in obese subjects.28 Consequently, slowing gastric emptying reduces food intake by prolongation of a vagal nerve-mediated satiety reflex.29 The exact mechanism of how pregnane glycosides affect gastric emptying remains to be investigated.
Oral administration of ikemagenin, the most abundant pregnane glycoside from milkweed root extract, at a single dose of 10 mg/kg reduced food intake in rats by 27% relative to vehicle-treated control group. While ikemagenin is analogous to the bioactive P57AS3 pregnane glycoside isolated from Hoodia,11 it possesses several unique structural properties. These include a bulky lipophilic cinnamoyl moiety attached at the C12 position, additional hydroxylation at C18 position, and four rare deoxysugar moieties (oleandrose, digitoxose, and cymarose) attached at the C3 position (Figure 3). A number of other bioactive compounds possess deoxysugars attached to their aglycones; in many cases, these deoxysugars are essential for their biological activity as in the case of cardiac glycosides,30 antibiotics,31 or antitumor agents.32 Although incarnatin treatment achieved stronger suppression of food intake in females than in males (Figure 5), its effect on body weight and body weight gain was similar for both genders, indicating that female rats compensated for reduced food intake with an unknown mechanism. It is generally recognized that males and females differ in eating behavior,33 and several gender differences in postprandial satiation and satiety responses have been described previously.34
The present study also shows that the effects of orally administered ikemagenin are also rapidly transmitted to central tissues including hypothalamus, where the expression of orexigenic and anorexic neuropeptides is affected. Ikemagenin caused a decrease in hypothalamic AgRP mRNA levels and increase in the BDNF mRNA levels without an effect on POMC and CART mRNA (Figure 3). AgRP is a crucial part of the melanocortin system involved in regulation of food intake and energy balance through antagonistic effects on melanocortin 3 and 4 receptors that stimulate a long-lasting increase in food intake.35 The NPY/AgRP and POMC systems alter metabolic homeostasis by regulation of gene transcription, excitability, and synaptic transmission by projecting to areas such as the paraventricular nucleus (PVN) and lateral hypothalamic area (LHA), where further integration influences circuits responsible for energy homeostasis. It is somewhat surprising that ikemagenin treatment reduced food intake even though NPY levels remained elevated (Figure 3). Because global deletion of the NPY gene produces a weak phenotype36 in comparison to transgenes that target the melanocortin system,37 it is likely that parallel induction of two anorexigenic neuromediators (AgRP and BDNF) overrides NPY increase.
On the other hand, hypothalamic BDNF, a neuronal survival, differentiation, and plasticity factor that serves as an essential constituent of central neural circuits involved in regulating energy homeostasis,38 increased following ikemagenin treatment (Figure 4). While we assayed BDNF expression in vivo only at the mRNA level (Figure 3C), cell culture data on changes in mRNA and protein levels of BDNF in response to ikemagenin treatment suggest that induction of BDNF protein is evident at a lower doses as compared to the mRNA induction (Figure 4). It is therefore likely that ikemagenin treatment may affect post-transcriptional control of BDNF mRNA in the brain.39 Several lines of evidence suggest a link between the melanocortin signaling and the BDNF/TrkB signaling. The obese phenotype displayed by TrkB hypomorph mice is strikingly similar to the one observed in mouse mutants with an impaired melanocortin signaling (Ay and MC4R−/−), and intracerebroventricular infusion of BDNF suppresses the hyperphagia and excessive weight gain observed on higher-fat diets in Ay mice with deficient MC4R signaling both in hypothalamus5 and in the brainstem satiety reflex center.40 In a previous study, pregnane glycoside was directly injected into the brain of the rats, and an increase in the ATP content in the hypothalamus region of the brain that controls the food intake has been proposed to be a possible mode of action.12 Our results neither confirm nor contradict these data since ATP is known to elicit BDNF gene expression41 and AgRP gene expression is inhibited by glucose, apparently mediated by AMP-activated protein kinase in cell culture.42
Collectively, the results presented here demonstrate that pregnane glycoside treatment has several effects on food intake that are possibly mediated by hypothalamic neuropeptides. Although the precise mechanism is not known, both genomic analysis and functional assays suggest that the cellular mechanisms operative in vivo in the hypothalamus to enhance satiety may be either directly affected by pregnane glycosides (as in case of BDNF) or may be secondary to the modulation of gastrointestinal transit through stimulation of vagal afferent terminals. Understanding the effect of pregnane glycosides could lead to a better understanding of the mechanisms underlying feeding behavior and could potentially lead to new therapeutic targets for weight loss.
Acknowledgments
Funding
The analyses were supported in part by the NIH Botanical Research Center Grant 2P50AT002776-06 from the National Center for Complementary and Alternative Medicine (NCCAM).
This manuscript is submitted in the loving memory of Dmitry Govorko and Joseph M. O’Neil who contributed to this work. We are grateful to Ivan Jenkins for greenhouse help and Reni Pouleva for technical assistance with cell culture.
Footnotes
The authors declare no competing financial interest.
References
- 1.Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray GA, Bouchard C. Handbook of Obesity: Ethiology and Pathophysiology. Marcel Dekker Inc; New York: 2005. p. 1046. [Google Scholar]
- 3.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 4.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 5.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang DJ, Chang YY, Hsu CL, Liu CW, Lin YL, Lin YH, Liu KC, Chen YC. Antiobesity and hypolipidemic effects of polyphenol-rich longan (Dimocarpus longans Lour.) flower water extract in hypercaloric-dietary rats. J Agric Food Chem. 2010;58:2020–2027. doi: 10.1021/jf903355q. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu K, Ida T, Tsutsui H, Asai T, Otsubo K, Oku N. Anti-obesity Effect of Phosphatidylinositol on Diet-Induced Obesity in Mice. J Agric Food Chem. 2010;58:11218–11225. doi: 10.1021/jf102075j. [DOI] [PubMed] [Google Scholar]
- 8.Yoon MY, Choi NH, Min BS, Choi GJ, Choi YH, Jang KS, Han SS, Cha B, Kim JC. Potent in vivo antifungal activity against powdery mildews of pregnane glycosides from the roots of Cynanchum wilfordii. J Agric Food Chem. 2011;59:12210–12216. doi: 10.1021/jf2039185. [DOI] [PubMed] [Google Scholar]
- 9.Holt S, Taylor TV. Hoodia gordonii: An overview of biological and botanical characteristics. Townsend Lett. 2006;280:104–113. [Google Scholar]
- 10.Kuriyan R, Raj T, Srinivas SK, Vaz M, Rajendran R, Kurpad AV. Effect of Caralluma fimbriata extract on appetite, food intake and anthropometry in adult Indian men and women. Appetite. 2007;48:338–344. doi: 10.1016/j.appet.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 11.van Heerden FR, Marthinus Horak R, Maharaj VJ, Vleggaar R, Senabe JV, Gunning PJ. An appetite suppressant from Hoodia species. Phytochemistry. 2007;68:2545–2553. doi: 10.1016/j.phytochem.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 12.MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: Studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020:1–11. doi: 10.1016/j.brainres.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 13.Woodson RE. The North American Species of Asclepias L. Ann Missouri Bot Garden. 1954;41:1–211. [Google Scholar]
- 14.Herrick JW. PhD Thesis. State University of New York; Albany: 1977. Iroquois Medical Botany. [Google Scholar]
- 15.Warashina T, Noro T. Steroidal glycosides from the aerial part of Asclepias incarnata. Phytochemistry. 2000;53:485–498. doi: 10.1016/s0031-9422(99)00560-9. [DOI] [PubMed] [Google Scholar]
- 16.Warashina T, Noro T. Cardenolide and oxypregnane glycosides from the root of Asclepias incarnata L. Chem Pharm Bull (Tokyo) 2000;48:516–524. doi: 10.1248/cpb.48.516. [DOI] [PubMed] [Google Scholar]
- 17.Aitova R, Maslennikova V, Abubakirov N. Determination of 2-deoxyaldoses and their natural glycosides with the aid of xanthydrol. Chem Nat Compd. 1973;9:605–609. [Google Scholar]
- 18.Stahl E. Drug Analysis by Chromatography and Microscopy. Ann Arbor Science Publishers; Ann Arbor, MI: 1973. p. 238. [Google Scholar]
- 19.Shi G, Leray V, Scarpignato C, Bentouimou N, Bruley des Varannes S, Cherbut C, Galmiche JP. Specific adaptation of gastric emptying to diets with differing protein content in the rat: Is endogenous cholecystokinin implicated? Gut. 1997;41:612–618. doi: 10.1136/gut.41.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komarnytsky S, Esposito D, Poulev A, Raskin I. Pregnane glycosides interfere with steroidogenic enzymes to down-regulate corticosteroid production in human adrenocortical H295R cells. J Cell Physiol. 2012 doi: 10.1002/jcp.24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Govorko D, Logendra S, Wang Y, Esposito D, Komarnytsky S, Ribnicky D, Poulev A, Wang Z, Cefalu WT, Raskin I. Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol Endocrinol Metab. 2007;293:E1503–E1510. doi: 10.1152/ajpendo.00420.2007. [DOI] [PubMed] [Google Scholar]
- 23.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 24.Madgula VL, Ashfaq MK, Wang YH, Avula B, Khan IA, Walker LA, Khan SI. Bioavailability, Pharmacokinetics, and Tissue Distribution of the Oxypregnane Steroidal Glycoside P57AS3 (P57) from Hoodia gordonii in Mouse Model. Planta Med. 2010;76:1582–1586. doi: 10.1055/s-0030-1249818. [DOI] [PubMed] [Google Scholar]
- 25.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman NS, Marciani L, Blackshaw E, Wright J, Parker M, Yano T, Yamazaki S, Chan PQ, Wilde K, Gowland PA, Perkins AC, Spiller RC. Effect of a novel 5-HT3 receptor agonist MKC-733 on upper gastrointestinal motility in humans. Aliment Pharmacol Ther. 2003;18:1039–1048. doi: 10.1046/j.1365-2036.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- 27.Cubeddu LX, Bönisch H, Göthert M, Molderings G, Racké K, Ramadori G, Miller KJ, Schwörer H. Effects of metformin on intestinal 5-hydroxytryptamine (5-HT) release and on 5-HT3 receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;361:85–91. doi: 10.1007/s002109900152. [DOI] [PubMed] [Google Scholar]
- 28.Tosetti C, Corinaldesi R, Stanghellini V, Pasquali R, Corbelli C, Zoccoli G, Di Febo G, Monetti N, Barbara L. Gastric emptying of solids in morbid obesity. Int J Obes Relat Metab Disord. 1996;20:200–205. [PubMed] [Google Scholar]
- 29.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 31.Salah-Bey K, Doumith M, Michel JM, Haydock S, Cortes J, Leadlay PF, Raynal MC. Targeted gene inactivation for the elucidation of deoxysugar biosynthesis in the erythromycin producer Saccharopolyspora erythraea. Mol Gen Genet. 1998;257:542–553. doi: 10.1007/s004380050680. [DOI] [PubMed] [Google Scholar]
- 32.Otten SL, Gallo MA, Madduri K, Liu X, Hutchinson CR. Cloning and characterization of the Streptomyces peucetius dnmZUV genes encoding three enzymes required for biosynthesis of the daunorubicin precursor thymidine diphospho-L-daunosamine. J Bacteriol. 1997;179:4446–4450. doi: 10.1128/jb.179.13.4446-4450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991;10:133–142. doi: 10.1037//0278-6133.10.2.133. [DOI] [PubMed] [Google Scholar]
- 34.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83:1297–1305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 35.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 36.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 37.Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- 38.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci US A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma B, Culver BP, Baj G, Tongiorgi E, Chao MV, Tanese N. Localization of BDNF mRNA with the Huntington’s disease protein in rat brain. Mol Neurodegener. 2010;5:22. doi: 10.1186/1750-1326-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–5620. doi: 10.1210/en.2005-0419. [DOI] [PubMed] [Google Scholar]
- 41.Takasaki I, Takarada S, Tatsumi S, Azegami A, Yasuda M, Fukuchi M, Tabuchi A, Kondo T, Tabuchi Y, Tsuda M. Extracellular adenosine 5′-triphosphate elicits the expression of brain-derived neurotrophic factor exon IV mRNA in rat astrocytes. Glia. 2008;56:1369–1379. doi: 10.1002/glia.20704. [DOI] [PubMed] [Google Scholar]
- 42.Lee K, Li B, Xi X, Suh Y, Martin RJ. Role of neuronal energy status in the regulation of adenosine 5′-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology. 2005;146:3–10. doi: 10.1210/en.2004-0968. [DOI] [PubMed] [Google Scholar]