Abstract
The increasing use of antipsychotics (APs) to treat pediatric psychiatric conditions has led to concerns over the long-term tolerability of these drugs. While the risk of cardiometabolic abnormalities has received most of the attention, preclinical and clinical studies provide preliminary evidence that APs can adversely impact bone metabolism. This would be most concerning in children and adolescents as suboptimal bone accrual during development may lead to increased fracture risk later in life. However, the potential mechanisms of action through which APs may impact bone turnover and, consequently, bone mineral content are not clear. Emerging data suggest that the skeletal effects of APs are complex, with APs directly and indirectly impacting bone cells through modulation of multiple signaling pathways, including those involving dopamine D2, serotonin, adrenergic, and prolactin receptors, as well as by affecting gonadotropins. Determining the action of APs on skeletal development is further complicated by polypharmacy. In children and adolescents, APs are frequently coprescribed with psychostimulants and selective serotonin reuptake inhibitors, which have also been linked to changes in bone metabolism. This review discusses the mechanisms by which APs may influence bone metabolism. Also covered are preclinical and pediatric findings concerning the impact of APs on bone turnover. However, the dearth of clinical information despite the potential public health significance of this issue underscores the need for further studies. The review ends with a call for clinicians to be vigilant about promoting optimal overall health in chronically ill youth with psychopathology, particularly when pharmacotherapy is unavoidable.
Keywords: antipsychotics, bone metabolism, hyperprolactinemia, osteoporosis, psychopathology, skeletal development
Introduction
The use of antipsychotic (AP) medications in children and adolescents has rapidly grown over the last two decades, with second-generation APs forming the lion’s share [Comer et al. 2010; Olfson et al. 2010, 2012]. In fact, within certain clinical groups, up to 4% of children are receiving an AP [Cooper et al. 2006; Crystal et al. 2009]. This widespread use likely reflects the increasing evidence supporting APs’ efficacy in a variety of psychiatric conditions, optimizing functioning, and possibly reducing the need for institutionalization [FDA, 2006, 2007, 2009; Zuddas et al. 2011]. However, concerns have been raised about the long-term safety of APs, particularly since many pediatric psychiatric conditions are chronic, often requiring extended treatment [Vitiello et al. 2009]. In fact, across a variety of disorders, symptoms recur following the discontinuation of the AP or even despite continued therapy [Research Units on Pediatric Psychopharmacology Autism Network, 2005; Reyes et al. 2006a; Findling et al. 2010].
Much attention has been paid to AP-related weight gain and cardiometabolic abnormalities, particularly in children and adolescents [Calarge et al. 2009a; Correll et al. 2009]. However, less research has explored other potential long-term side effects such as impaired skeletal development. This is of significance in light of accumulating evidence in adults implicating APs in suboptimal bone mineral density (BMD) [Bilici et al. 2002; Abraham et al. 2003; Becker et al. 2003; Meaney et al. 2004; Howes et al. 2005; Jung et al. 2006; Meaney and O’Keane, 2007; Kishimoto et al. 2008]. If AP treatment were to begin earlier in life, children and adolescents may be prevented from optimizing their peak bone mass and placed at a heightened risk for the later emergence of osteoporosis [NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, 2001]. Osteoporosis is a taxing condition both financially, with costs estimated at US$10–15 billion annually in the USA for treatment of fractures alone, as well as personally due to reduced quality of life and increased morbidity and mortality [NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, 2001].
This paper briefly describes skeletal development to highlight the importance of optimizing peak bone mass, reviews the mechanisms through which APs might affect bone metabolism, summarizes the evidence linking APs to skeletal health in animals as well as in children and adolescents, and ends by underscoring the need for clinicians to be mindful of the potential long-term implications of the skeletal effects of psychotropics.
Bone mineral density during development
Peak bone mass achieved by early adulthood is a strong predictor of future BMD [NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, 2001]. More than 85% of peak skeletal mass is accrued before age 18, making bone development during this phase critical for lifelong skeletal health [Theintz et al. 1992; Rauch and Schoenau, 2001]. Importantly, failure to achieve peak bone mass before young adulthood (e.g. in youth with prolactin-secreting adenomas) cannot necessarily be compensated for at a later stage [Colao et al. 2000]. Moreover, age-related bone loss is directly correlated with peak bone mass and even a 5–10% reduction in peak BMD (equivalent to a reduction of BMD between 0.5 and 1 SD) can increase the incidence of future fractures substantially [Matkovic et al. 1995; Matkovic, 1996]. In sum, whether genetic or environmental, processes that impact bone mass accrual during development have the potential to increase the lifetime risk of osteoporosis and fractures [Carrie Fassler and Bonjour, 1995; Duntas, 2001].
Mechanisms potentially linking antipsychotics to bone metabolism
Most APs block the dopamine D2 receptors [Richelson and Souder, 2000]. Dopamine released by tuberoinfundibulum neurons in the arcuate nucleus of the mediobasal hypothalamus activate dopamine D2 receptors on pituitary lactotrophs, tonically inhibiting prolactin release [Halbreich et al. 2003; Shibli-Rahhal and Schlechte, 2009]. Thus, during AP treatment, hyperprolactinemia often ensues, particularly since lactotrophs dopamine D2 receptors are highly sensitive to the D2-blocking activity of APs [Langer et al. 1977]. Amenorrhea due to prolactin-secreting pituitary adenomas is associated with low spinal bone mass [Shibli-Rahhal and Schlechte, 2009]. Hyperprolactinemia may inhibit the pulsatile secretion of gonadotropin-releasing hormone, thereby impairing gonadotropin secretion and causing hypogonadism [Klibanski et al. 1980; Biller et al. 1992; Schlechte et al. 1992; Shibli-Rahhal and Schlechte, 2009]. Sex hormones play a critical role in bone metabolism and hypogonadism (e.g. menopause) is associated with a drastic reduction in bone mass [Phillip and Lazar, 2003]. Therefore, concerns have been raised that, similar to prolactin-secreting pituitary adenomas, AP-induced hyperprolactinemia may lead to bone loss by causing hypogonadism [Abraham et al. 2003].
However, the mechanism by which hyperprolactinemia leads to bone loss is likely not limited to its effects on the hypothalamic–pituitary–gonadal axis since eugonadal patients with hyperprolactinemia may exhibit bone loss and fail to completely recover bone mass after treatment [Schlechte et al. 1983; Greenspan et al. 1989]. Moreover, adolescents with prolactin-secreting adenomas exhibit significantly reduced BMD for age despite progressing through puberty normally [Colao et al. 1998, 2000].
Of note, prolactin appears to directly affect the skeleton through the prolactin receptor expressed by osteoblasts [Clement-Lacroix et al. 1999; Seriwatanachai et al. 2008a, 2008b, 2009]. In fact, knockout mice lacking the prolactin receptor gene exhibit a dramatic reduction in bone formation and, consequently, low BMD [Clement-Lacroix et al. 1999]. Conversely, activation of the prolactin receptor inhibits osteoblast differentiation and matrix mineralization, with reduced alkaline phosphatase concentration [Coss et al. 2000; Seriwatanachai et al. 2009]. Thus, while a certain basal level of prolactin is necessary for skeletal health, excess prolactin seems detrimental.
Importantly, findings across preclinical studies vary depending on their design, including duration of prolactin exposure, cell culture, and the stage of differentiation of osteoblasts when exposed to prolactin [Charoenphandhu et al. 2008; Seriwatanachai et al. 2008a, 2008b, 2009]. For instance, the receptor activator of nuclear factor κB ligand to osteoprotegerin (RANKL/OPG) ratio increased when osteoblast-like MG-63 cells were incubated with prolactin for 48 h in a nondexamethasone-enriched medium, denoting increased osteoclastic bone resorption [Seriwatanachai et al. 2008b]. However, human pre-osteoblasts (SV-HFO) showed no change in the RANKL/OPG ratio following a 21-day exposure to prolactin in a dexamethasone-enriched medium [Seriwatanachai et al. 2009]. In addition, Charoenphandhu and colleagues found that cultured primary osteoblasts treated with prolactin for 48 h showed decreased expression of certain genes involved in osteoblast differentiation, including runx2, a transcriptional regulator of the alkaline phosphatase gene [Charoenphandhu et al. 2008]. Furthermore, exposure to prolactin for 5 days did not change osteoblast mineralization capacity [Charoenphandhu et al. 2008]. In contrast, in the human pre-osteoblast cell line, Seriwatanachai and colleagues found increased runx2 expression at day 5 of osteoblast differentiation, while mineralization capacity was decreased after 14 days of prolactin exposure [Seriwatanachai et al. 2009].
Further evidence implicating prolactin in bone turnover comes from studies in rats showing reduced bone turnover following treatment with bromocriptine, an inhibitor of prolactin secretion [Lotinun et al. 1998, 2003]. Interestingly, while endogenous prolactin levels increase bone turnover in pregnant rats, exogenous prolactin administration decreases it [Lotinun et al. 1998].
In addition to its direct effect on bone cells, prolactin may alter bone mass by enhancing the absorption of calcium by the small intestine [Mainoya, 1975; Charoenphandhu et al. 2001; Tudpor et al. 2005]. This effect is also complex since, beyond a certain concentration, prolactin may actually decrease calcium absorption [Manna et al. 2001; Tanrattana et al. 2004]. In fact, excessive prolactin is thought to inactivate its own receptor by inhibiting dimerization [Fuh et al. 1993; Ilondo et al. 1994].
An alternative, under-investigated, mechanism linking hyperprolactinemia to low BMD may involve parathyroid hormone related peptide (PTH-RP), a hormone which activates bone resorption in selected contexts. PTH-RP increases in lactating women, whose circulating prolactin concentration is also elevated, and is inversely associated with lumbar BMD in patients with prolactin-secreting tumors [Kovacs and Chik, 1995; Stiegler et al. 1995]. Thus, by increasing prolactin and, consequently, promoting the release of PTH-RP, APs may adversely affect bone metabolism. However, this mechanism has not been explored in AP-treated patients.
Furthermore, APs may affect bone health independently of their effect on prolactin. In fact, APs, particularly second-generation APs, modulate serotoninergic and adrenergic receptors [Richelson and Souder, 2000]. A number of functional serotonin receptors [e.g. 5-hydroxytryptamine 1A (5-HT1A), 5-HT1B, 5-HT2A, 5-HT2B] along with the serotonin transporter have been identified in bone cells [Bliziotes et al. 2001; Westbroek et al. 2001; Yadav et al. 2008]. This has recently stimulated great interest in understanding the role of serotonin in bone metabolism [Warden et al. 2010]. In fact, circulating serotonin has been implicated in reduced bone formation [Yadav et al. 2008; Modder et al. 2010], by activating the 5-HT1B receptor on osteoblasts [Yadav et al. 2008]. This activation reduces the expression of the transcription factor cyclic adenosine monophosphate response element binding protein, which in turn decreases the expression of cyclin D1, reducing osteoblast proliferation [Yadav et al. 2008]. Consistent with this finding, 5-HT1B knockout mice display increased bone formation and bone mass [Yadav et al. 2008]. While the affinity for the 5-HT1B receptor of commonly used APs is generally low, it does vary across the different compounds [Roth et al. 2004]. Therefore, it is conceivable that some APs may promote bone formation by blocking the 5-HT1B receptor. The skeletal effect of blocking the 5-HT2A receptor, which most APs have a high affinity for [Roth et al. 2004], is less clear. This receptor is expressed on osteoblasts [Bliziotes et al. 2001] but 5-HT2A receptor knockout mice display normal bone formation and resorption and no deficit in osteoblast proliferation [Yadav et al. 2008]. In contrast, 5-HT2B receptor knockout mice exhibit impaired osteoblast recruitment and proliferation resulting in deficient cortical and trabecular BMD [Locker et al. 2006; Collet et al. 2008; Baudry et al. 2010]. Thus, APs may also interfere with bone mineralization by blocking the 5-HT2B receptor [Bymaster et al. 1999]. However, by blocking the 5-HT2C receptors in neurons of the ventromedial hypothalamus, APs may attenuate the inhibition of the sympathetic nervous system by serotoninergic signaling from the raphe nuclei [Ducy, 2011]. This, in turn, activates skeletal β2-adrenergic receptors, which negatively impact bone metabolism [Karsenty and Ducy, 2006]. Of note is that most APs have a very low affinity for β-adrenergic receptors [Leysen et al. 1992]. However, several APs have high affinity for α1A- and α1B-adrenergic receptors [Roth et al. 2004]. Human osteoblasts express α1A-adrenergic receptors and stop proliferating when treated with α1 antagonists [Huang et al. 2009]. Expression of α1B-adrenergic receptor mRNA has also been demonstrated in human osteoclasts [Togari, 2002], suggesting that these receptors may have a role in osteoclast regulation. This would be consistent with recent evidence linking risperidone to increased osteoclast proliferation in mouse cell culture [Motyl et al. 2012].
Finally, it is also possible for APs to hinder bone metabolism by impairing muscular function, either directly or indirectly (e.g. by inducing sedation, thereby reducing physical activity) [Safer, 2011]. In fact, muscular contractions are the major cause of physiological loading, which, in turn, determines bone modeling and remodeling activity [Frost, 1987]. Therefore, reduced bone mass during AP treatment may reflect, at least in part, the decreased load bearing strain placed on the skeleton [Frost, 1987, Schoenau, 2005; Fricke and Schoenau, 2007].
In sum, APs may influence skeletal development through various mechanisms with overlapping or opposing effects (Figure 1). These include the release of prolactin, with or without secondary hypogonadism, and the modulation of serotoninergic and adrenergic signaling, sympathetic nervous system activity, and perhaps muscular function as well. The overall impact on bone metabolism is difficult to predict but is necessary to establish empirically due to the potential long-term health sequelae.
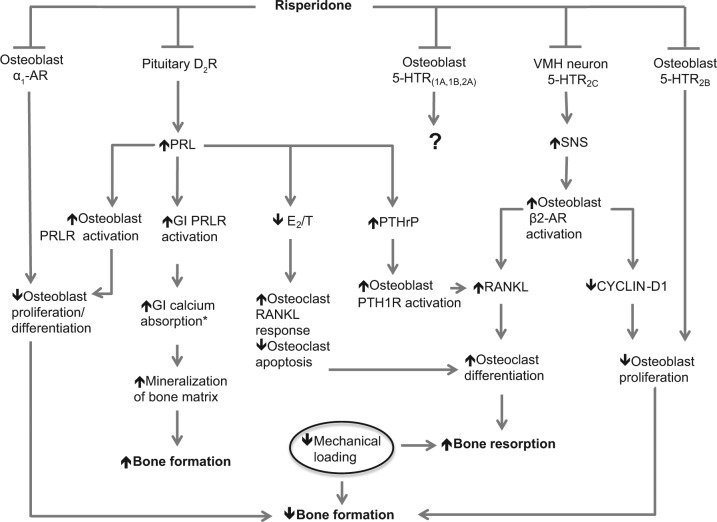
Figure 1.
Mechanisms by which risperidone impacts bone formation and resorption. Risperidone inhibits osteoblastic α1-adrenergic receptors (α1-ARs) and serotonin [5-hydroxytryptamine (5-HT)] receptors. Inhibition of α1-ARs negatively regulates osteoblasts. The impact of osteoblastic 5-HT receptor inhibition remains to be elucidated. Inhibition of dopamine D2 receptors (D2Rs) on pituitary lactotrophs leads to increased prolactin (PRL) concentration, impacting multiple signaling pathways. PRL activates osteoblastic prolactin receptors (PRLRs), negatively regulating osteoblasts. PRL activates PRLRs in the gastrointestinal (GI) tract to increase calcium absorption, allowing for increased bone mineralization (*absorption is enhanced by PRL only up to a certain point, beyond which absorption is decreased). PRL decreases estrogen (E2) and testosterone (T) concentrations, promoting bone resorption. It increases levels of parathyroid hormone-related peptide (PTHrP), activating the PTH type 1 receptor (PTH1R) and stimulating the production of receptor activator of nuclear factor κB ligand (RANKL); RANKL positively regulates osteoclasts. Inhibition of the 5-HT2C receptors of ventromedial hypothalamic (VMH) neurons leads to increased sympathetic nervous system (SNS) signaling, activation of osteoblastic β2 adrenergic receptors (β2-ARs), and decreased production of cyclin D1 by osteoblasts; this negatively regulates osteoblasts. Similarly, blockade of the 5-HT2B receptor may reduce osteoblast recruitment and proliferation. Conversely, blockade of the 5-HT1B receptor may promote bone formation by increasing cyclin D1 and, consequently, osteoblast proliferation. Risperidone may also reduce mechanical loading, thereby affecting bone modeling and remodeling.
Preclinical studies
Understanding the mechanisms of AP-induced skeletal changes is necessary for developing treatment strategies that might minimize the impact of these drugs on growth and bone mass. However, the majority of preclinical studies to date describe the effects of APs on bone and related systems but have not tested specific mechanisms with loss or gain of function approaches. Nonetheless, the following reports support clinical observations and provide clues as to how APs may directly or indirectly modulate bone metabolism.
In a study of 9- to 24 month-old male pigtail macaques (equivalent to 4–8-year-old children), neither risperidone nor quetiapine had any significant effect on body weight or skeletal growth measurements during the 6 months of treatment (3 months at low dose, followed by 3 months at high dose) or the 3 months of post-drug monitoring [Sackett et al. 2010]. However, low-dose risperidone (0.025 mg/kg) transiently reduced total areal BMD and significantly elevated prolactin compared with placebo and 2 mg/kg quetiapine. Despite increasing the dose of risperidone to 0.05 mg/kg, areal BMD recovered within the high-dose treatment period and prolactin concentrations decreased, albeit they remained above those of placebo or quetiapine (4 mg/kg). These findings are promising in that risperidone did not permanently hinder skeletal growth or BMD. However, the small sample size per treatment group (n = 10) might have limited statistical power, particularly since risperidone-treated macaques appeared to have numerically lower BMD in the post-drug phase. Furthermore, because drug metabolism varies by species, it is not clear whether the oral doses of the medications, which are similar to those used in humans, resulted in comparable serum concentrations. Moreover, the effects of hyperprolactinemia-induced hypogonadism could not be addressed as the macaques were prepubertal. Finally, the length of treatment was relatively short; many patients prescribed APs might take them throughout childhood and adolescence.
Work in rodents has also helped shed light on the skeletal impact of APs. For instance, Costa and colleagues found that male Sprague–Dawley rats treated daily, from 6 to 12 weeks of age, with the prolactin-sparing second-generation AP clozapine (10 mg/kg) had reduced total areal BMD compared with vehicle and haloperidol (0.25 mg/kg) treated rats [Costa et al. 2011]. In addition, both haloperidol and clozapine lowered body weight and clozapine alone transiently lowered testosterone levels. Clozapine reduced tibia trabecular bone volume/total volume (BV/TV) and trabecular number, as well as cortical perimeter but not thickness. The investigators then examined whether clozapine could cause any direct effects on bone turnover by treating bone cells with clozapine and haloperidol in vitro. Clozapine, but not haloperidol, at concentrations comparable to human serum levels (1 μM), significantly reduced osteoblast proliferation ([3H]-thymidine incorporation) and differentiation (% mineralized area). In addition, osteoclast differentiation from primary and RAW264.7 (osteoclast cell line) cells was significantly reduced only with supraphysiolgoic levels of clozapine (5–10 μM) but not with haloperidol. Therefore, if clozapine is directly modulating bone remodeling, it is likely inhibiting osteoblast proliferation and differentiation. However, indirect activation of osteoclasts could not be ruled out in vivo because the primary outcomes of the study were metabolic and did not include histomorphometric measurements.
Recently, Motyl and colleagues characterized the bone effects of risperidone with two different drug delivery strategies: male 3.5-week-old mice were administered 1 mg/kg/day of risperidone in their food; and female 8-week-old mice were administered 0.5 mg/kg/day of risperidone by subcutaneous osmotic minipump [Motyl et al. 2012]. Both studies were focused on metabolic complications and whether they were associated with bone loss. Oral administration of risperidone reduced body mass, percent body fat, and trabecular BV/TV, which was largely attributed to increased osteoclast parameters by histomorphometry. Consistent with this, in vitro application of risperidone (concentrations comparable to human serum concentrations) to primary osteoclast cultures increased osteoclast differentiation. Because bone loss could be associated with lower body mass, the investigators then treated female mice (which are more likely to gain weight on atypical APs) using osmotic minipump delivery. In this case, risperidone did not alter body composition, insulin or glucose tolerance, or uterine weight, but did decrease BV/TV and bone formation parameters, leaving resorption parameters unchanged. Due to the substantial differences in study design, it is not possible to determine what factors (age, gender, dose, delivery method) contributed to the disparate findings. The authors concluded that bone changes could not be solely related to metabolic dysfunction or body composition changes.
In addition to the effects of some second-generation APs on bone turnover, some studies have also found significant changes from typical APs. For example, Oh-ie and colleagues found that 10 mg/kg/day of chlorpromazine (CPZ) reduced serum and marrow alkaline phosphatase activity and increased serum acid phosphatase activity in 25-day-old rats, suggesting reduced bone formation and increased resorption respectively [Oh-ie et al. 2002]. Interestingly, these serum changes were completely blocked and marrow changes were ameliorated by coadministration of CPZ with 25 ng/kg 1α-hydroxyvitamin D3. Unfortunately, this study only examined serum markers of remodeling, but did not address changes in trabecular or cortical bone mass.
In another related study, Kunimatsu and colleagues examined the effects of long-term (daily oral gavage for 6 months) CPZ and haloperidol on prolactin and BMD in female rats [Kunimatsu et al. 2010]. They administered 2 and 10 mg/kg haloperidol and 25 and 50 mg/kg CZP to induce changes in reproductive organs. As expected, all dosing strategies increased serum prolactin and caused significant mammary gland acinous hyperplasia, as well as uterine atrophy and a trend toward low estradiol, suggesting hypogonadism. In addition, CPZ increased osteocalcin and both CPZ and haloperidol increased urinary deoxypyridinoline, suggesting increased bone turnover. Consistent with this notion, trabecular, but not cortical, BMD in the femur was significantly reduced by all treatments compared with that of untreated rats. Hyperprolactinemia and indicators of hypogonadism improved after a 3-month drug-free phase; however, trabecular BMD did not normalize. Importantly, the medicated rats were less active and gained less weight than untreated rats, both of which could cause significant changes in trabecular BMD.
In sum, preclinical studies suggest that both typical and second-generation APs can alter bone metabolism. However, the mechanism(s) of these effects remain elusive since, as noted above, the drugs may affect bone cells directly and indirectly. Future, hypothesis-driven studies examining loss or gain of function models or cotreatment strategies will be essential for better understanding potential underlying mechanisms.
Clinical studies in children and adolescents
Hyperprolactinemia
Hyperprolactinemia commonly follows the onset of AP treatment in children and adolescents [Sikich et al. 2008; Roke et al. 2009; Safer, 2011]. The incidence of this adverse event varies across APs due to their differential neuroreceptor binding affinity as well as differences in study design, duration of follow up, age and sex composition of the study population, dose of the AP, and the concurrent use of other psychotropics. For instance, it has been estimated that acute (i.e. ≤12 weeks) treatment with risperidone in children and adolescents is associated with an average increase in prolactin concentration of nearly 21 ng/ml compared with placebo [Pringsheim et al. 2011]. With prolactin reference values typically ranging from 3 to 25 ng/ml, such an increase is substantial, placing many individuals above the upper limit of normal. Similarly, compared with placebo, treatment for 3 and 6 weeks with olanzapine was associated with a nearly 31-fold increase in the risk of hyperprolactinemia, although the magnitude of the elevation is smaller than that observed with risperidone [Tohen et al. 2007; Kryzhanovskaya et al. 2009; Pringsheim et al. 2011]. Quetiapine has also been associated with hyperprolactinemia while ziprasidone and clozapine tend to be prolactin sparing [Roke et al. 2009]. In contrast, aripiprazole reduces prolactin concentration below normal in nearly two-thirds of treated children, likely due to its partial dopamine agonist activity [Safer et al. 2013]. Of note, changes in prolactin concentration have been observed during AP treatment across a variety of psychiatric disorders [Roke et al. 2009; Pringsheim et al. 2011].
Over more extended periods of exposure, prolactin concentration decreases although hyperprolactinemia persists in a substantial number of individuals [Findling et al. 2003; Calarge et al. 2009b; Kryzhanovskaya et al. 2009]. For example, between 30 and 50% of children and adolescents treated with risperidone continue to exhibit this side effect [Findling et al. 2003; Calarge et al. 2009b]. Hyperprolactinemia is also common during long-term treatment with typical APs as well as with olanzapine [Kryzhanovskaya et al. 2009; Roke et al. 2009].
In order to explore the tolerability of risperidone during long-term treatment in children and adolescents, Calarge and colleagues recruited 7–17 year-old patients who had received risperidone for at least 6 months [Calarge et al. 2009b, 2010]. At study enrollment, psychotropic polypharmacy was allowed but treatment with APs other than risperidone led to study exclusion. A morning fasting blood sample was used to measure prolactin and sex hormones. Nearly 3 years after the onset of risperidone treatment, hyperprolactinemia was present in 50% of the participants. Older age, more advanced pubertal development, and a higher oral dose of risperidone were associated with higher prolactin concentrations whereas treatment with psychostimulants, which potentiates dopaminergic signaling, lowered prolactin [Calarge et al. 2009b]. However, these predictors became non-significant when the combined serum concentration of risperidone and 9-hydroxyrisperidone, its major active metabolite, was considered, accounting for about 40% of the variability in prolactin. In addition, prolactin concentration was also associated with variants of the dopamine D2 receptor gene [Calarge et al. 2009b], although this association could not be established by others [Anderson et al. 2007]. More recently, we have also linked risperidone-induced hyperprolactinemia to body iron stores, with iron deficiency being associated with higher prolactin concentration [Calarge and Ziegler, 2013]. Iron deficiency impairs central dopaminergic signaling and studies in rodents have shown a potentiation of prolactin elevation during AP treatment in the context of iron deficiency [Barkey et al. 1985].
Sex hormones
Sex hormones play a key role in the growth spurt and bone mineralization [Phillip and Lazar, 2003]. In a combined analysis of children involved in several long-term clinical trials with risperidone, puberty appeared to have progressed normally [Dunbar et al. 2004]. This was also suggested by two long-term open-label risperidone studies [Reyes et al. 2006b, 2006c]. In none of these studies, however, were serum concentrations of sex hormones directly measured. Moreover, only patients who were able to tolerate risperidone during acute treatment were included, raising questions about the generalizability of the findings. In our work, we found no association between the serum concentration of prolactin and testosterone in risperidone-treated boys, after accounting for pubertal development [Calarge et al. 2009b].
The effect of APs on pubertal development and sex hormones is complex since obesity has been associated with earlier onset of puberty. However, as noted above, hyperprolactinemia could induce a hypogonadal state.
Bone mineral density
Little work has been conducted to directly explore skeletal health in children and adolescents receiving AP treatment. In the study cited earlier from our group, risperidone-treated participants underwent dual x-ray absorptiometry (DXA) of the lumbar spine and a peripheral quantitative computed tomography (pQCT) scan of the nondominant ultra-distal radius [Calarge et al. 2010]. DXA was used because of its versatility and the availability of age- and gender-specific BMD reference values for children [Zemel et al. 2004; Kelly et al. 2005]. However, DXA is a projectional technique. Thus, its measurements are based on the bidimensional projection of a tridimensional structure, resulting in only an approximation of bone size. This limitation makes it vulnerable to inaccuracies in estimating BMD, particularly in youth on either extremes of the height percentile curve. In addition, imprecision could also result from inhomogeneities in body composition. In contrast, pQCT defines the tridimensional bone structure allowing measurement of the true total volumetric BMD, which is less influenced by body size [Pitukcheewanont and Chen, 2005]. The other major advantage of pQCT is its unique ability to differentiate trabecular from cortical bone. Cortical bone is present in the shaft of long bones. In contrast, trabecular bone is present in the metaphyses of long bones and in short bones (like the vertebrae). Trabecular bone is more metabolically active and, consequently, more vulnerable to the effect of factors that alter bone turnover, including hyperprolactinemia [Schlechte et al. 1992; Pitukcheewanont and Chen, 2005; Specker and Schoenau, 2005]. Thus, pQCT offers the potential to improve the sensitivity to detect early changes in BMD.
In addition to measuring BMD and serum prolactin concentration, the participants’ physical activity and dietary intake were assessed [Calarge et al. 2010]. Since sex is a major determinant of BMD and since boys composed the vast majority of our sample (88%), we excluded girls from the analyses. The mean age of the participants was 11.9 years [standard deviation (SD) = 2.8] with 36% being prepubertal. The rest were distributed across the other four Tanner stages of pubertal development. The participants had received risperidone for a mean of 2.9 years (SD = 1.9). Most participants suffered from attention deficit hyperactivity disorder (87%) and disruptive behavior disorder (64%), although a wide range of disorders were present, often concurrently. As is frequently the case in AP-treated children and adolescents, polypharmacy was prevalent with psychostimulants (67%), selective serotonin reuptake inhibitors (SSRIs, 54%), and α2 agonists (27%) being the most commonly co prescribed. After taking into account the stage of sexual development (p > 0.1), age–sex-adjusted body mass index (z score (p < 0.002), and age–sex-adjusted height z score (p < 0.02), prolactin was inversely associated with trabecular BMD at the ultradistal radius. Similar findings were obtained when the analysis was restricted to non-Hispanic white boys. We also found a suggestion for an inverse association between DXA-based total lumbar bone mineral content and prolactin (p < 0.07) in white boys but this association was not significant after adjusting for stage of sexual development, vitamin D intake, and estimated physical activity. No significant associations were found between prolactin and the DXA-based cross-sectional area or age–sex-adjusted BMD z score of the lumbar spine.
Importantly, the duration of risperidone treatment did not significantly predict BMD but it may be that this was not a valid surrogate for the duration of hyperprolactinemia. As noted earlier, prolactin concentration is correlated with serum risperidone concentration, which in turn is closely associated with the oral dose [Calarge et al. 2009b]. However, over extended periods of treatment, prolactin concentration could significantly vary depending on the duration of treatment, changes in the oral dose in relation to the growing body weight in youth, treatment adherence, and concurrent medications.
Of note is that the association between prolactin concentration and trabecular BMD at the ultra-distal radius was attenuated (p < 0.07) when we also considered treatment with SSRIs, which was inversely correlated with trabecular BMD (p < 0.04). However, including SSRI treatment in the model did not alter the significant inverse association of prolactin with trabecular BMD in non-Hispanic white boys. SSRIs were also negatively associated with lumbar BMD z score (p < 0.05), after accounting for Tanner stage, sex–age-adjusted height and weight z scores, daily intake of calcium, physical activity, and prolactin. Moreover, this association was moderated by variants of the serotonin transporter gene [Calarge et al. 2011].
Finally, several studies have reported elevations in alkaline phosphatase during AP treatment [Kumra et al. 1996, 1997; Erdogan et al. 2008; Pavuluri et al. 2010; Geller et al. 2012]. In children and adolescents, most circulating alkaline phosphatase consists of the bone isoenzyme [Yang and Grey, 2006]. Therefore, an increase in the concentration of total alkaline phosphatase might reflect a direct effect of APs on bone turnover. This, however, cannot be confirmed (or ruled out) since the bone-specific isoenzyme was not measured in these studies. Alternatively, it is equally likely that the hepatic isoenzyme accounts for this increase due to AP-induced weight gain, potentially leading to steatosis, or due to direct hepatotoxicity [Kumra et al. 1996, 1997; Erdogan et al. 2008, 2010].
Discussion
Most, albeit not all, APs exhibit a strong affinity for dopamine D2 receptors [Richelson and Souder, 2000]. By blocking these receptors in the anterior pituitary, APs increase circulating prolactin. This, in turn, could interfere with bone metabolism through direct and indirect effects. In addition, APs may affect bone health via several other processes, including the modulation of serotoninergic and adrenergic signaling. In children and adolescents, impaired skeletal mineralization could have lasting consequences since bone mass acquired by young adulthood is a significant determinant of lifetime fracture risk [NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, 2001].
In general, when an AP is initiated, the immediate concern is symptom control. While APs might be predominantly used to treat psychotic and severe mood disorders in adults, they are commonly used to treat explosive behavior in children and adolescents, in the context of disruptive behavior disorders, pervasive developmental disorders, mood disorders, or psychosis [Findling et al. 2008; Comer et al. 2010]. Therefore, optimizing the safety of the child and their environment is the absolute priority initially. It is when the acute circumstances dissipate and the child’s behavior improves that attention ought to focus on the long-term tolerability of the treatment. Unfortunately, extended use of APs is often necessary lest the underlying psychiatric symptoms return. This makes side effects like hyperprolactinemia particularly challenging since they often go unnoticed, yet might lead to long-term sequelae. In fact, APs in children and adolescents are disproportionately prescribed to boys [Olfson et al. 2010, 2012]. In this group, as well as in prepubertal girls, amenorrhea is not a useful marker for problematic hyperprolactinemia. Moreover, some other prolactin-related side effects, like gynecomastia, are difficult to attribute directly to hyperprolactinemia since these can occur in untreated peripubertal youth undergoing normal development [Styne, 2002]. Even galactorrhea can be challenging to identify since stimulation of the nipple is often needed for the release of milk. All of these challenges make it difficult to ‘clinically’ suspect hyperprolactinemia in children and adolescents before proceeding to laboratory confirmation.
Practice guidelines do not recommend universal testing for prolactin during AP treatment [Marder et al. 2004; Correll and Carlson, 2006]. This is understandable as there is only a weak correlation between prolactin concentration and side effects [Findling et al. 2003]. Moreover, relatively mild hyperprolactinemia, in the absence of hypogonadism, has not been definitively linked to deleterious skeletal sequelae [Shibli-Rahhal and Schlechte, 2009]. In addition, the studies exploring the skeletal effects of AP-induced hyperprolactinemia in adults are conflicting and have largely failed to use state-of-the-art methods to assess trabecular BMD or to adequately account for important confounding variables, such as physical activity, dietary intake, vitamin D concentration, smoking status, and gonadal activity [Bilici et al. 2002; Abraham et al. 2003; Becker et al. 2003; Meaney et al. 2004; Howes et al. 2005; Jung et al. 2006; Meaney and O’Keane, 2007; Kishimoto et al. 2008]. As reviewed earlier, the dearth of information in children and adolescents is also problematic, prohibiting the field from drawing firm conclusions.
What is particularly concerning in children and adolescents is that polypharmacy is increasingly prevalent, particularly among AP-treated patients [dosReis et al. 2005; Duffy et al. 2005; Crystal et al. 2009; Comer et al. 2010]. A recent analysis of the National Ambulatory Medical Care Survey data revealed that medications for attention deficit hyperactivity disorder, antidepressants, and APs are the classes of psychotropics most often used in combination [Comer et al. 2010]. For example, nearly two-thirds of children receiving risperidone were concurrently prescribed other psychotropics. In our sample of risperidone-treated youth, once risperidone was started, only 1% of the children received risperidone monotherapy for their entire treatment period. Thus, with accumulating evidence linking various psychotropics to bone health, clinicians ought to consider the potential skeletal impact of polypharmacy as opposed to that of individual psychotropics. This task is challenging, particularly in light of evidence suggesting that some psychotropics, like lithium, may promote bone mineralization [Zamani et al. 2009; Warden et al. 2010].
To complicate matters further, childhood psychopathology is itself associated with lower socioeconomic status, single-parent homes, and poor parental supervision. These factors, in turn, are correlated with deleterious lifestyle habits, including suboptimal diet and physical activity. The essential role adequate nutrition, including calcium and vitamin D intake, and physical activity play in healthy bone development is well established [NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, 2001]. Moreover, a history of maltreatment is relatively common in psychiatrically ill children and adolescents, particularly those treated with APs. Such factors, along with obesity whether idiopathic or iatrogenic due to APs, can affect the onset of puberty and induce subclinical inflammation, thereby influencing bone mineralization.
Albeit somewhat controversial, guidelines issued by the Pediatric Position Development Conference of the International Society of Clinical Densitometry recommend densitometry in certain clinical situations (e.g. osteogenesis imperfecta) [Baim et al. 2008; Lewiecki et al. 2009]. These include anorexia nervosa but not other psychiatric conditions or treatments with psychotropics. In fact, there is presently insufficient scientific evidence to support routine bone densitometry for ‘screening’ or ‘case finding’ in children and adolescents undergoing psychotropic treatment. Nonetheless, the decision to pursue testing is ultimately a clinical one and must take into account such factors as the number of risk factors, personal history of fractures, and family history. Such a decision may be best made in close collaboration with a specialist since the optimal skeletal site to scan and the appropriate interpretation of the densitometry results can be challenging in children, requiring consideration of factors like pubertal development and height [Baim et al. 2008; Bachrach and Sills, 2011].
In sum, the risk factors that place a child at an elevated risk for receiving an AP overlap with those contributing to polypharmacy, suboptimal lifestyle habits, obesity, and early pubertal development. The overall result is a situation where multiple risk factors for impaired skeletal development converge (Figure 2). Therefore, clinicians have a challenging task to not only treat the imminent psychiatric problems but also to optimize health during long-term care to prevent chronic diseases, including osteoporosis. This would, in turn, increase the longevity and quality of life of individuals suffering with mental illness.
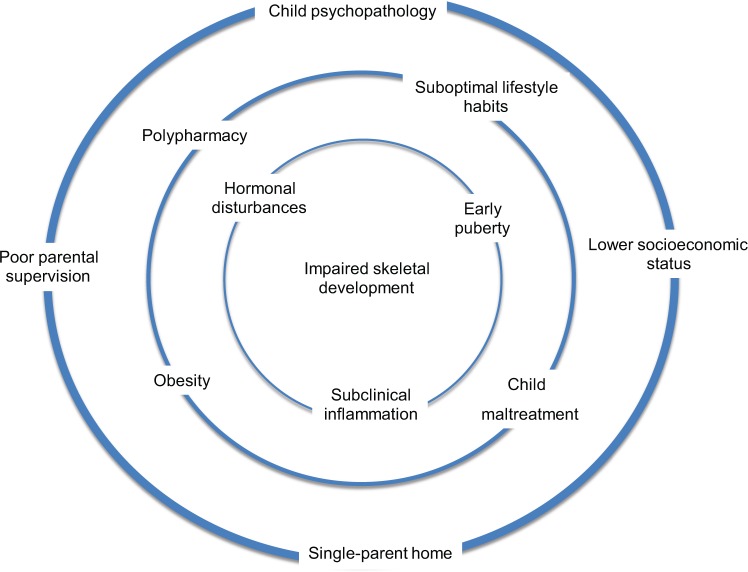
Figure 2.
Psychotropic treatment, particularly the use of antipsychotics, is associated with a multitude of factors that might, directly or indirectly, impair bone development in children and adolescents. For instance, childhood psychopathology may be associated with suboptimal lifestyle habits, hormonal changes (e.g. hypercortisolemia), and the concurrent use of several medications that may affect bone metabolism.
Footnotes
Funding: This work was funded by a 2005 NARSAD Young Investigator Award and by the National Institute of Health (RR024979, R21MH080968, and K23MH085005). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Chadi A. Calarge, Associate Professor, Departments of Psychiatry and Pediatrics, University of Iowa Carver College of Medicine, Psychiatry Research, 2-209 MEB, 500 Newton Road, Iowa City, IA 52242, USA
Stephanie D. Ivins, University of Iowa Carver College of Medicine, Iowa City, IA, USA
Katherine J. Motyl, Maine Medical Center Research Institute, Scarborough, ME, USA
Amal A. Shibli-Rahhal, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA, USA
Michael M. Bliziotes, Oregon Health and Science University, Portland, OR, USA
Janet A. Schlechte, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA, USA
References
- Abraham G., Paing W., Kaminski J., Joseph A., Kohegyi E., Josiassen R. (2003) Effects of elevated serum prolactin on bone mineral density and bone metabolism in female patients with schizophrenia: a prospective study. Am J Psychiatry 160: 1618–1620 [DOI] [PubMed] [Google Scholar]
- Anderson G., Scahill L., McCracken J., McDougle C., Aman M., Tierney E., et al. (2007) Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry 61: 545–550 [DOI] [PubMed] [Google Scholar]
- Bachrach L., Sills I. (2011) Clinical report-bone densitometry in children and adolescents. Pediatrics 127: 189–194 [DOI] [PubMed] [Google Scholar]
- Baim S., Leonard M., Bianchi M., Hans D., Kalkwarf H., Langman C., et al. (2008) Official positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Pediatric Position Development Conference. J Clin Densitom 11: 6–21 [DOI] [PubMed] [Google Scholar]
- Barkey R., Ben-Shachar D., Amit T., Youdim M. (1985) Increased hepatic and reduced prostatic prolactin (PRL) binding in iron deficiency and during neuroleptic treatment: correlation with changes in serum PRL and testosterone. Eur J Pharmacol 109: 193–200 [DOI] [PubMed] [Google Scholar]
- Baudry A., Bitard J., Mouillet-Richard S., Locker M., Poliard A., Launay J., et al. (2010) Serotonergic 5-HT(2b) receptor controls tissue-nonspecific alkaline phosphatase activity in osteoblasts via eicosanoids and phosphatidylinositol-specific phospholipase C. J Biol Chem 285: 26066–26073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Liver O., Mester R., Rapoport M., Weizman A., Weiss M. (2003) Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry 64: 761–766 [DOI] [PubMed] [Google Scholar]
- Bilici M., Cakirbay H., Guler M., Tosun M., Ulgen M., Tan U. (2002) Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci 112: 817–828 [DOI] [PubMed] [Google Scholar]
- Biller B., Baum H., Rosenthal D., Saxe V., Charpie P., Klibanski A. (1992) Progressive trabecular osteopenia in women with hyperprolactinemic amenorrhea. J Clin Endocrinol Metab 75: 692–697 [DOI] [PubMed] [Google Scholar]
- Bliziotes M., Eshleman A., Zhang X., Wiren K. (2001) Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone 29: 477–486 [DOI] [PubMed] [Google Scholar]
- Bymaster F., Nelson D., DeLapp N., Falcone J., Eckols K., Truex L., et al. (1999) Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophrenia research 37: 107–122 [DOI] [PubMed] [Google Scholar]
- Calarge C., Acion L., Kuperman S., Tansey M., Schlechte J. (2009a) Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol 19: 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge C., Ellingrod V., Acion L., Miller D., Moline J., Tansey M., et al. (2009b) Variants of the dopamine D2 receptor gene and risperidone-induced hyperprolactinemia in children and adolescents. Pharmacogenet Genomics 19: 373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge C., Ellingrod V., Zimmerman B., Bliziotes M., Schlechte J. (2011) Variants of the serotonin transporter gene, selective serotonin reuptake inhibitors, and bone mineral density in risperidone-treated boys: a reanalysis of data from a cross-sectional study with emphasis on pharmacogenetics. J Clin Psychiatry 72: 1685–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge C., Ziegler E. (2013) Iron deficiency in pediatric patients in long-term risperidone treatment. J Child Adolesc Psychopharmacol 23: 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge C., Zimmerman B., Xie D., Kuperman S., Schlechte J. (2010) A cross-sectional evaluation of the effect of risperidone and selective serotonin reuptake inhibitors on bone mineral density in boys. J Clin Psychiatry 71: 338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie Fassler A., Bonjour J. (1995) Osteoporosis as a pediatric problem. Pediatr Clin N Am 42: 811–824 [DOI] [PubMed] [Google Scholar]
- Charoenphandhu N., Limlomwongse L., Krishnamra N. (2001) Prolactin directly stimulates transcellular active calcium transport in the duodenum of female rats. Can J Physiol Pharmacol 79: 430–438 [PubMed] [Google Scholar]
- Charoenphandhu N., Teerapornpuntakit J., Methawasin M., Wongdee K., Thongchote K., Krishnamra N. (2008) Prolactin decreases expression of Runx2, osteoprotegerin, and RANKL in primary osteoblasts derived from tibiae of adult female rats. Can J Physiol Pharmacol 86: 240–248 [DOI] [PubMed] [Google Scholar]
- Clement-Lacroix P., Ormandy C., Lepescheux L., Ammann P., Damotte D., Goffin V., et al. (1999) Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology 140: 96–105 [DOI] [PubMed] [Google Scholar]
- Colao A., Di Somma C., Loche S., Di Sarno A., Klain M., Pivonello R., et al. (2000) Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf) 52: 319–327 [DOI] [PubMed] [Google Scholar]
- Colao A., Loche S., Cappa M., Di Sarno A., Landi M., Sarnacchiaro F., et al. (1998) Prolactinomas in children and adolescents. clinical presentation and long-term follow-up. J Clin Endocrinol Metab 83: 2777–2780 [DOI] [PubMed] [Google Scholar]
- Collet C., Schiltz C., Geoffroy V., Maroteaux L., Launay J., de Vernejoul M. (2008) The serotonin 5-HT2b receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J 22: 418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer J., Olfson M., Mojtabai R. (2010) National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996–2007. J Am Acad Child Adolesc Psychiatry 49: 1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper W., Arbogast P., Ding H., Hickson G., Fuchs D., Ray W. (2006) Trends in prescribing of antipsychotic medications for US children. Ambulatory Pediatr 6: 79–83 [DOI] [PubMed] [Google Scholar]
- Correll C., Carlson H. (2006) Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry 45: 771–791 [DOI] [PubMed] [Google Scholar]
- Correll C., Manu P., Olshanskiy V., Napolitano B., Kane J., Malhotra A. (2009) Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302: 1765–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D., Yang L., Kuo C., Xu X., Luben R., Walker A. (2000) Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am J Physiol Endocrinol Metab 279: E1216–E1225 [DOI] [PubMed] [Google Scholar]
- Costa J., Smith G., Watson M., Lin J., Callon K., Gamble G., et al. (2011) The atypical anti-psychotic clozapine decreases bone mass in rats in vivo. Schizophr Res 126: 291–297 [DOI] [PubMed] [Google Scholar]
- Crystal S., Olfson M., Huang C., Pincus H., Gerhard T. (2009) Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood) 28: w770–w781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dosReis S., Zito J., Safer D., Gardner J., Puccia K., Owens P. (2005) Multiple psychotropic medication use for youths: a two-state comparison. J Child Adolesc Psychopharmacol 15: 68–77 [DOI] [PubMed] [Google Scholar]
- Ducy P. (2011) 5-HT and bone biology. Curr Opin Pharmacol 11: 34–38 [DOI] [PubMed] [Google Scholar]
- Duffy F., Narrow W., Rae D., West J., Zarin D., Rubio-Stipec M., et al. (2005) Concomitant pharmacotherapy among youths treated in routine psychiatric practice. J Child Adolesc Psychopharmacol 15: 12–25 [DOI] [PubMed] [Google Scholar]
- Dunbar F., Kusumakar V., Daneman D., Schulz M. (2004) Growth and sexual maturation during long-term treatment with risperidone. Am J Psychiatry 161: 918–920 [DOI] [PubMed] [Google Scholar]
- Duntas L. (2001) Prolactinomas in children and adolescents – consequences in adult life. J Pediatr Endocrinol Metab 14(Suppl 5): 1227–1232; discussion 1261–1222. [PubMed] [Google Scholar]
- Erdogan A., Atasoy N., Akkurt H., Ozturk D., Karaahmet E., Yalug I., et al. (2008) Risperidone and liver function tests in children and adolescents: a short-term prospective study. Prog Neuropsychopharmacol Biol Psychiatry 32: 849–857 [DOI] [PubMed] [Google Scholar]
- Erdogan A., Karaman M., Ozdemir E., Yurteri N., Tufan A., Kurcer M. (2010) Six months of treatment with risperidone May be associated with nonsignificant abnormalities of liver function tests in children and adolescents: a longitudinal, observational study from Turkey. J Child Adolesc Psychopharmacol 20: 407–413 [DOI] [PubMed] [Google Scholar]
- FDA (2006) FDA approves the first drug to treat irritability associated with autism, risperdal. Available at: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01485.html [PubMed]
- FDA (2007) Recommendation of approvable action for risperidone (Risperdal®) for the treatment of schizophrenia and bipolar I disorder in pediatric patients (response to PWR). Available at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM163241.pdf
- FDA (2009) Seroquel® (quetiapine fumarate) tablets for the treatment of pediatric patients with schizophrenia (13-17 years old) or bipolar mania (10–17 years old). Briefing document for Psychopharmacologic Drugs Advisory Committee Meeting, 9–10 June 2009 Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/UCM164280.pdf [Google Scholar]
- Findling R., Johnson J., McClellan J., Frazier J., Vitiello B., Hamer R., et al. (2010) Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) study. J Am Acad Child Adolesc Psychiatry 49: 583–594; quiz 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling R., Kusumakar V., Daneman D., Moshang T., De Smedt G., Binder C. (2003) Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 64: 1362–1369 [DOI] [PubMed] [Google Scholar]
- Findling R., Robb A., Nyilas M., Forbes R., Jin N., Ivanova S., et al. (2008) A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry 165: 1432–1441 [DOI] [PubMed] [Google Scholar]
- Fricke O., Schoenau E. (2007) The ‘functional muscle-bone unit’: probing the relevance of mechanical signals for bone development in children and adolescents. Growth Horm IGF Res 17: 1–9 [DOI] [PubMed] [Google Scholar]
- Frost H. (1987) Bone ‘mass’ and the ‘mechanostat’: a proposal. Anat Rec 219: 1–9 [DOI] [PubMed] [Google Scholar]
- Fuh G., Colosi P., Wood W.I., Wells J.A. (1993) Mechanism-Based Design of Prolactin Receptor Antagonists. J Biol Chem 268: 5376–5381 [PubMed] [Google Scholar]
- Geller B., Luby J., Joshi P., Wagner K., Emslie G., Walkup J., et al. (2012) A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Arch Gen Psychiatry 69: 515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan S., Oppenheim D., Klibanski A. (1989) Importance of gonadal steroids to bone mass in men with hyperprolactinemic hypogonadism. Ann Intern Med 110: 526–531 [DOI] [PubMed] [Google Scholar]
- Halbreich U., Kinon B., Gilmore J., Kahn L. (2003) Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinology 28(Suppl. 1): 53–67 [DOI] [PubMed] [Google Scholar]
- Howes O., Wheeler M., Meaney A., O’Keane V., Fogelman I., Blake G., et al. (2005) Bone mineral density and its relationship to prolactin levels in patients taking antipsychotic treatment. J Clin Psychopharmacol 25: 259–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Brennan T., Muir M., Mason R. (2009) Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J Cell Physiol 220: 267–275 [DOI] [PubMed] [Google Scholar]
- Ilondo M., Damholt A., Cunningham B., Wells J., De Meyts P., Shymko R. (1994) Receptor dimerization determines the effects of growth hormone in primary rat adipocytes and cultured human IM-9 lymphocytes. Endocrinology 134: 2397–2403 [DOI] [PubMed] [Google Scholar]
- Jung D., Conley R., Kelly D., Kim D., Yoon S., Jang J., et al. (2006) Prevalence of bone mineral density loss in Korean patients with schizophrenia: a cross-sectional study. J Clin Psychiatry 67: 1391–1396 [DOI] [PubMed] [Google Scholar]
- Karsenty G., Ducy P. (2006) The hypothalamic control of bone mass, implication for the treatment of osteoporosis. Ann Endocrinol (Paris) 67: 123. [DOI] [PubMed] [Google Scholar]
- Kelly T., Specker B., Binkley T., Zemel B., Leonard M., Kalkward H., et al. (2005) Pediatric BMD reference database for U.S. white children. Bone 36(Suppl. 1): S30 [Google Scholar]
- Kishimoto T., Watanabe K., Shimada N., Makita K., Yagi G., Kashima H. (2008) Antipsychotic-induced hyperprolactinemia inhibits the hypothalamo-pituitary-gonadal axis and reduces bone mineral density in male patients with schizophrenia. J Clin Psychiatry 69: 385–391 [DOI] [PubMed] [Google Scholar]
- Klibanski A., Neer R., Beitins I., Ridgway E., Zervas N., McArthur J. (1980) Decreased bone density in hyperprolactinemic women. N Engl J Med 303: 1511–1514 [DOI] [PubMed] [Google Scholar]
- Kovacs C., Chik C. (1995) Hyperprolactinemia caused by lactation and pituitary adenomas is associated with altered serum calcium, phosphate, parathyroid hormone (PTH), and PTH-related peptide levels. J Clin Endocrinol Metab 80: 3036–3042 [DOI] [PubMed] [Google Scholar]
- Kryzhanovskaya L., Schulz S., McDougle C., Frazier J., Dittmann R., Robertson-Plouch C., et al. (2009) Olanzapine versus placebo in adolescents with schizophrenia: a 6-week, randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 48: 60–70 [DOI] [PubMed] [Google Scholar]
- Kumra S., Frazier J., Jacobsen L., McKenna K., Gordon C., Lenane M., et al. (1996) Childhood-onset schizophrenia. a double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry 53: 1090–1097 [DOI] [PubMed] [Google Scholar]
- Kumra S., Herion D., Jacobsen L., Briguglia C., Grothe D. (1997) Case study: risperidone-induced hepatotoxicity in pediatric patients. J Am Acad Child Adolesc Psychiat 36: 701–705 [DOI] [PubMed] [Google Scholar]
- Kunimatsu T., Kimura J., Funabashi H., Inoue T., Seki T. (2010) The antipsychotics haloperidol and chlorpromazine increase bone metabolism and induce osteopenia in female rats. Regul Toxicol Pharmacol 58: 360–368 [DOI] [PubMed] [Google Scholar]
- Langer G., Sachar E., Halpern F., Gruen P., Solomon M. (1977) The prolactin response to neuroleptic drugs. a test of dopaminergic blockade: neuroendocrine studies in normal men. J Clin Endocrinol Metab 45: 996–1002 [DOI] [PubMed] [Google Scholar]
- Lewiecki E., Baim S., Langman C., Bilezikian J. (2009) Official positions of the International Society for Clinical Densitometry: perceptions and commentary. J Clin Densitom 12: 267–271 [DOI] [PubMed] [Google Scholar]
- Leysen J., Janssen P., Gommeren W., Wynants J., Pauwels P., Janssen P. (1992) In vitro and in vivo receptor binding and effects on monoamine turnover in rat brain regions of the novel antipsychotics risperidone and ocaperidone. Mol Pharmacol 41: 494–508 [PubMed] [Google Scholar]
- Locker M., Bitard J., Collet C., Poliard A., Mutel V., Launay J., et al. (2006) Stepwise control of osteogenic differentiation by 5-HT(2B) receptor signaling: nitric oxide production and phospholipase A2 activation. Cell Signal 18: 628–639 [DOI] [PubMed] [Google Scholar]
- Lotinun S., Limlomwongse L., Krishnamra N. (1998) The study of a physiological significance of prolactin in the regulation of calcium metabolism during pregnancy and lactation in rats. Can J Physiol Pharmacol 76: 218–228 [PubMed] [Google Scholar]
- Lotinun S., Limlomwongse L., Sirikulchayanonta V., Krishnamra N. (2003) Bone calcium turnover, formation, and resorption in bromocriptine- and prolactin-treated lactating rats. Endocrine 20: 163–170 [DOI] [PubMed] [Google Scholar]
- Mainoya J. (1975) Further studies on the action of prolactin on fluid and ion absorption by the rat jejunum. Endocrinology 96: 1158–1164 [DOI] [PubMed] [Google Scholar]
- Manna P., El-Hefnawy T., Kero J., Huhtaniemi I. (2001) Biphasic action of prolactin in the regulation of murine Leydig tumor cell functions. Endocrinology 142: 308–318 [DOI] [PubMed] [Google Scholar]
- Marder S., Essock S., Miller A., Buchanan R., Casey D., Davis J., et al. (2004) Physical health monitoring of patients with schizophrenia. Am J Psychiatry 161: 1334–1349 [DOI] [PubMed] [Google Scholar]
- Matkovic V. (1996) Skeletal development and bone turnover revisited. J Clin Endocrinol Metab 81: 2013–2016 [DOI] [PubMed] [Google Scholar]
- Matkovic V., Ilich J., Skugor M., Saracoglu M. (1995) Primary prevention of osteoporosis. Phys Med Rehab Clin North Am 6: 595–627 [Google Scholar]
- Meaney A., O’Keane V. (2007) Bone mineral density changes over a year in young females with schizophrenia: relationship to medication and endocrine variables. Schizophr Res 93: 136–143 [DOI] [PubMed] [Google Scholar]
- Meaney A., Smith S., Howes O., O’Brien M., Murray R., O’Keane V. (2004) Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. Br J Psychiatry 184: 503–508 [DOI] [PubMed] [Google Scholar]
- Modder U., Achenbach S., Amin S., Riggs B., Melton L., 3rd, Khosla S. (2010) Relation of serum serotonin levels to bone density and structural parameters in women. J Bone Miner Res 25: 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl K., Dick-de-Paula I., Maloney A., Lotinun S., Bornstein S., de Paula F., et al. (2012) Trabecular bone loss after administration of the second-generation antipsychotic risperidone is independent of weight gain. Bone 50: 490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285: 785–79511176917 [Google Scholar]
- Oh-ie K., Miyazaki T., Koyama I., Hokari S., Komoda T. (2002) Altered bone turnover in chlorpromazine-challenged rats and its effect on 1alpha-hydroxyvitamin D3 administration in vivo. J Bone Miner Metab 20: 21–27 [DOI] [PubMed] [Google Scholar]
- Olfson M., Blanco C., Liu S., Wang S., Correll C. (2012) National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatry 69: 1247-1256 [DOI] [PubMed] [Google Scholar]
- Olfson M., Crystal S., Huang C., Gerhard T. (2010) Trends in antipsychotic drug use by very young, privately insured children. J Am Acad Child Adolesc Psychiatry 49: 13–23 [DOI] [PubMed] [Google Scholar]
- Pavuluri M., Henry D., Findling R., Parnes S., Carbray J., Mohammed T., et al. (2010) Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder. Bipolar Dis 12: 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillip M., Lazar L. (2003) The regulatory effect of hormones and growth factors on the pubertal growth spurt. Endocrinologist 13: 465–469 [Google Scholar]
- Pitukcheewanont P., Chen P. (2005) Bone density measurements in children and adolescents. quantitative computed tomography versus dual-energy x-ray absorptiometry. Endocrinologist 15: 232–239 [Google Scholar]
- Pringsheim T., Lam D., Ching H., Patten S. (2011) Metabolic and neurological complications of second-generation antipsychotic use in children: a systematic review and meta-analysis of randomized controlled trials. Drug Safety 34: 651–668 [DOI] [PubMed] [Google Scholar]
- Rauch F., Schoenau E. (2001) Changes in bone density during childhood and adolescence: an approach based on bone’s biological organization. J Bone Miner Res 16: 597–604 [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network (2005) Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry 162: 1361–1369 [DOI] [PubMed] [Google Scholar]
- Reyes M., Buitelaar J., Toren P., Augustyns I., Eerdekens M. (2006a) A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. Am J Psychiatry 163: 402–410 [DOI] [PubMed] [Google Scholar]
- Reyes M., Croonenberghs J., Augustyns I., Eerdekens M. (2006b) Long-term use of risperidone in children with disruptive behavior disorders and subaverage intelligence: efficacy, safety, and tolerability. J Child Adolesc Psychopharmacol 16: 260–272 [DOI] [PubMed] [Google Scholar]
- Reyes M., Olah R., Csaba K., Augustyns I., Eerdekens M. (2006c) Long-term safety and efficacy of risperidone in children with disruptive behaviour disorders. results of a 2-year extension study. Eur Child Adolesc Psychiatry 15: 97–104 [DOI] [PubMed] [Google Scholar]
- Richelson E., Souder T. (2000) Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 68: 29–39 [DOI] [PubMed] [Google Scholar]
- Roke Y., van Harten P., Boot A., Buitelaar J. (2009) Antipsychotic medication in children and adolescents: a descriptive review of the effects on prolactin level and associated side effects. J Child Adolesc Psychopharmacol 19: 403–414 [DOI] [PubMed] [Google Scholar]
- Roth B., Sheffler D., Kroeze W. (2004) Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 3: 353–359 [DOI] [PubMed] [Google Scholar]
- Sackett G., Unis A., Crouthamel B. (2010) Some effects of risperidone and quetiapine on growth parameters and hormone levels in young pigtail macaques. J Child Adolesc Psychopharmacol 20: 489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer D. (2011) Age-grouped differences in adverse drug events from psychotropic medication. J Child Adolesc Psychopharmacol 21: 299–309 [DOI] [PubMed] [Google Scholar]
- Safer D., Calarge C., Safer A. (2013) Prolactin serum concentrations during aripiprazole treatment in youth. J Child Adolesc Psychopharmacol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlechte J., Sherman B., Martin R. (1983) Bone density in amenorrheic women with and without hyperprolactinemia. J Clin Endocrinol Metab 56: 1120–1123 [DOI] [PubMed] [Google Scholar]
- Schlechte J., Walkner L., Kathol M. (1992) A longitudinal analysis of premenopausal bone loss in healthy women and women with hyperprolactinemia. J Clin Endocrinol Metab 75: 698–703 [DOI] [PubMed] [Google Scholar]
- Schoenau E. (2005) From mechanostat theory to development of the ‘functional muscle-bone-unit’. J Musculoskelet Neuronal Interact 5: 232–238 [PubMed] [Google Scholar]
- Seriwatanachai D., Charoenphandhu N., Suthiphongchai T., Krishnamra N. (2008a) Prolactin decreases the expression ratio of receptor activator of nuclear factor kappaB ligand/osteoprotegerin in human fetal osteoblast cells. Cell Biol Int 32: 1126-1135 [DOI] [PubMed] [Google Scholar]
- Seriwatanachai D., Krishnamra N., van Leeuwen J. (2009) Evidence for direct effects of prolactin on human osteoblasts: inhibition of cell growth and mineralization. J Cell Biochem 107: 677–685 [DOI] [PubMed] [Google Scholar]
- Seriwatanachai D., Thongchote K., Charoenphandhu N., Pandaranandaka J., Tudpor K., Teerapornpuntakit J., et al. (2008b) Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone 42: 535–546 [DOI] [PubMed] [Google Scholar]
- Shibli-Rahhal A., Schlechte J. (2009) The effects of hyperprolactinemia on bone and fat. Pituitary 12: 96–104 [DOI] [PubMed] [Google Scholar]
- Sikich L., Frazier J., McClellan J., Findling R., Vitiello B., Ritz L., et al. (2008) Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study. Am J Psychiatry 165: 1420–1431 [DOI] [PubMed] [Google Scholar]
- Specker B., Schoenau E. (2005) Quantitative bone analysis in children: current methods and recommendations. J Pediatr 146: 726–731 [DOI] [PubMed] [Google Scholar]
- Stiegler C., Leb G., Kleinert R., Warnkross H., Ramschak-Schwarzer S., Lipp R., et al. (1995) Plasma levels of parathyroid hormone-related peptide are elevated in hyperprolactinemia and correlated to bone density status. J Bone Miner Res 10: 751–759 [DOI] [PubMed] [Google Scholar]
- Styne D. (2002) The testes, disorders of sexual differentiation and puberty in the male. In: Sperling M. (ed.), Pediatric endocrinology, 2nd ed. Philadelphia, PA: Sauders [Google Scholar]
- Tanrattana C., Charoenphandhu N., Limlomwongse L., Krishnamra N. (2004) Prolactin directly stimulated the solvent drag-induced calcium transport in the duodenum of female rats. Biochim Biophys Act 1665: 81–91 [DOI] [PubMed] [Google Scholar]
- Theintz G., Buchs B., Rizzoli R., Slosman D., Clavien H., Sizonenko P., et al. (1992) Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab 75: 1060–1065 [DOI] [PubMed] [Google Scholar]
- Togari A. (2002) Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microscopy Res Technique 58: 77–84 [DOI] [PubMed] [Google Scholar]
- Tohen M., Kryzhanovskaya L., Carlson G., Delbello M., Wozniak J., Kowatch R., et al. (2007) Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry 164: 1547–1556 [DOI] [PubMed] [Google Scholar]
- Tudpor K., Charoenphandhu N., Saengamnart W., Krishnamra N. (2005) Long-term prolactin exposure differentially stimulated the transcellular and solvent drag-induced calcium transport in the duodenum of ovariectomized rats. Exp Biol Med 230: 836–844 [DOI] [PubMed] [Google Scholar]
- Vitiello B., Correll C., van Zwieten-Boot B., Zuddas A., Parellada M., Arango C. (2009) Antipsychotics in children and adolescents: increasing use, evidence for efficacy and safety concerns. Eur Neuropsychopharmacol 19: 629–635 [DOI] [PubMed] [Google Scholar]
- Warden S., Hassett S., Bond J., Rydberg J., Grogg J., Hilles E., et al. (2010) Psychotropic drugs have contrasting skeletal effects that are independent of their effects on physical activity levels. Bone 46: 985–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden S., Robling A., Haney E., Turner C., Bliziotes M. (2010) The emerging role of serotonin (5-hydroxytryptamine) in the skeleton and its mediation of the skeletal effects of low-density lipoprotein receptor-related protein 5 (LRP5). Bone 46: 4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbroek I., van der Plas A., de Rooij K., Klein-Nulend J., Nijweide P. (2001) Expression of serotonin receptors in bone. J Biol Chem 276: 28961–28968 [DOI] [PubMed] [Google Scholar]
- Yadav V., Ryu J., Suda N., Tanaka K., Gingrich J., Schutz G., et al. (2008) LRP5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135: 825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Grey V. (2006) Pediatric reference intervals for bone markers. Clin Biochem 39: 561–568 [DOI] [PubMed] [Google Scholar]
- Zamani A., Omrani G., Nasab M. (2009) Lithium’s effect on bone mineral density. Bone 44: 331–334 [DOI] [PubMed] [Google Scholar]
- Zemel B., Leonard M., Kalkward H., Specker B., Moyer-Mileur L., Shepherd J., et al. (2004) Reference data for the whole body, lumbar spine, and proximal femur for American children relative to age, gender, and body size. J Bone Miner Res 19: S231 [Google Scholar]
- Zuddas A., Zanni R., Usala T. (2011) Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol 21: 600–620 [DOI] [PubMed] [Google Scholar]