Abstract
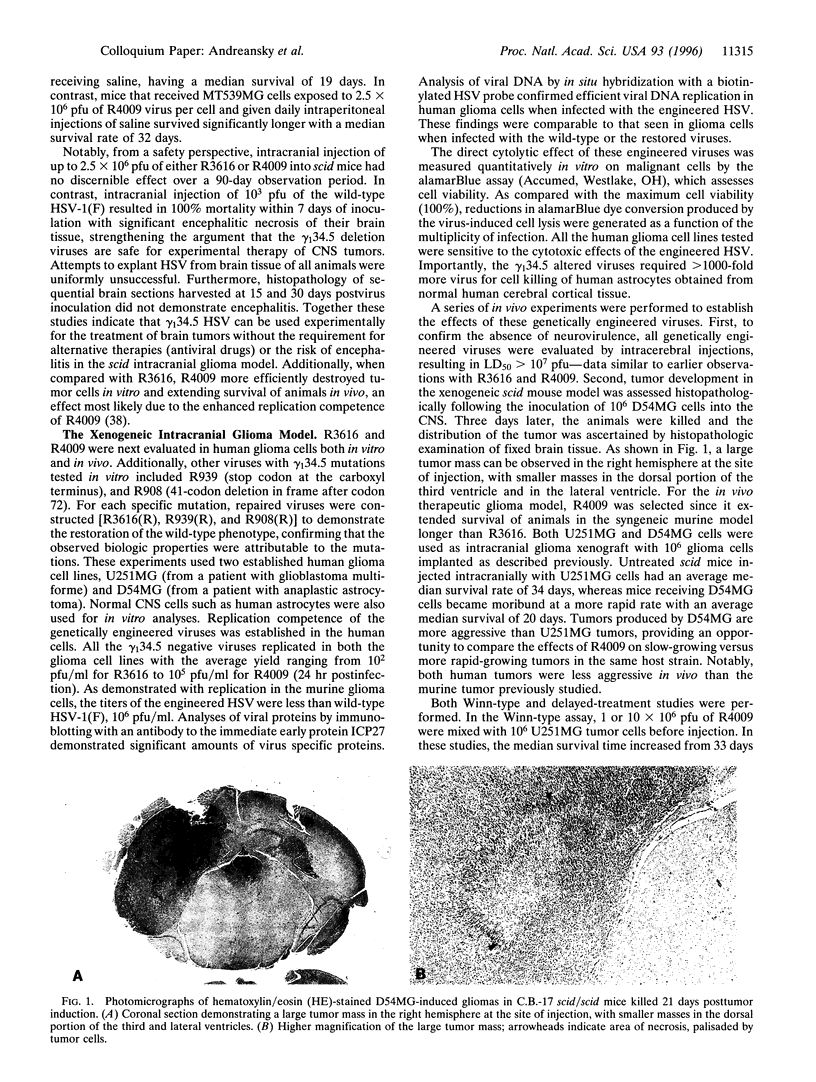
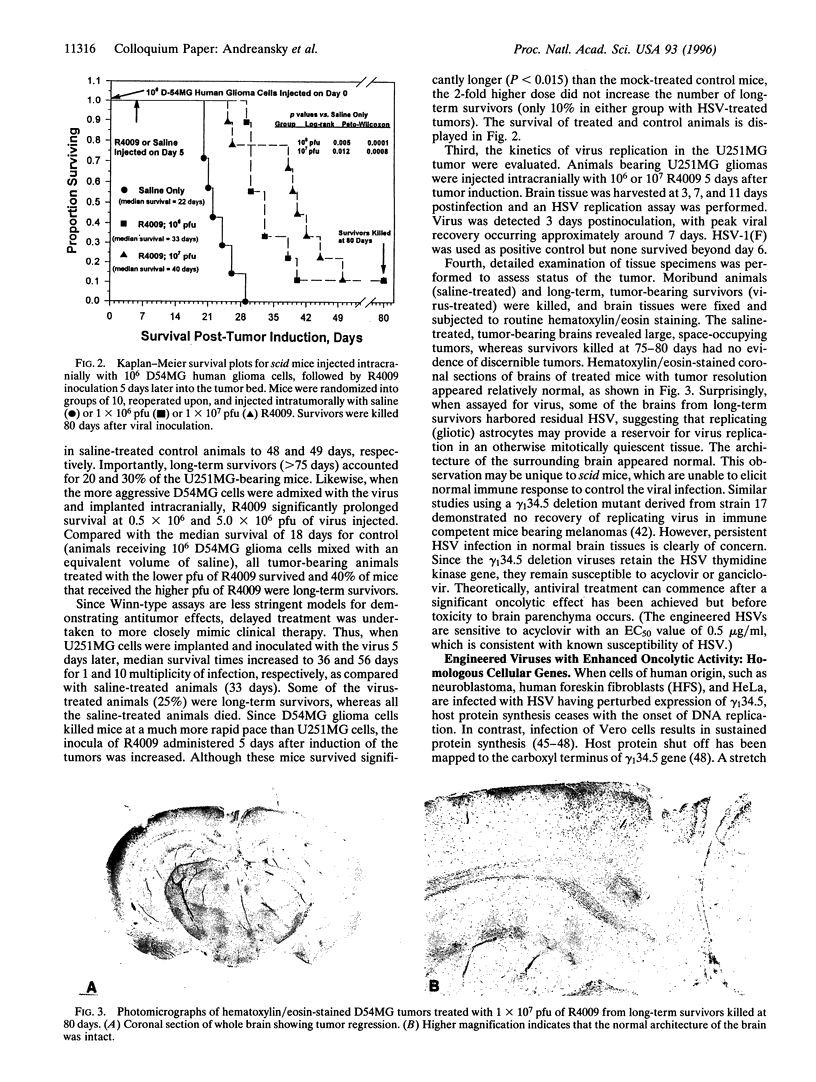
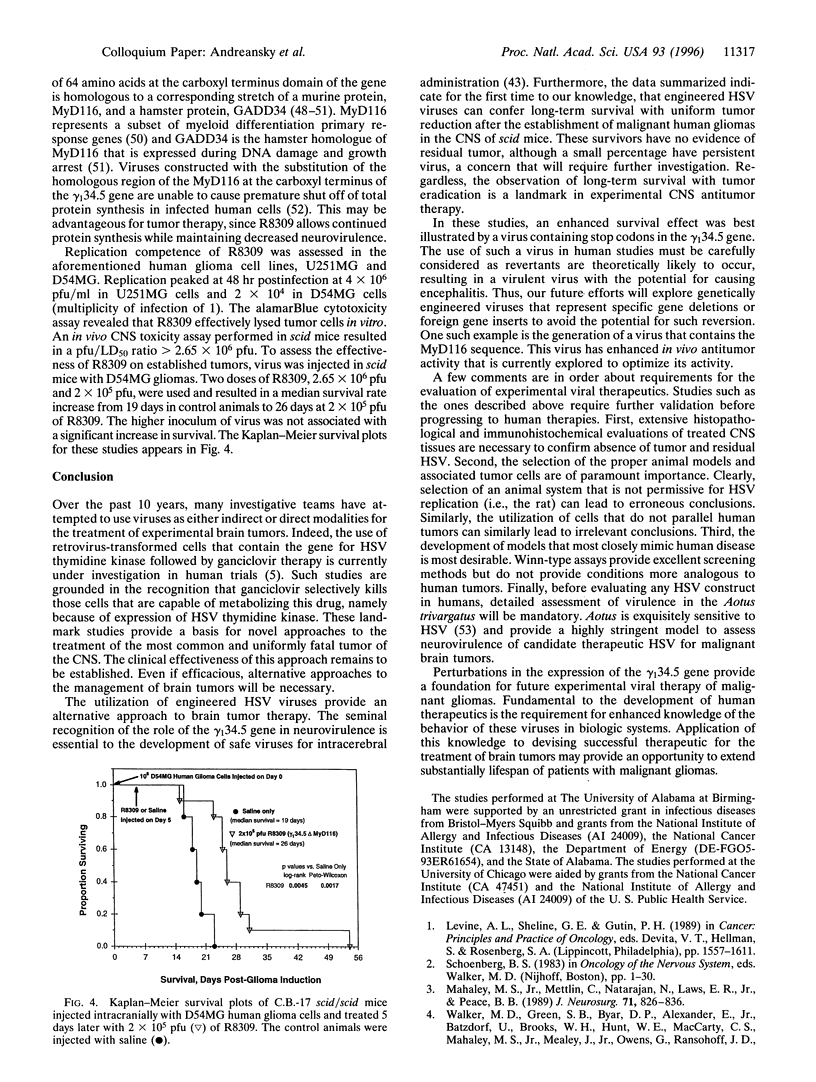
Due to lack of effective therapy, primary brain tumors are the focus of intense investigation of novel experimental approaches that use vectors and recombinant viruses. Therapeutic approaches have been both indirect, whereby vectors are used, or direct to allow for direct cell killing by the introduced virus. Genetically engineered herpes simplex viruses are currently being evaluated as an experimental approach to eradicate malignant human gliomas. Initial studies with gamma (1)34.5 mutants, R3616 (from which both copies of the gamma (1)34.5 gene have been deleted) and R4009 (a construct with two stop codons inserted into the gamma (1)34.5 gene), have been assessed. In a syngeneic scid mouse intracranial tumor model, recombinant herpes simplex virus can be experimentally used for the treatment of brain tumors. These viruses and additional engineered viruses were subsequently tested in human glioma cells both in vitro and in vivo. Using a xenogeneic scid mouse intracranial glioma model, R4009 therapy of established tumors significantly prolonged survival. Most importantly, long-term survival was achieved, with histologic evidence that R4009 eradicated intracranial tumors in this model. Furthermore, the opportunity to evaluate gamma (1)34.5 mutants that have enhanced oncolytic activity, e.g., R8309 where the carboxyl terminus of the gamma (1)34.5 gene has been replaced by the murine homologue, MyD116, are considered.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Tashiro K., Miyatake S., Kinashi T., Nakano T., Oda Y., Kikuchi H., Honjo T. Expression of murine interleukin 7 in a murine glioma cell line results in reduced tumorigenicity in vivo. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3850–3854. doi: 10.1073/pnas.89.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai A., Miyagi Y., Hashimoto H., Lee S. H., Mishima K., Sugiyama A., Tanaka H., Mochizuki T., Yasuda T., Kuchino Y. Modulation of tumor immunogenicity of rat glioma cells by s-Myc expression: eradication of rat gliomas in vivo. Cell Growth Differ. 1994 Nov;5(11):1153–1158. [PubMed] [Google Scholar]
- Badie B., Drazan K. E., Kramar M. H., Shaked A., Black K. L. Adenovirus-mediated p53 gene delivery inhibits 9L glioma growth in rats. Neurol Res. 1995 Jun;17(3):209–216. doi: 10.1080/01616412.1995.11740314. [DOI] [PubMed] [Google Scholar]
- Boviatsis E. J., Scharf J. M., Chase M., Harrington K., Kowall N. W., Breakefield X. O., Chiocca E. A. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994 Sep;1(5):323–331. [PubMed] [Google Scholar]
- Chambers R., Gillespie G. Y., Soroceanu L., Andreansky S., Chatterjee S., Chou J., Roizman B., Whitley R. J. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Shine H. D., Goodman J. C., Grossman R. G., Woo S. L. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Chen J. J., Gross M., Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5- mutants of herpes simplex virus 1. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Kern E. R., Whitley R. J., Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990 Nov 30;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Chou J., Poon A. P., Johnson J., Roizman B. Differential response of human cells to deletions and stop codons in the gamma(1)34.5 gene of herpes simplex virus. J Virol. 1994 Dec;68(12):8304–8311. doi: 10.1128/jvi.68.12.8304-8311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. Herpes simplex virus 1 gamma(1)34.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5247–5251. doi: 10.1073/pnas.91.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo B. M., Benedetti S., Ottolenghi S., Mora M., Pollo B., Poli G., Finocchiaro G. The "bystander effect": association of U-87 cell death with ganciclovir-mediated apoptosis of nearby cells and lack of effect in athymic mice. Hum Gene Ther. 1995 Jun;6(6):763–772. doi: 10.1089/hum.1995.6.6-763. [DOI] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Chou J., Liebermann D. A., Hoffman B., Roizman B. The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the gamma(1)34.5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J Virol. 1996 Jan;70(1):84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B. E., Austin E. A., Richards C. A., Davis S. T., Good S. S. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W. W., McDermott M., Goldie J., Cynader M., Tan J., Tufaro F. Selective destruction of gliomas in immunocompetent rats by thymidine kinase-defective herpes simplex virus type 1. J Natl Cancer Inst. 1994 Aug 17;86(16):1209–1215. doi: 10.1093/jnci/86.16.1209. [DOI] [PubMed] [Google Scholar]
- Kaplitt M. G., Tjuvajev J. G., Leib D. A., Berk J., Pettigrew K. D., Posner J. B., Pfaff D. W., Rabkin S. D., Blasberg R. G. Mutant herpes simplex virus induced regression of tumors growing in immunocompetent rats. J Neurooncol. 1994;19(2):137–147. doi: 10.1007/BF01306455. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Kim S. H., Brown S. L., Freytag S. O. Selective enhancement by an antiviral agent of the radiation-induced cell killing of human glioma cells transduced with HSV-tk gene. Cancer Res. 1994 Dec 1;54(23):6053–6056. [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Sequence of MyD116 cDNA: a novel myeloid differentiation primary response gene induced by IL6. Nucleic Acids Res. 1990 May 11;18(9):2823–2823. doi: 10.1093/nar/18.9.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaley M. S., Jr, Mettlin C., Natarajan N., Laws E. R., Jr, Peace B. B. National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989 Dec;71(6):826–836. doi: 10.3171/jns.1989.71.6.0826. [DOI] [PubMed] [Google Scholar]
- Markert J. M., Malick A., Coen D. M., Martuza R. L. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993 Apr;32(4):597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Martuza R. L., Malick A., Markert J. M., Ruffner K. L., Coen D. M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991 May 10;252(5007):854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Barnett B. C. Neurovirulence factor. Nature. 1991 Oct 17;353(6345):609–609. doi: 10.1038/353609b0. [DOI] [PubMed] [Google Scholar]
- Meignier B., Martin B., Whitley R. J., Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus). J Infect Dis. 1990 Aug;162(2):313–321. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- Mineta T., Rabkin S. D., Martuza R. L. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994 Aug 1;54(15):3963–3966. [PubMed] [Google Scholar]
- Mineta T., Rabkin S. D., Yazaki T., Hunter W. D., Martuza R. L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995 Sep;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Mullen C. A., Coale M. M., Lowe R., Blaese R. M. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 1994 Mar 15;54(6):1503–1506. [PubMed] [Google Scholar]
- Mullen C. A., Kilstrup M., Blaese R. M. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):33–37. doi: 10.1073/pnas.89.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E. H., Ram Z., Culver K. W., Blaese R. M., DeVroom H. L., Anderson W. F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- Perez-Cruet M. J., Trask T. W., Chen S. H., Goodman J. C., Woo S. L., Grossman R. G., Shine H. D. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res. 1994 Nov 1;39(4):506–511. doi: 10.1002/jnr.490390417. [DOI] [PubMed] [Google Scholar]
- Raffel C., Culver K., Kohn D., Nelson M., Siegel S., Gillis F., Link C. J., Villablanca J. G., Anderson W. F. Gene therapy for the treatment of recurrent pediatric malignant astrocytomas with in vivo tumor transduction with the herpes simplex thymidine kinase gene/ganciclovir system. Hum Gene Ther. 1994 Jul;5(7):863–890. doi: 10.1089/hum.1994.5.7-863. [DOI] [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Frank J. A., Blaese R. M., Oldfield E. H. Toxicity studies of retroviral-mediated gene transfer for the treatment of brain tumors. J Neurosurg. 1993 Sep;79(3):400–407. doi: 10.3171/jns.1993.79.3.0400. [DOI] [PubMed] [Google Scholar]
- Ram Z., Walbridge S., Heiss J. D., Culver K. W., Blaese R. M., Oldfield E. H. In vivo transfer of the human interleukin-2 gene: negative tumoricidal results in experimental brain tumors. J Neurosurg. 1994 Mar;80(3):535–540. doi: 10.3171/jns.1994.80.3.0535. [DOI] [PubMed] [Google Scholar]
- Randazzo B. P., Kesari S., Gesser R. M., Alsop D., Ford J. C., Brown S. M., Maclean A., Fraser N. W. Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus 1 mutant. Virology. 1995 Aug 1;211(1):94–101. doi: 10.1006/viro.1995.1382. [DOI] [PubMed] [Google Scholar]
- Redekop G. J., Naus C. C. Transfection with bFGF sense and antisense cDNA resulting in modification of malignant glioma growth. J Neurosurg. 1995 Jan;82(1):83–90. doi: 10.3171/jns.1995.82.1.0083. [DOI] [PubMed] [Google Scholar]
- Resnicoff M., Sell C., Rubini M., Coppola D., Ambrose D., Baserga R., Rubin R. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994 Apr 15;54(8):2218–2222. [PubMed] [Google Scholar]
- Takamiya Y., Short M. P., Moolten F. L., Fleet C., Mineta T., Breakefield X. O., Martuza R. L. An experimental model of retrovirus gene therapy for malignant brain tumors. J Neurosurg. 1993 Jul;79(1):104–110. doi: 10.3171/jns.1993.79.1.0104. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Mulé J. J. Experimental and clinical studies of cytokine gene-modified tumor cells. Hum Gene Ther. 1994 Feb;5(2):153–164. doi: 10.1089/hum.1994.5.2-153. [DOI] [PubMed] [Google Scholar]
- Tjuvajev J., Gansbacher B., Desai R., Beattie B., Kaplitt M., Matei C., Koutcher J., Gilboa E., Blasberg R. RG-2 glioma growth attenuation and severe brain edema caused by local production of interleukin-2 and interferon-gamma. Cancer Res. 1995 May 1;55(9):1902–1910. [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Ilan J., Tykocinski M. L., Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993 Jan 1;259(5091):94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- Van Meir E. G., Polverini P. J., Chazin V. R., Su Huang H. J., de Tribolet N., Cavenee W. K. Release of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nat Genet. 1994 Oct;8(2):171–176. doi: 10.1038/ng1094-171. [DOI] [PubMed] [Google Scholar]
- Van Meir E. G., Roemer K., Diserens A. C., Kikuchi T., Rempel S. A., Haas M., Huang H. J., Friedmann T., de Tribolet N., Cavenee W. K. Single cell monitoring of growth arrest and morphological changes induced by transfer of wild-type p53 alleles to glioblastoma cells. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1008–1012. doi: 10.1073/pnas.92.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. D., Green S. B., Byar D. P., Alexander E., Jr, Batzdorf U., Brooks W. H., Hunt W. E., MacCarty C. S., Mahaley M. S., Jr, Mealey J., Jr Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980 Dec 4;303(23):1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- Wei M. X., Tamiya T., Hurford R. K., Jr, Boviatsis E. J., Tepper R. I., Chiocca E. A. Enhancement of interleukin-4-mediated tumor regression in athymic mice by in situ retroviral gene transfer. Hum Gene Ther. 1995 Apr;6(4):437–443. doi: 10.1089/hum.1995.6.4-437. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R., Hallahan D. E., Beckett M. A., Mauceri H. J., Lee H., Sukhatme V. P., Kufe D. W. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells. Cancer Res. 1994 Aug 15;54(16):4266–4269. [PubMed] [Google Scholar]
- Whitley R. J., Kern E. R., Chatterjee S., Chou J., Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest. 1993 Jun;91(6):2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. K., Cano W. G., Meylaerts S. A., Qi P., Vrionis F., Cherington V. Bystander tumoricidal effect in the treatment of experimental brain tumors. Neurosurgery. 1994 Dec;35(6):1094–1103. doi: 10.1227/00006123-199412000-00012. [DOI] [PubMed] [Google Scholar]
- Yagi K., Hayashi Y., Ishida N., Ohbayashi M., Ohishi N., Mizuno M., Yoshida J. Interferon-beta endogenously produced by intratumoral injection of cationic liposome-encapsulated gene: cytocidal effect on glioma transplanted into nude mouse brain. Biochem Mol Biol Int. 1994 Jan;32(1):167–171. [PubMed] [Google Scholar]
- Yu J. S., Wei M. X., Chiocca E. A., Martuza R. L., Tepper R. I. Treatment of glioma by engineered interleukin 4-secreting cells. Cancer Res. 1993 Jul 1;53(13):3125–3128. [PubMed] [Google Scholar]




