Abstract
Background
Downsizing strategies are often attempted for patients with hepatocellular carcinoma (hcc) before liver transplantation (lt). The objective of the present study was to determine clinical predictors of favourable survival outcomes after transarterial chemoembolization (tace) before lt for hcc outside the Milan criteria, so as to better select candidates for this strategy.
Methods
In this retrospective study, patients with hcc tumours either beyond Milan criteria (single lesion > 5 cm, 3 lesions with 1 or more > 3 cm) or at the upper limit of Milan criteria (single lesions between 4.1 cm and 5.0 cm), with a predicted waiting time of more than 3 months, received carboplatin-based tace treatments. Exclusion criteria for tace included Child–Pugh C cirrhosis or the presence of portal vein invasion or extrahepatic disease on imaging. Only patients without tumour progression after tace underwent lt.
Results
Of 160 hcc patients who received liver grafts between 1997 and 2010, 35 were treated with tace preoperatively. The median of the sum of tumour diameters was 6.7 cm (range: 4.8–8.5 cm), which decreased with tace to 5.0 cm (range: 3.3–7.0 cm) at transplantation (p < 0.0004). The percentage drop in alpha-fetoprotein (αfp) was a positive predictor (p = 0.0051) and the time from last tace treatment to transplantation was a negative predictor (p < 0.0001) for overall survival.
Conclusions
The percentage drop in αfp and a shorter time from the final tace treatment to transplantation significantly predicted improved overall survival after lt for hcc downsized with tace. As a serum marker, αfp should be followed when tace is used as a strategy to stabilize or downsize hcc lesions before lt.
Keywords: Hepatocellular carcinoma, transarterial chemoembolization, alpha-fetoprotein, liver transplantation
1. INTRODUCTION
The incidence of hepatocellular carcinoma (hcc) in North America has nearly doubled since the early 1990s, paralleling the rising incidence of hepatitis C virus–related cirrhosis1–3. Hepatocellular carcinoma is often diagnosed late and has a 5-year survival rate of 11% in the United States4.
Transplantation is a therapeutic option unique to hcc among malignancies, having been brought to the forefront in 19965. Transplantation has been adopted as standard practice when a patient has both underlying liver dysfunction and hcc fulfilling the Milan criteria (single lesion ≤ 5 cm, or 2–3 lesions ≤ 3 cm)5. Some centres—for example, San Francisco6 (tumour volume < 115 cm3) and, most recently, Toronto7—have extended those criteria. Liver transplantation for advanced hcc using poor tumour differentiation on biopsy as an exclusion criterion is also practiced, because this approach better correlates with pre-transplantation radiology, potentially includes a greater number of candidates, and has comparable survival outcomes8. However, it is thought that the foregoing criteria may be too restrictive, because hcc tumour biology is variable and does not necessarily correlate with size.
A novel prognostic model, based on individual tumour characteristics, has been developed to more precisely identify patients beyond Milan criteria who could benefit from transplantation9. Transplant centres have also used chemoembolization to attempt to downsize large or multiple tumours that do not conform to Milan criteria, permitting subsequent liver transplantation. This downsizing strategy has been successful in carefully selected patients, showing survival rates comparable to those achieved with transplantation within the Milan criteria6,10–13. However, the waiting time for transplantation is especially crucial in hcc, and studies revealed a high dropout rate because of progression in tumour size14,15.
The Milan criteria do not integrate response to tace treatment, and hence the goal of the present study was to assess whether dynamic changes in clinical and laboratory parameters such as alpha-fetoprotein (αfp) might predict tumour stabilization or response to tace as a modality to downsize hcc before liver transplantation. Establishing such predictors of success might lead to improved outcomes after transplantation.
2. METHODS
2.1. Patients
Patients were included in our retrospective study if they were treated with tace before liver transplantation, having met one of these criteria6,14:
Single lesion greater than 5 cm
Three hcc lesions, with one or more being greater than 3 cm
More than 3 hcc lesions
Just within all of the foregoing criteria, with a tumour at risk for aggressive tumour biology (rapid growth of more than 2 cm within 6 months or size greater than 4 cm, with expected wait for transplantation of more than 3 months)
Patients were excluded from consideration of tace in the presence of portal vein invasion or extra-hepatic disease based on chest, abdomen, and pelvis computed tomography (ct) imaging, gallium scan, and bone scan. Additionally, patients deemed not to be fit for transplantation and those with Child–Pugh C cirrhosis were excluded. After transplantation, patients were followed by the transplant team, which included the transplant surgeon, transplant hepatologist, and transplant nursing coordinator.
The diagnosis of hcc was established based on
a new mass on ct imaging in a patient with cirrhosis, associated with elevated or rising serum αfp;
a mass compatible with hcc on ct imaging, corroborated by magnetic resonance imaging (mri) or ethiodized oil (Lipiodol: Guerbet Group, Paris, France) ct; or
a hepatic mass increasing in size on serial imaging in patients with underlying cirrhosis.
All tumour measurements were standardized and performed by an experienced imaging team using multiphasic ct–mri, with thin cuts through the liver, combined with ultrasonography (us) in a standardized protocol. Ethics approval to conduct the study was obtained from the McGill University Health Centre Institutional Research Ethics board.
2.2. Treatment Protocol
In at-risk hcc patients, tace was used to stabilize disease and prevent its progression during the wait for liver transplantation. Hepatic vascular anatomy was assessed by cross-sectional imaging (ct–mri) and corroborated using a superior mesenteric artery or splenic artery angiogram (or both). The hepatic artery was catheterized, and an emulsion prepared using carboplatin (300 mg/m2: Faulding Canada, Montreal, QC), 10 mL ethiodized oil (Lipiodol Ultra-Fluid: Guerbet Group), and iodine contrast was injected selectively into the arterial branches feeding the hcc tumours. The emulsion was injected slowly under fluoroscopic monitoring. Subsequently, the arterial branches were embolized using small gelatin pellets (Gelfoam: Pharmacia and Upjohn, Mississauga, ON). Before liver transplantation, chemotherapy was thus delivered every 4–6 weeks via embolization of the hepatic artery branches supplying the tumour or tumours. The goal of the treatment was to restrict arterial flow without causing total obstruction or reflux of drug into areas of unaffected liver. Antibacterial prophylaxis with ciprofloxacin (500 mg twice daily) was given for 5 days. Each patient received tace treatments for as long as viable tumours appeared on imaging studies (ct or mri, as evidenced by persistent enhancement with contrast) or αfp failed to drop16.
Baseline assessment in the patients consisted of physical examination; laboratory tests, including serum αfp; and imaging, including abdominal ct and us. Upon starting tace treatment, serum αfp was determined monthly. Abdominal us and ct were performed every 4–6 weeks to follow the response to tace. Only patients with stable or decreasing tumour size were placed on the transplant list. If patients experienced acute decompensation during the tace treatments or showed no response by any of the imaging criteria, they were not offered transplantation.
Baseline tumour size was defined as the sum of the maximum diameters of all lesions noted initially in imaging. Final tumour size was defined as the sum of the maximum diameters of all lesions identified during histopathologic examination of the explanted specimen (including lesions not identified preoperatively). Change in tumour size is expressed as a percentage of baseline size over final tumour size. Similarly, change in serum αfp is expressed as a percentage of the baseline level.
Because ethiodized oil was used for the chemoembolization treatment, contrast uptake from the tumour during follow-up ct imaging was not thought to be a sensitive criterion for changes in tumour size. Nevertheless, ct evidence of tumour progression was an indication for further diagnostic work-up with mri, which has high sensitivity for hcc staging17–19.
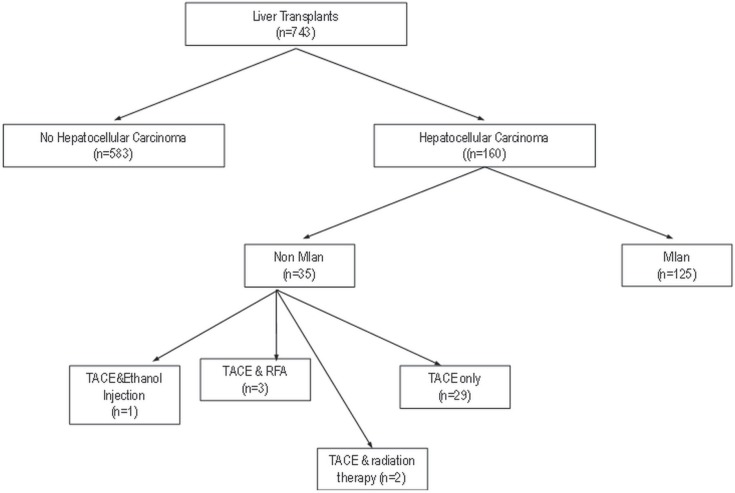
After liver transplantation, each patient underwent serial serum αfp measurements. Plain chest radiography and us were performed every 6 months for 3 years and then annually. Any evidence of disease recurrence, demonstrated by a rise in serum αfp or a mass evident on imaging, prompted a full metastatic work-up. Figure 1 depicts the algorithm of the study protocol, accounting for all patients undergoing downsizing tace.
FIGURE 1.
Flowchart of patients having undergone liver transplantation for hepatocellular carcinoma at the McGill University Health Centre. tace = transarterial chemoembolization; rfa = radiofrequency ablation.
The preoperative clinical diagnosis of hcc was cross-validated with the pathology findings in all transplanted patients. An experienced liver pathologist examined all explanted liver specimens. The number of tumours, the maximum tumour diameter, the histologic grade (modified Edmonson criteria)20, and the presence of microvascular invasion were noted. Pathologic staging used in the study was based on the classification of the International Union Against Cancer and the American Joint Committee on Cancer21.
2.3. Statistical Analysis
Statistical analysis was performed using the SAS software application (version 9.2: SAS Institute, Cary, NC, U.S.A.). The percentage drop in serum αfp was calculated as:
Survival in tace-treated patients was compared with survival in patients whose hcc was within and outside the Milan criteria and who did not undergo tace before liver transplantation.
One-way anova and independent t-test were used to measure change in continuous variables. Wilcoxon nonparametric testing was used for asymmetrically distributed variables. Logistic regression was used to assess the true associations of variables with recurrence and survival. Survival rates were calculated using the Kaplan–Meier method. A value of p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient Demographics
Between 1997 and 2011, 35 patients underwent tace for hcc before liver transplantation at the McGill University Health Center Liver Transplant Program (Figure 1). All were followed after transplantation.
Table i shows the demographic characteristics of the patient cohort. Mean age of the liver graft recipients was 61.7 ± 7.2 years, and 65.7% of the patients receiving grafts had hepatitis C or B as the underlying cause of their liver disease. Cirrhosis was present in 91.4% of patients.
TABLE I.
Baseline demographic and clinical characteristics of the liver transplant recipients
| Characteristic | Value |
|---|---|
| Patients (n) | 35 |
| Sex [n (%)] | |
| Men | 31 (88.6) |
| Women | 4 (11.4) |
| Mean age (years) | 61.7±7.2 |
| Follow-up (months) | |
| Mean | 27±22 |
| Range | 1–72 |
| Mean bmi (kg/m2) | 26.5±4.4 |
| Cirrhosis [n (%)] | |
| Present | 32 (91.4) |
| Cause | |
| Hepatitis C virus | 15 (42.9) |
| Hepatitis B virus | 8 (22.9) |
| nash | 8 (22.9) |
| aat deficiency | 1 (2.9) |
| Autoimmune hepatitis | 1 (2.9) |
| Hemochromatosis | 1 (2.9) |
| Mean meld score | 21.1±1.8 |
| Serum αfp (ng/mL) | |
| Median | 43 |
| Range | 8.55–118.25 |
bmi = body mass index; aat deficiency = α1-antitrypsin deficiency; nash = nonalcoholic steatohepatitis; meld = model for end-stage liver disease; αfp = alpha-fetoprotein.
3.2. HCC and Downsizing with TACE
Most patients (48.6%) had stage ii hcc (by TNM staging), with 42.9% having stage iii disease, and 8.6%, stage i disease (Table ii).
TABLE II.
Histopathologic features of hepatomas in 35 patients undergoing liver transplantation after transarterial chemoembolization (tace) treatment
| Feature | Value |
|---|---|
| Lesions [n (%)] | |
| Single | 9 (25.7) |
| Multiplea | 26 (74.3) |
| Sum of tumour diameters (cm) | |
| Before tace | |
| Median | 6.7 |
| Range | 4.8–8.5 |
| After tace (in explant) | |
| Median | 5.0 |
| Range | 3.3–7.0 |
| Pathologic TNM [n (%)] | |
| i | 3 (8.6) |
| ii | 17 (48.6) |
| iii | 15 (42.9) |
| iva | 0 (0) |
| Histologic grade [n (%)] | |
| 1 | 5 (14.3) |
| 2 | na |
| 3 | na |
| Lymph node involvement [n (%)] | 2 (5.7) |
| Invasion [n (%)] | |
| Microvascular | 11 (31.4) |
| Macrovascular | 0 (0) |
Defined as 2–8 lesions.
na = not interpretable because of necrosis.
The median of the sum of tumour diameters at baseline was 6.7 cm (range: 4.8–8.5 cm), which decreased with tace to 5.0 cm (range: 3.3–7.0 cm) at transplantation (p < 0.0004). Micro- or macro-vascular invasion was present in 31.4% of explant specimens. Recurrent hcc was diagnosed in 25.7% by newly detectable serum αfp in addition to confirmatory findings on imaging performed as part of the routine post-transplantation follow-up. Patients had received a median of 2 tace treatments (interquartile range: 1–3 treatments), with 28.2% receiving 1 treatment; 25.6%, 2 treatments; and 46.2%, 3–4 treatments. Concomitantly with tace, 5 patients received systemic chemotherapy, which was part of the practice in the late 1990s. One patient received 4 alcohol-injection treatments in addition to a single tace treatment, which together succeeded in downsizing the tumour. In addition to tace treatments, 2 patients received focused external-beam radiation therapy once, and 3 patients received a single radiofrequency ablation (rfa) treatment followed by tace for residual tumour post-ablation.
The median αfp level before tace was 43 ng/mL (range: 8.6–118.3 ng/mL), which decreased after tace to a median of 14.5 ng/mL (range: 5.7–33 ng/mL; p = 0.0003). The difference translated into a 43.0% drop in serum αfp, which correlated with less hcc recurrence (p = 0.012) and a trend toward improved overall survival (p = 0.086). The number of tace treatments (p = 0.04) and age (p = 0.0006) were also significantly associated with the slope of the drop in serum αfp. However, when logistic regression was performed, none of the foregoing variables were found to be predictive of the serum αfp slope.
3.3. Liver Transplantation
The median wait time between the last tace treatment and the date of transplantation was 76 days (range: 34–167 days). The average cold ischemia time of the transplanted organs was 8. 8 ± 3.0 hours, with a donor risk index of 1.54 ± 0.33.
3.4. HCC Recurrence and Predictors of Recurrence-Free Interval
Of the 35 patients, 9 (25.7%) experienced hcc recurrence at a median of 701 days after transplantation (interquartile range: 182.75–1536 days). In an effort to identify predictors of hcc recurrence after liver transplantation, we analyzed the predictive value of pretransplant serum αfp, demographic variables, and other clinical characteristics.
On univariate analysis, the cause of liver disease (p = 0.033), the pathology grade of hcc (p = 0.012), receipt of systemic chemotherapy before transplantation (p = 0.036), cold ischemia time (p = 0.02), and serum αfp exceeding 1000 ng/mL before transplantation (p = 0.008) were all significantly associated with recurrence. When subjected to a multivariate logistic regression analysis, only a preoperative serum αfp exceeding 1000 ng/mL (p = 0.033) and cause of liver disease (p = 0.042) were found to be predictive of hcc recurrence. The model held significance with a p value of 0.0025.
3.5. Patient Survival Analysis
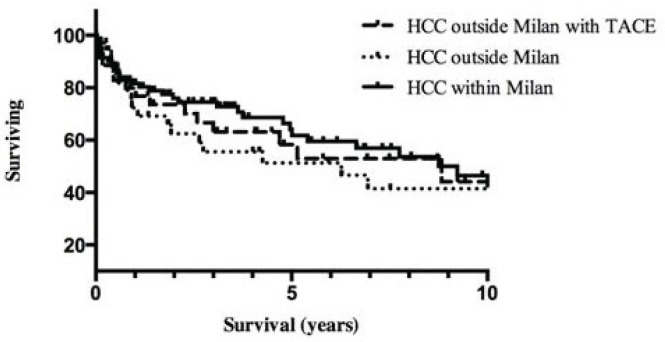
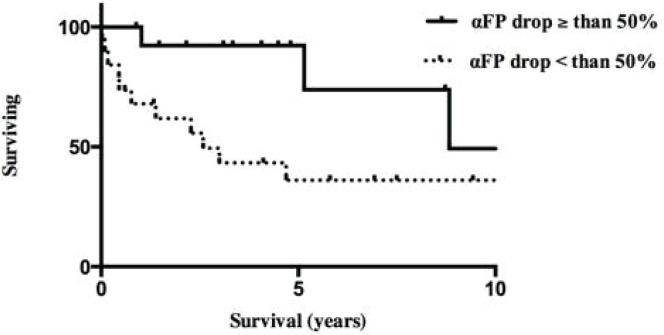
After transplantation, survival in all patients whose hcc tumours were downsized with tace was 88%, 78%, and 50% at 1, 3, and 5 years respectively. Those survivals are comparable to those for patients with hcc within the Milan criteria (Figure 2). In the univariate analysis, the percentage drop in αfp (p = 0.039), the time elapsed between the final tace and transplantation (p < 0.0001), sex (p = 0.003), age (p = 0.0001), body mass index (p = 0.0001), the presence of a single hcc lesion (p = 0.021), cold ischemia time (p = 0.0003), and donor risk index (p = 0.004) were significantly associated with overall patient survival. The logistic regression model revealed that the percentage drop in αfp (p = 0.0051) and a shorter elapsed time between the final tace and transplantation (p < 0.0001) were predictive of improved overall survival. The model was significant with a p value of 0.0015. As shown in Figure 3, patients with a drop in αfp exceeding 50% had a median survival of more than 8 years; the remaining patients, with a drop in αfp of less than 50%, survived for a median of 2.5 years (p = 0.031).
FIGURE 2.
Survival of hepatocellular carcinoma (hcc) patients downsized with transarterial chemoembolization compared with that of patients within and outside the Milan criteria.
FIGURE 3.
Median survival of hepatocellular carcinoma (hcc) patients with drop in serum alpha-fetoprotein (αfp) greater and less than 50%.
4. DISCUSSION
The variability in hcc tumour biology makes treatment of this malignancy using a “one size fits all” approach difficult. Liver transplantation in hcc patients beyond the Milan criteria has been shown to achieve outcomes comparable to those for patients within the Milan criteria when the total tumour volume is less than 115 cm3 and when less aggressive tumours can be downsized as defined by response to tace in selected patients. Thus, it appears that neoadjuvant therapy can expand the criteria under which liver transplantation can be offered to patients with hcc. However, treatment with tace to stabilize or downstage a tumour is often performed without a clear idea of the tumour’s biology. Through sampling error, even routine biopsies of hcc can fail to provide precise information about the tumour’s characteristics and differentiation, given that not all parts of the same tumour mass necessarily demonstrate the same histologic grade22. Treating patients with hepatomas beyond the Milan criteria with tace therefore permits dynamic selection of tumours with more favourable biology and might predict whether a good outcome can be expected after transplantation.
We hypothesized that a reduction in serum αfp might be able to be used as a surrogate for hcc tumour biology and response to tace. In our cohort of hcc patients, the percentage drop in serum αfp and a shorter wait time between the final tace and transplantation were strongly predictive of survival after liver transplantation. Those findings indicate that the trend in serum αfp during tace treatment can be used to gauge whether a liver graft would produce a favourable survival outcome in a specific patient. We also found that the presence of a preoperative αfp exceeding 1000 ng/mL predicts hcc recurrence after transplantation regardless of a significant drop during tace treatment, indicating that even this approach has its limitations. Because a longer wait time between tace and transplantation was associated with decreased survival, some type of prioritization of these patients might be warranted.
Successfully targeted tace should lead to a decrease in the amount of viable tumour, but corresponding changes in size on cross-sectional imaging may lag behind. Given that a diminished viable tumour burden is known to correlate with a decline in serum αfp23,24, serum αfp may be a more rapid indicator of the type of response produced by tace. If serum αfp fails to decrease, then one of three factors is possible:
Technical or anatomic issues are preventing the treatment from reaching the tumour.
The tumour has a very aggressive biology and is not affected by the treatment. In fact, poor tumour differentiation has been used as a criterion to exclude patients with downstaged advanced hcc from transplantation, with excellent survival rates7.
Undetected tumours are present in other parts of the liver or outside the liver, or both.
All of those factors would preclude patients from liver transplantation, because outcomes would be poor. Thus, the change in serum αfp during a tace protocol might select patients who are most likely to benefit from transplantation.
We initially started performing the tace procedure at our institution to downstage hcc or to prevent hcc disease progression. On the basis of stabilization or response, the decision was made to transplant. Instituting a carboplatin-based tace protocol for patients with hcc tumours exceeding Milan criteria, just within Milan criteria, or seen to be rapidly growing on sequential imaging results in a larger number of candidates proceeding to transplantation with excellent outcomes, as our study shows.
Many transplant programs have noted that not all patients whose tumours are beyond Milan have a dismal outcome after transplantation, and they would like to offer the opportunity for transplantation to this subcategory of patients; however, the challenge has been to identify and characterize this specific group.
Several research groups have used neoadjuvant treatments to address this challenge. A prospective study by Yao et al.6,14 downsized large-volume hcc to achieve conventional Milan criteria before liver transplantation. Those authors carefully selected eligible patients who had a single lesion of 8 cm maximal size or up to 3 lesions no greater than 5 cm in diameter. Downsizing with tace or rfa was achieved in 43 of 61 patients, who then went on to transplantation. No hcc recurred, and the 4-year survival rate was excellent at 92.1%. A pretreatment serum αfp exceeding 1000 ng/mL was discovered to be predictive of treatment failure. The authors concluded that the upper limits of tumour size and number, together with criteria for response to tace, had to be strictly enforced to achieve these stellar post-transplantation outcomes. A second smaller study by Chapman et al.10 enrolled 76 stage iii/iv hcc patients and was able to downstage 18 of them to Milan criteria using tace. Of those 18 patients, 16 were alive at 20 months after transplantation, and 1 patient experienced hcc recurrence (1 patient died from an unrelated cause). A prospective Italian study enrolled 48 hcc patients slightly beyond the Milan criteria (1 tumour ≤6 cm in diameter; or multiple tumours with none ≤5 cm and the sum diameter ≤12 cm) for a downsizing protocol. The recurrence-free survival rates were comparable for the downsized and Milan-criteria groups, with survival rates of 78% and 80% respectively at 1 year, and 71% and 71% at 3 years.
Radiofrequency ablation has also been tested on its own for downsizing to respect the Milan criteria5. A proof-of-concept study used rfa to downsize hccs to T2 from T3 in 19 of 34 patients, achieving a 1-year survival of 84% and a 3-year survival of 27%25. Radiofrequency ablation has also been directly compared with tace for success of downsizing, with 43 patients and a comparable median tumour diameter of 5.6 cm in each group. The 1-year recurrence-free survival was 89% for rfa compared with 73% for tace, and overall survival was 41.6 months compared with 19.2 months (p = 0.008), favouring rfa26. In our patient population, a decreasing serum αfp after tace appears to result in post-transplantation survival rates comparable to those in patients within the Milan criteria.
The principal limitations of our study are those inherent to its retrospective nature. Also, the number of patients whose hcc tumours were downsized with tace was small, although the number was comparable to that in other studies evaluating tace for the purpose of downsizing hcc. Furthermore, 11 patients received additional treatments such as systemic chemotherapy, rfa, external-beam radiation, and ethanol injection in parallel with tace, which was nevertheless the common denominator in terms of the downsizing strategy. Given the clinical significance and utility of our findings, the conclusions drawn merit further study in clinical practice. Although we noted that differences in the percentage change in serum αfp (above or below a 50% decrease) assist in anticipating prognosis in patients after liver transplantation, validation in a larger cohort of patients is required before that finding can be applied in clinical practice.
5. CONCLUSIONS
Treatment of hcc tumours with tace has enabled transplantation in a group of patients previously thought to be ineligible because of poor outcomes. Providing therapeutic tace sessions as part of our neoadjuvant treatment strategy allowed such patients to pass the valuable “test of time” in assessing the biologic behavior of the tumour. In that context, our study revealed that the percentage drop in serum αfp is an excellent marker of response to tace and outcomes after liver transplantation, thus leading to a rational selection of patients who should go on to transplantation.
6. ACKNOWLEDGMENTS
This work was supported by a Fonds de Recherche en Santé du Québec Fellowship to MB.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Kaczynski J, Oden A. The rising incidence of hepatocellular carcinoma. N Engl J Med. 1999;341:451. doi: 10.1056/NEJM199908053410613. [DOI] [PubMed] [Google Scholar]
- 3.Ince N, Wands JR. The increasing incidence of hepatocellular carcinoma. N Engl J Med. 1999;340:798–9. doi: 10.1056/NEJM199903113401009. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY, Hirose R, LaBerge JM, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–14. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 7.DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166–72. doi: 10.1097/SLA.0b013e31820508f1. [DOI] [PubMed] [Google Scholar]
- 8.Toso C, Trotter J, Wei A, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107–15. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 10.Chapman WC, Majella Doyle MB, Stuart JE, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617–25. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 11.Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–57. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 12.Yao FY, Kerlan RK, Jr, Hirose R, et al. Excellent outcome following down staging of hepatocellular carcinoma prior to liver transplantation: an intention to treat analysis. Hepatology. 2008;48:819–27. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanje AJ, Yao FY. Current approach to down-staging of hepatocellular carcinoma prior to liver transplantation. Curr Opin Organ Transplant. 2008;13:234–40. doi: 10.1097/MOT.0b013e3282fc2633. [DOI] [PubMed] [Google Scholar]
- 14.Yao FY, Bass NM, Nikolai B, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention to treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–83. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 15.Maddala YK, Stadheim L, Andrews JC, et al. Drop out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl. 2004;10:449–55. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 16.Kubota K, Hisa N, Nishikawa T, et al. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of lipiodol-ct, power Doppler sonography, and dynamic mri. Abdom Imaging. 2001;26:184–90. doi: 10.1007/s002610000139. [DOI] [PubMed] [Google Scholar]
- 17.Tsui EY, Chan JH, Cheung YK, et al. Evaluation of therapeutic effectiveness of transarterial chemoembolization for hepatocellular carcinoma: correlation of dynamic susceptibility contrast-enhanced echoplanar imaging and hepatic angiography. Clin Imaging. 2000;24:210–16. doi: 10.1016/S0899-7071(00)00204-7. [DOI] [PubMed] [Google Scholar]
- 18.De Santis M, Alborino S, Tartoni PL, Torricelli P, Casolo A, Romagnoli R. Effects of lipiodol retention on mri signal intensity from hepatocellular carcinoma and surrounding liver treated by chemoembolization. Eur Radiol. 1997;7:10–16. doi: 10.1007/s003300050099. [DOI] [PubMed] [Google Scholar]
- 19.Libbrecht L, Bielen D, Verslype C, et al. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749–61. doi: 10.1053/jlts.2002.34922. [DOI] [PubMed] [Google Scholar]
- 20.Kemeny F, Vadrot J, Wu A, Smadja C, Meakins JL, Franco D. Morphological and histological features of resected hepatocellular carcinoma in cirrhotic patients in the West. Hepatology. 1989;9:253–7. doi: 10.1002/hep.1840090215. [DOI] [PubMed] [Google Scholar]
- 21.Vauthey JN, Sobin LH. On the uniform use of the ajcc/uicc staging system for hepatocellular carcinoma. Surgery. 2000;128:870. doi: 10.1067/msy.2000.110225. [DOI] [PubMed] [Google Scholar]
- 22.Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg. 2007;245:435–42. doi: 10.1097/01.sla.0000250420.73854.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan SL, Mo FKF, Johnson PJ, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446–52. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 24.Pan CC, Huang ZL, Li W, et al. Serum alpha-fetoprotein measurement in predicting clinical outcome related to autologous cytokine-induced killer cells in patients with hepatocellular carcinoma undergone minimally invasive therapy. Chin J Cancer. 2010;29:596–602. doi: 10.5732/cjc.009.10580. [DOI] [PubMed] [Google Scholar]
- 25.Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium 90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, rfa and bridge to transplantation. J Surg Oncol. 2006;94:572–86. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 26.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–8. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]