Abstract
Background
Recent studies have suggested a controversial role of Helicobacter pylori infection in gastric cancer prognosis. The aim of the present study was to investigate the potential impact of H. pylori status on the prognosis of patients with gastric cancer in a Chinese prospective cohort.
Methods
Between 2007 and 2009, 261 patients with curatively resected gastric cancer were enrolled in the study. H. pylori status was defined by means of immunohistochemical staining in tumour and non-neoplastic tissues. Treatment prognosis was measured in terms of cancer-specific survival and disease-free survival (dfs). Univariate and multivariate Cox regression models were used to assess the association between H. pylori status and patient prognosis.
Results
Positivity for H. pylori infection was observed in 188 of the 261 patients (72.0%). In patients positive for H. pylori, mean cancer-specific survival was 55.2 months [95% confidence interval (ci): 53.4 to 56.9 months] and mean dfs was 53.9 months (95% ci: 51.8 to 56.0 months); the same survivals were, respectively, 45.1 months (95% ci: 42.2 to 47.9 months) and 43.7 months (95% ci: 40.4 to 47.0 months) in patients negative for H. pylori. In univariate analysis, positive H. pylori status was associated with better cancer-specific survival [hazard ratio (hr): 0.486; 95% ci: 0.271 to 0.870; p = 0.015] and dfs (hr: 0.540; 95% ci: 0.307 to 0.950; p = 0.033). In multivariate analysis, H. pylori was an independent prognostic factor for cancer-specific survival (hr: 0.485; 95% ci: 0.265 to 0.889; p = 0.019).
Conclusions
Our study demonstrates that positive H. pylori status is a beneficial prognostic indicator in patients with gastric cancer and might suggest possible therapeutic approaches for gastric cancer. Further research is required to better understand inflammation mechanisms and cancer progression.
Keywords: Helicobacter pylori, gastric cancer, survival, relapse
1. INTRODUCTION
Cancer development is characterized by stepwise accumulation of various genetic and epigenetic alterations of the genome1,2. Approximately 10%–15% of cancers have been related to chronic infections with bacteria, viruses, or parasites. Chronic inflammation, particularly in the digestive organs, plays an important role in cancer development—for example, Helicobacter pylori–associated gastric cancer.
Gastric cancer is one of the most frequently reported cancers in the world and is characterized by invasivity, metastatic potential, and poor outcomes. Despite promising developments in multimodal treatment strategies, gastric cancer maintains a profile of low survival rates and lack successful therapies, which may be a result of high rates of tumour invasion and lymph node metastasis3,4. The addition of trastuzumab to combination chemotherapy can achieve remarkable survival advantages in patients with advanced gastric cancer positive for the human epidermal growth factor receptor 25. The avagast trial evaluating bevacizumab in combination with chemotherapy demonstrated a higher response rate and a longer progression-free survival time in patients with gastric cancer6.
Gastric cancer is a heterogeneous disease that results from complex interactions involving host genetic, environmental, bacterial, and molecular mechanisms7. Currently, there is an urgent need to identify novel molecular predictive and prognostic biomarkers that will contribute to the effective use of targeted therapy in patients with gastric cancer, facilitate an understanding of gastric carcinogenesis, and reveal new molecular targets for therapeutic intervention7,8.
First discovered by Warren and Marshall in 19829, H. pylori is known to colonize the gastric epithelium in a noninvasive way10. H. pylori infection is a major cause of chronic gastritis, peptic ulcer, mucosa-associated lymphoid tissue (malt) lymphoma, and gastric cancer. In 1994, H. pylori was classified as a definite group 1 carcinogen by the International Agency for Research on Cancer11. Since then, infection with H. pylori has been well established to be a crucial event associated with the risk of gastric cancer. H. pylori infection is so common that more than 50% of the global population harbours the bacterium12. However, only a limited proportion of H. pylori–infected subjects have gastric cancer, which can be explained mainly by host factors, bacterial factors, and environmental factors13,14.
Based on an association between H. pylori infection and the risk of gastric cancer, some investigations have focused on the relationship between H. pylori status and gastric cancer prognosis15–19. Currently, the available data suggest a controversial role of H. pylori infection in the prognosis of patients with gastric cancer, and because of regional differences in H. pylori prevalence and gastric cancer incidence, the effect of H. pylori status on prognosis should be determined in various areas. To the best of our knowledge, only one retrospective study, by Qiu et al.18, has been reported in China, which is well-known as a H. pylori endemic area. In that study, overall survival (os) in 157 gastric cancer patients had no correlation with H. pylori status. Further studies with additional clinically relevant data from prospective cohorts are required to evaluate the prognostic significance of H. pylori infection in patients with gastric cancer. The aim of the present study was to investigate, in a Chinese prospective cohort, the potential impact of H. pylori status on the prognosis of patients undergoing curative resection for gastric cancer.
2. METHODS
2.1 Patients
The cohort consisted of 261 consecutive patients who underwent curative resection for gastric cancer between November 5, 2007, and November 30, 2009, at the First Affiliated Hospital of Anhui Medical University, Hefei, PR China. After surgery, all patients enrolled in the study had a histologically confirmed diagnosis of primary gastric adenocarcinoma. Patients were excluded if they had undergone non-resective surgery and if they had received neoadjuvant treatment before surgery. Cases with Siewert type i cardia adenocarcinoma or distant metastasis were also excluded from the study.
In all patients, the surgeries performed were partial or total gastrectomy. In accordance with the General Rules for the Gastric Cancer Study in Surgery and Pathology of the Japanese Research Society for Gastric Cancer20, a standardized technique was used for surgical resection and lymphadenectomy. Pathology staging followed the International Union Against Cancer (uicc) criteria, 6th edition21. Histology of the gastric cancer was classified according to Lauren’s criteria22. Adjuvant treatments—including chemotherapy and radiotherapy—were chosen by the oncologist based on the pathology findings and patient comorbidities, without knowledge of the patient’s H. pylori status. A database was used to record age, sex, tumour location, uicc stage, tumour grade, Lauren classification, and adjuvant treatments for each patient.
The study protocol was approved by the Institutional Ethics Committee of Anhui Medical University and was conducted according to the principles of the Declaration of Helsinki. Collection of tissue samples and clinical information was undertaken after written informed consent had been obtained from all participants.
2.2. Follow-Up Evaluation
After discharge from our hospital, all patients entered a follow-up program that was conducted according to a standard protocol23. The patients were reviewed every 3 months for 2 years, every 6 months for 2 years, and once again after another 12 months.
The follow-up program consisted of clinical examination, hematologic analyses, measurement of tumour markers, abdominal ultrasonography and chest radiography (every 6 months), and endoscopy of the upper digestive tract (once annually). To complete staging, abdominal computed tomography imaging was performed in cases of suspected recurrence and after diagnosis of recurrence. Recurrences were documented by clinical or radiologic assessment (or both) and were categorized as local, systemic, or combined.
All patients also received monthly telephone follow-up. In cases with presentation of symptoms or suspected indicators of recurrence, further check-ups were conducted immediately. Follow-up was closed on October 16, 2012.
2.3. Endpoints
Analyses for cancer-specific survival and disease-free survival (dfs) were performed. Cancer-specific survival was measured from time of diagnosis to death from gastric cancer–related complications or to the last follow-up. The dfs was calculated from time of diagnosis to disease recurrence or last follow-up.
2.4. Histopathologic Examinations
All resected stomach specimens were fixed in neutral-buffered 10% formalin, embedded in paraffin, and cut into 4-μm sections. The sections were stained with hematoxylin–eosin for histology.
Tumour and non-neoplastic tissues were collected from the resected specimens of stomach for all patients. Non-neoplastic tissue was sampled from the antrum and corpus mucosa at least 5 cm from the tumour. When tumour involved the entire antrum, non-neoplastic mucosa was removed from the middle or upper third of the stomach. Harvested samples were immediately placed in formalin and embedded in paraffin.
Evaluation of H. pylori status was performed using immunohistochemical (ihc) staining. The density of H. pylori was classified as negative (normal) or positive (mild, moderate, marked), using the visual analog scale of the Updated Sydney System24. Patients were regarded as negative for H. pylori if they were negative on tests of both tumour and nonneoplastic samples. Otherwise, they were regarded positive for H. pylori.
2.5. Immunohistochemistry
Results from the ihc staining were independently evaluated by 2 pathologists who had no prior knowledge of the clinical features and outcomes of the patients. Discrepancies between the pathologists were resolved by consensus after discussion.
Tissue sections were deparaffinized, rehydrated, and pretreated by autoclave for antigen retrieval. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide for 10 minutes at room temperature. Incubation with a polyclonal rabbit anti-H. pylori primary antibody (B0471: Dako Corporation, Glostrup, Denmark) at a dilution of 1:50 was conducted at room temperature for 1 hour. After samples had been washed 3 times with phosphate-buffered saline, the Dako EnVision Dual Link System–HRP (K4065: Dako Corporation) was applied for 30 minutes. Finally, sections were incubated in diaminobenzidine for 10 minutes, followed by hematoxylin counterstaining and mounting. H. pylori– infected gastric mucosa from chronic gastritis patients served as positive controls. Negative controls were obtained by replacing the primary antibody with phosphate-buffered saline. H. pylori infection in the tissue sections was confirmed when short, curved, or spiral bacilli resting on the epithelial surface, in the mucus layer, or deep in the gastric pits were observed by light microscopy with ihc staining.
2.5. Statistical Analysis
The association of H. pylori infection with clinical and demographic characteristics was determined using the chi-square test. The prognostic influence of H. pylori infection was assessed by univariate and multivariate Cox proportional hazards regression models. Hazard ratios (hrs) together with 95% confidence intervals (cis) are presented for cancer-specific survival and dfs. All statistical analyses were performed using the SPSS statistical software package (version 13.0: SPSS, Chicago, IL, U.S.A.). Values of p less than 0.05 were considered statistically significant.
3. RESULTS
3.1. Patient Characteristics
Table i shows the main characteristics of the cohort, which included 201 men (77.0%) and 60 women (23.0%), with a median age of 61 years (range: 26–87 years). The uicc staging yielded 79 stage i cases, 77 stage ii cases, 100 stage iii cases, and 5 stage iv cases. After surgery, 182 patients received adjuvant treatment postoperatively: 3 received radiotherapy, and 182 received chemotherapy. At the end of the follow-up, 51 patients had experienced tumour recurrence or distant metastasis, 48 patients had died of gastric cancer–related complications, and 2 patients had been lost to follow-up.
TABLE I.
Clinical and demographic characteristics of gastric cancer patients assessed for Helicobacter pylori status
| Variable | H. pylori status [n (%)]
|
p Value | |
|---|---|---|---|
| Positive | Negative | ||
| Patients (n) | 188 | 73 | |
| Age | 0.034 | ||
| <61 Years | 97 (51.6) | 27 (37.0) | |
| ≥61 Years | 91 (48.4) | 46 (63.0) | |
| Sex | 0.117 | ||
| Women | 48 (25.5) | 12 (16.4) | |
| Men | 140 (74.5) | 61 (83.6) | |
| Tumour location | 0.880 | ||
| Distal (antrum or corpus) | 153 (81.4) | 60 (82.2) | |
| Proximal (cardia or fundus) | 35 (18.6) | 13 (17.8) | |
| uicc stage | 0.337 | ||
| i | 59 (31.4) | 20 (27.4) | |
| ii | 58 (30.9) | 19 (26.0) | |
| iii | 69 (36.7) | 31(42.5) | |
| iv | 2 (1.1) | 3 (4.1) | |
| Grade | 0.516 | ||
| Well differentiated | 6 (3.2) | 4 (5.5) | |
| Moderately differentiated | 64 (34.0) | 28 (38.4) | |
| Poorly differentiated | 118 (62.8) | 41 (56.2) | |
| Lauren classification | 0.231 | ||
| Intestinal | 116 (61.7) | 53 (72.6) | |
| Diffuse | 60 (31.9) | 16 (21.9) | |
| Mixed | 12 (6.4) | 4 (5.5) | |
| Adjuvant treatment | 0.241 | ||
| Yes | 135 (71.8) | 47 (64.4) | |
| No | 53 (28.2) | 26 (35.6) | |
| Status at last follow-up | 0.043 | ||
| Alive | 158 (84.0) | 53 (72.6) | |
| Dead | 29 (15.4) | 19 (26.0) | |
| Unknown | 1 (0.5) | 1 (1.4) | |
| Relapse | 0.042 | ||
| Yes | 31 (16.5) | 20 (27.4) | |
| No | 156 (83.0) | 52 (71.2) | |
| Unknown | 1 (0.5) | 1 (1.4) | |
uicc = Union Internationale Contre le Cancer.
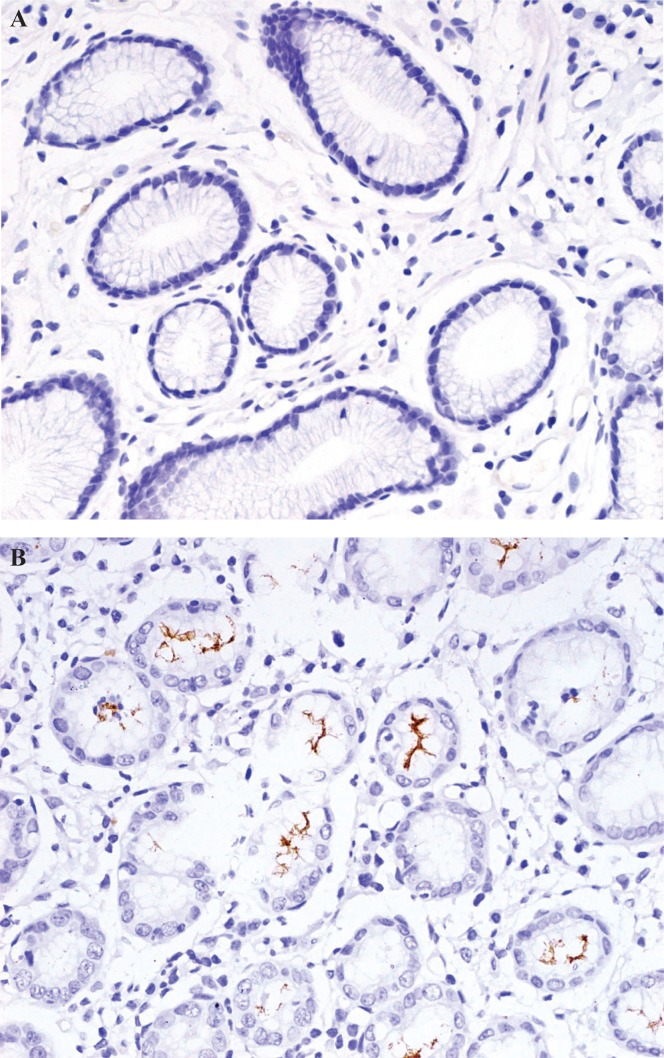
Quantitative detection of H. pylori was performed in tumour and non-neoplastic tissues in all patients. In evaluating the results of H. pyloriihc staining, 188 patients (72.0%) were found to be H. pylori–positive, and 73 (28.0%) were found to be H. pylori–negative (Figure 1). Of the positive patients, 42 (22.3%) showed H. pylori in tumour tissue; 123 (65.4%), in non-neoplastic tissue; and 23 (12.2%), in both types of tissue.
FIGURE 1.
Immunohistochemical staining for Helicobacter pylori. (A) H. pylori–negative (B) H. pylori–positive. 400× original magnification.
Positivity for H. pylori was significantly associated with younger age (p = 0.034). Patients who were negative for H. pylori had a significantly higher frequency of disease relapse (p = 0.042) and death (p = 0.043). With respect to sex, tumour location, uicc stage, grade, Lauren classification, and adjuvant treatment, we observed no statistically significant differences between the groups by chi-square test.
3.2. Association of H. pylori Status with Survival
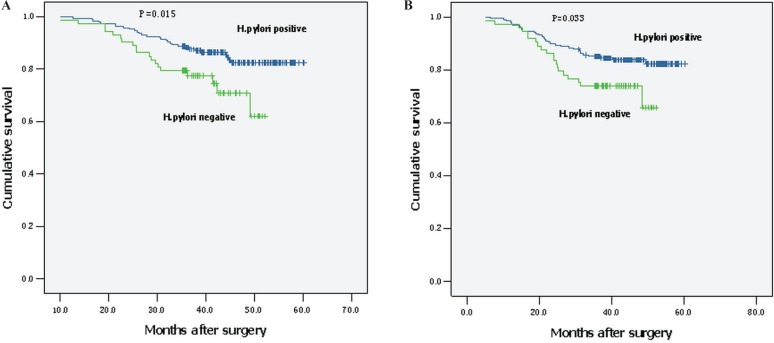
In the univariate Cox regression analysis, positive H. pylori status was associated with better cancer-specific survival (hr: 0.486; 95% ci: 0.271 to 0.870; p = 0.015) and dfs (hr: 0.540; 95% ci: 0.307 to 0.950; p = 0.033; Table ii). Mean cancer-specific survival and mean dfs were 55.2 months (95% ci: 53.4 to 56.9 months) and 53.9 months (95% ci: 51.8 to 56.0) respectively in H. pylori–positive patients; they were 45.1 months (95% ci: 42.2 to 47.9 months) and 43.7 months (95% ci: 40.4 to 47.0 months) in those who were H. pylori–negative. Figure 2 shows Kaplan–Meier survival curves for cancer-specific survival and dfs.
TABLE II.
Univariate and multivariate Cox regression analysis of survival by Helicobacter pylori status
| Survival type |
Cox regression analysis
|
|||||
|---|---|---|---|---|---|---|
|
Univariate
|
Multivariatea
|
|||||
| hr | 95% ci | p Value | hr | 95%ci | p Value | |
| Cancer-specific survival | ||||||
| H. pylori–negative | 1.0 | Reference | 1.0 | Reference | ||
| H. pylori–positive | 0.486 | 0.271 to 0.870 | 0.015 | 0.485 | 0.265 to 0.889 | 0.019 |
| Disease-free survival | ||||||
| H. pylori–negative | 1.0 | Reference | 1.0 | Reference | ||
| H. pylori–positive | 0.540 | 0.307 to 0.950 | 0.033 | 0.557 | 0.310 to 1.004 | 0.051 |
Adjusted for age, sex, tumour location, Union Internationale Contre le Cancer stage, grade, Lauren classification, and adjuvant treatment.
hr = hazard ratio; ci = confidence interval.
FIGURE 2.
Patient survival according to Helicobacter pylori status. (A) Cancer-specific survival. (B) Disease-free survival.
Multivariate analysis was used to further evaluate the potential for H. pylori infection to be as an independent prognostic biomarker. A multivariate Cox proportional hazards model demonstrated that H. pylori positivity was associated with improved survival (hr: 0.485; 95% ci: 0.265 to 0.889; p = 0.019) independent of other clinical covariates, which included age, sex, tumour location, uicc stage, grade, Lauren classification, and adjuvant treatment (Table ii). Compared with H. pylori–negative patients, H. pylori–positive patients showed a trend toward improved dfs (hr: 0.557; 95% ci: 0.310 to 1.004; p = 0.051; Table ii), but the difference was not statistically significant.
4. DISCUSSION
In the present study, we identified H. pylori infection as an independent beneficial prognostic factor for patients with curatively resected gastric cancer. Patients negative for H. pylori had a poorer outlook. Multivariate analysis confirmed the prognostic value of H. pylori infection, even after adjustments for other well-known prognostic factors such as uicc stage, Lauren classification, and adjuvant treatment. To the best of our knowledge, this prospective study is the first in China designed to assess the potential role of H. pylori infection in the prognosis of patients with gastric cancer.
H. pylori infection may be localized in or around the tumour (classified as diffuse, localized, or none). As a result, a histologic diagnosis may be more successful than other methods in detecting the relationship between H. pylori infection and prognosis in gastric cancer patients. The reported rates of H. pylori positivity vary from 17.5%25 to 86.2%17. An overall positivity rate of 72.0% was observed in our patients. That value is higher than the rates reported in other Asian studies18,26, which might be explained by epidemiologic factors and the sensitivity of the various methods used to detect H. pylori infection. Moreover, it is well known that a high prevalence of H. pylori is always accompanied by a high incidence of gastric cancer27.
In agreement with most studies in the literature, we found that the rate of H. pylori positivity was higher in non-neoplastic tissue than in tumour tissue18. The possible biologic basis might be elucidated as follows: Once cancer developed, sustained injury to the gastric mucosa persisted. The microenvironment in gastric mucosa was then no longer suitable for H. pylori survival18. In addition, we identified a higher frequency of H. pylori infection in younger patients (age < 61 years: 78.2% vs. 66.4%). A possible explanation might be that inclusion of patients with gastric cancer only after curative resection led to selection bias.
Several clinicopathologic factors have been proposed to predict the prognosis of patients with gastric cancer. Among those factors, pathologic stage appears to be the most reliable28,29. In the univariate analysis in our study, patients positive for H. pylori experienced better survival. Further findings in the multivariate analysis confirmed the role of H. pylori as an independent beneficial prognostic factor for patients with curatively resected gastric cancer. Stratified analysis according to uicc stage indicated that positive H. pylori status might be a beneficial prognostic factor mainly for patients with advanced-stage gastric cancer. Univariate Cox regression analysis showed that infection with H. pylori was associated with a high dfs in patients with gastric cancer. Meanwhile, multivariate analysis also identified a role for infection with H. pylori as a predictive factor for improved dfs (with marginal significance).
To the best of our knowledge, two prospective studies have investigated the relationship between H. pylori status and prognosis in gastric cancer patients. Meimarakis and colleagues16 identified H. pylori as an independent beneficial prognostic factor for os and relapse-free survival in both univariate and multivariate analysis. The effect was pronounced in patients with early-stage cancer. Patients positive for H. pylori showed improved os and relapse-free survival. A prospective study by Marrelli et al.17 indicated that negative H. pylori status was an indicator of poor prognosis in patients with gastric cancer.
We believe that the current prospective study is the first to confirm H. pylori status as a favourable prognostic factor in a large number of patients with gastric cancer in China, thus validating the effect of H. pylori infection status on survival in a Chinese prospective cohort. The main findings of the present study are consistent with those in previous reports. However, in our study, the association between H. pylori infection and survival was observed mainly in patients with advanced gastric cancer, a finding that requires further validation. Moreover, our results contrast with those from a retrospective Chinese study by Qiu et al.18, who used real-time polymerase chain reaction to detect H. pylori in 157 gastric cancer patients and found no significant association between H. pylori infection and os or relapse-free survival in patients who underwent curative surgery. The difference might be attributable to some combination of the different approaches used in the two studies, different H. pylori detection methods, or different sample sizes.
To date, the mechanisms of the reported differences in prognosis between patients with gastric cancer who are positive for H. pylori and those who are negative are still unclear. Several explanations for the role of H. pylori infection in improving the prognosis of patients with gastric cancer are plausible. The most recognized virulence markers for H. pylori are CagA (cytotoxin-associated gene A), VacA (vacuolating toxin A), and BabA (blood-group antigen-binding adhesion)30. Bacterial virulence factors, host genetic factors, and environmental factors constitute a complex regulatory network for persistent immune and inflammatory responses. Infection with H. pylori might promote the processes of tissue damage and lead to gastric malignancy. Once a multi-step process leading to gastric carcinogenesis is initiated, persistent infection with H. pylori and infiltration with certain leucocyte subsets seem to correlate with a favourable prognosis31–33. For example, high numbers of T-bet–positive tumour-infiltrating lymphocytes, a key marker for the type 1 immune response, can serve as a favourable prognostic indicator for gastric cancer and a potential biomarker for immunotherapy34. Evidence has shown that a microbe-induced cellular immune response, which is identified as involving mainly type 1 T-helper (Th1) cells, may modulate antitumour immunity35. In a Th1 environment, elevated proinflammatory cytokines (interleukins 1α, 1β, and 6) may participate in cancer eradication by recruiting leucocytes from the circulation and by stimulating CD4+ T-cell functions36,37. There is also proof-of-principle evidence for dual antitumour activity of Th1-derived interferon γ. On the one hand, Th1-derived interferon γ has been shown to render macrophages directly cytotoxic to cancer cells. On the other, it can induce macrophages to secret the angiostatic chemokines cxcl9/mig and cxcl10/ip-10, which may halt tumour progression by inhibiting angiogenesis38.
Autoimmunity is one of the important features consequent to chronic H. pylori infection. To a certain extent, the presence of autoreactive antibodies may play an active role in improving cellular immune status and the prognosis of patients with gastric cancer14. Some investigators have focused on the relationship between H. pylori status and microsatellite instability (msi). A few studies have shown that H. pylori cause impaired dna mismatch repair in gastric epithelial cells. Infection with H. pylori occurs more frequently in individuals with msi-positive than with msi-negative gastric cancers39. Compared with stable or low-level msi tumours, high-level msi tumours are associated with a better os rate in gastric cancer patients after curative surgery40,41.
The poor prognosis of patients with a negative H. pylori status might be the result of a more aggressive form of gastric cancer26. The further progression of gastric cancer results in increased destruction of parietal cells in the gastric mucosa42. H. pylori is an organism that can live only in a relatively narrow pH band in vitro—namely, pH 6.5–7.5. As acid secretion falls, the lumen of the stomach becomes an alkaline microenvironment, which is unfavorable for H. pylori. For that reason, cancer might be more advanced in patients with negative H. pylori status. Further studies are required to provide more evidence for these hypotheses.
Despite the demonstration of strong prognostic significance for H. pylori infection, our study has some limitations that should be addressed. First, although all patients in this prospective study entered a routine hospital follow-up program and received telephone follow-up monthly, there were difficulties in determining the exact date of disease recurrence. The lack of significance for the association between H. pylori status and dfs might perhaps be partly attributable to those difficulties. Second, in current study, we used only ihc to detect H. pylori. Other methods, such as the urea breath test, real-time polymerase chain reaction, serologic tests, and bacterial culture are also used for H. pylori detection. The combination of two or more methods might increase the sensitivity and specificity of a diagnosis of H. pylori infection. However, in clinical practice, performing all of those tests is impractical, given the costs and time required. Furthermore, no method of H. pylori detection is perfect. For example, Marrelli et al.17 reported that 14% of polymerase chain reaction– positive gastric cancer patients demonstrated negative serology for H. pylori. The role of serology tests therefore remains questionable for other than epidemiologic purposes. In the present study, histologic examination provided insights about the status of the gastric mucosa. Compared with other staining methods, ihc for the detection of H. pylori therefore makes the screening time much shorter and increases specificity, helping to eliminate other similarly shaped organisms43,44.
5. CONCLUSIONS
The present study demonstrates that H. pylori positivity is a beneficial prognostic indicator in patients with gastric cancer, independent of other clinicopathologic variables. In clinical practice, patients with curatively resected gastric cancer who are negative for H. pylori may need more careful follow-up and more aggressive antitumour treatment to prolong life expectancy. Further research is required to elucidate the exact mechanisms of inflammation and tumour suppression, which might provide new opportunities for personalized treatment options.
6. ACKNOWLEDGMENTS
We thank everyone at our institution who helped with this study. This work was supported by grants from the National Natural Science Foundation of China (81071986, 81001283, 81272739).
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Sjöblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 2.Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albulescu R, Neagu M, Albulescu L, Tanase C. Tissular and soluble mirnas for diagnostic and therapy improvement in digestive tract cancers. Expert Rev Mol Diagn. 2011;11:101–20. doi: 10.1586/erm.10.106. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Sun GP, Zou YF, Hao JQ, Zhong F, Ren WJ. Micrornas as promising biomarkers for gastric cancer. Cancer Biomark. 2012;11:259–67. doi: 10.3233/CBM-2012-00284. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of her2-positive advanced gastric or gastro-oesophageal junction cancer (toga): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase iii study. J Clin Oncol. 2011;29:3968–76. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 7.Wong H, Yau T. Targeted therapy in the management of advanced gastric cancer: are we making progress in the era of personalized medicine? Oncologist. 2012;17:346–58. doi: 10.1634/theoncologist.2011-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JG. Molecular targeted therapy for advanced gastric cancer. Korean J Intern Med. 2013;28:149–55. doi: 10.3904/kjim.2013.28.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–5. [PubMed] [Google Scholar]
- 10.Ricci V, Zarrilli R, Romano M. Voyage of Helicobacter pylori in human stomach: odyssey of a bacterium. Dig Liver Dis. 2002;34:2–8. doi: 10.1016/S1590-8658(02)80051-2. [DOI] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer . Monographs on the Evaluation of Carcinogenic Risk to Humans. Vol. 61. Lyon, France: World Health Organization; 1994. pp. 1–241. [Google Scholar]
- 12.Beswick EJ, Pinchuk IV, Minch K, et al. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-κB activation and interleukin-8 production. Infect Immun. 2006;74:1148–55. doi: 10.1128/IAI.74.2.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mégraud F, Lehours P. Helicobacter pylori and gastric cancer prevention is possible. Cancer Detect Prev. 2004;28:392–8. doi: 10.1016/j.cdp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Xue LJ, Su QS, Yang JH, Lin Y. Autoimmune responses induced by Helicobacter pylori improve the prognosis of gastric carcinoma. Med Hypotheses. 2008;70:273–6. doi: 10.1016/j.mehy.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Kurtenkov O, Klaamas K, Sergeyev B, Chuzmarov V, Miljukhina L, Shljapnikova L. Better survival of Helicobacter pylori infected patients with early gastric cancer is related to a higher level of Thomsen–Friedenreich antigen-specific antibodies. Immunol Invest. 2003;32:83–93. doi: 10.1081/IMM-120019210. [DOI] [PubMed] [Google Scholar]
- 16.Meimarakis G, Winter H, Assmann I, et al. Helicobacter pylori as a prognostic indicator after curative resection of gastric carcinoma: a prospective study. Lancet Oncol. 2006;7:211–22. doi: 10.1016/S1470-2045(06)70586-1. [DOI] [PubMed] [Google Scholar]
- 17.Marrelli D, Pedrazzani C, Berardi A, et al. Negative Helicobacter pylori status is associated with poor prognosis in patients with gastric cancer. Cancer. 2009;115:2071–80. doi: 10.1002/cncr.24253. [DOI] [PubMed] [Google Scholar]
- 18.Qiu HB, Zhang LY, Keshari RP, et al. Relationship between H. pylori infection and clinicopathological features and prognosis of gastric cancer. BMC Cancer. 2010;10:374. doi: 10.1186/1471-2407-10-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos RS, Lourenço JE, Herbella FA, Del Grande JC, Patti MG. Helicobacter pylori has no influence on distal gastric cancer survival. Arq Gastroenterol. 2011;48:109–11. doi: 10.1590/S0004-28032011000200005. [DOI] [PubMed] [Google Scholar]
- 20.The General Rules for the Gastric Cancer Study in Surgery and Pathology. Part ii. Histological classification of gastric cancer. Jpn J Surg. 1981;11:140–5. [PubMed] [Google Scholar]
- 21.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 6th ed. Hoboken, NJ: John Wiley and Sons; 2002. [Google Scholar]
- 22.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 23.Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of cea, ca 19-9, and ca 72-4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001;181:16–9. doi: 10.1016/S0002-9610(00)00549-3. [DOI] [PubMed] [Google Scholar]
- 24.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Chen HY, Zhu BH, Zhang CH, et al. High CpG island methylator phenotype is associated with lymph node metastasis and prognosis in gastric cancer. Cancer Sci. 2012;103:73–9. doi: 10.1111/j.1349-7006.2011.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hur H, Lee SR, Xuan Y, et al. The Effects of Helicobacter pylori on the prognosis of patients with curatively resected gastric cancers in a population with high infection rate. J Korean Surg Soc. 2012;83:203–11. doi: 10.4174/jkss.2012.83.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077–83. doi: 10.2169/internalmedicine.47.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YF, Shi J, Yu HP, et al. Factors predicting survival in patients with proximal gastric carcinoma involving the esophagus. World J Gastroenterol. 2012;18:3602–9. doi: 10.3748/wjg.v18.i27.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Li Z, Ma Y, Zhu G, Zhang H, Xue Y. Prognostic predictors of patients with carcinoma of the gastric cardia. Hepatogastroenterology. 2012;59:930–3. doi: 10.5754/hge09356. [DOI] [PubMed] [Google Scholar]
- 30.Höcker M, Hohenberger P. Helicobacter pylori virulence factors—one part of a big picture. Lancet. 2003;362:1231–3. doi: 10.1016/S0140-6736(03)14547-3. [DOI] [PubMed] [Google Scholar]
- 31.Rad R, Prinz C, Schmid RM. Helicobacter pylori and prognosis of gastric carcinoma. Lancet Oncol. 2006;7:364–5. doi: 10.1016/S1470-2045(06)70672-6. [DOI] [PubMed] [Google Scholar]
- 32.Klintrup K, Mäkinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645–54. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Lee AH, Gillett CE, Ryder K, Fentiman IS, Miles DW, Millis RR. Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology. 2006;48:692–701. doi: 10.1111/j.1365-2559.2006.02410.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen LJ, Zheng X, Shen YP, et al. Higher numbers of T-bet+ intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother. 2013;62:553–61. doi: 10.1007/s00262-012-1358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bamford KB, Fan X, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92. doi: 10.1016/S0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 36.Von Stebut E, Ehrchen JM, Belkaid Y, et al. Interleukin 1α promotes Th1 differentiation and inhibits disease progression in Leishmania major–susceptible BALB/c mice. J Exp Med. 2003;198:191–9. doi: 10.1084/jem.20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Sasson SZ, Hu–Li J, Quiel J, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–24. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haabeth OA, Lorvik KB, Hammarström C, et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. doi: 10.1038/ncomms1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JJ, Tao H, Carloni E, Leung WK, Graham DY, Sepulveda AR. Helicobacter pylori impairs dna mismatch repair in gastric epithelial cells. Gastroenterology. 2002;123:542–53. doi: 10.1053/gast.2002.34751. [DOI] [PubMed] [Google Scholar]
- 40.Lee HS, Choi SI, Lee HK, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002;15:632–40. doi: 10.1038/modpathol.3880578. [DOI] [PubMed] [Google Scholar]
- 41.Fang WL, Chang SC, Lan YT, et al. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J Surg. 2012;36:2131–8. doi: 10.1007/s00268-012-1652-7. [DOI] [PubMed] [Google Scholar]
- 42.Hobsley M, Tovey FI, Holton J. Helicobacter pylori and gastric cancer: neither friend nor foe. Gastroenterology. 2007;132:2076. doi: 10.1053/j.gastro.2007.03.088. [DOI] [PubMed] [Google Scholar]
- 43.Eshun JK, Black DD, Casteel HB, et al. Comparison of immunohistochemistry and silver stain for the diagnosis of pediatric Helicobacter pylori infection in urease-negative gastric biopsies. Pediatr Dev Pathol. 2001;4:82–8. doi: 10.1007/s100240010129. [DOI] [PubMed] [Google Scholar]
- 44.Wang XI, Zhang S, Abreo F, Thomas J. The role of routine immunohistochemistry for Helicobacter pylori in gastric biopsy. Ann Diagn Pathol. 2010;14:256–9. doi: 10.1016/j.anndiagpath.2010.05.002. [DOI] [PubMed] [Google Scholar]