Abstract
Drug-induced lupus erythematosus (dile) syndromes are documented complications of chemotherapeutic agents, including paclitaxel. Subacute cutaneous lupus erythematosus (scle) is a distinct dile syndrome presenting with characteristic annular or papulosquamous skin lesions in a photosensitive distribution with associated high anti-ssa titres. Previously, dile syndromes complicating paclitaxel therapy have been attributed to polyethoxylated castor oil (Kolliphor EL: BASF, Ludwigshafen, Germany), the biologic solvent included in the drug’s original formulation (Taxol: Bristol–Myers Squibb, Montreal, QC), rather than the parent chemotherapy molecule. Here, we report a characteristic case of drug-induced scle complicating treatment with nanoparticle albumin bound (nab)–paclitaxel (Abraxane: Celgene, Summit, NJ, U.S.A.), a solvent-free taxane formulation. The pertinent English-language literature is also discussed. This case report is the first to link solvent-free paclitaxel with scle, and it suggests that the parent molecule is responsible for the reaction.
Keywords: Nanoparticle albumin-bound paclitaxel, nab-paclitaxel, subacute cutaneous lupus erythematosus, cutaneous drug reactions
1. CASE DESCRIPTION
A previously healthy 62-year-old woman was seen in the outpatient medical oncology clinic after presenting with right-sided inflammatory breast cancer.
Diagnostic core biopsy of the breast mass demonstrated poorly differentiated invasive ductal carcinoma, which was estrogen receptor–negative, weakly progesterone receptor–positive by immunohistochemistry, and negative for her2 (human epidermal growth factor receptor 2) amplification by fluorescence in situ hybridization. Computed tomography imaging revealed lesions in keeping with small-volume lung and liver metastases and bilateral ovarian metastases.
The patient was therefore started on first-line systemic therapy for metastatic breast cancer, using nanoparticle albumin bound (nab)–paclitaxel 260 mg/m2 intravenously every 21 days. She initially tolerated the nab-paclitaxel well, with toxicities limited to alopecia and mild fatigue. After the third infusion, she developed a new skin eruption consisting of several ill-defined erythematous papules located symmetrically over the extensor surfaces of her arms. No pruritus, pain, or constitutional symptoms accompanied these skin lesions. In addition, the patient had no history of a known inciting exposure, significant sun exposure, or local trauma. She had no past history of photosensitivity, dermatoses, smoking, or rheumatic disease.
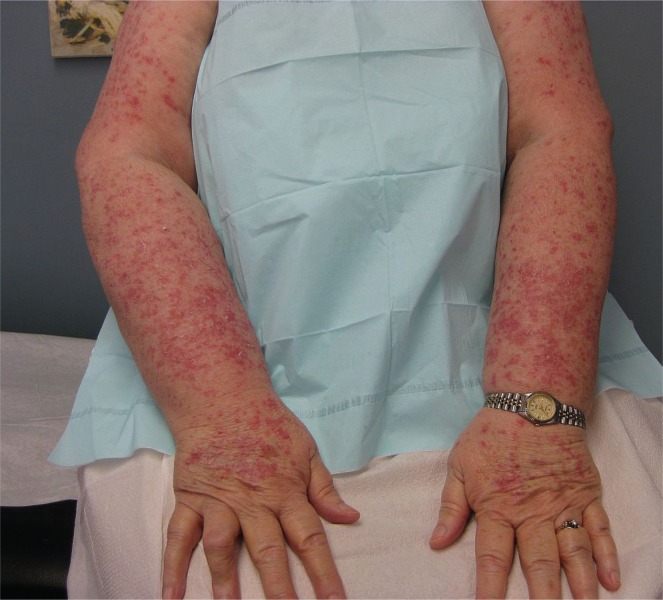
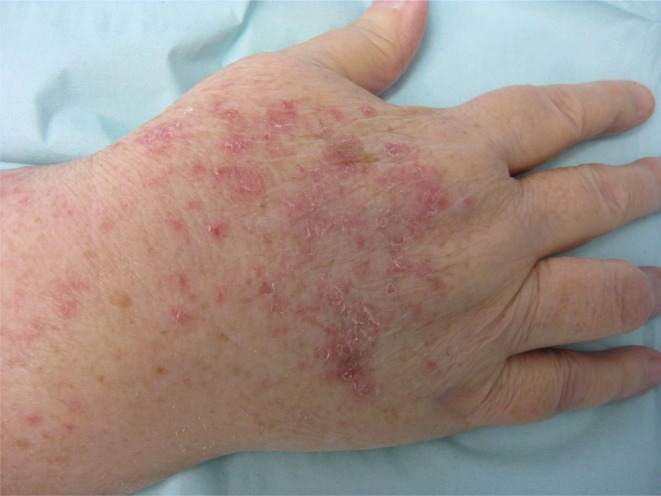
The skin lesions progressed over the subsequent weeks, though the patient remained otherwise asymptomatic. At the assessment preceding the patient’s 5th cycle of nab-paclitaxel, the skin eruption was noted to have progressed dramatically. Physical examination now showed a symmetrical, photodistributed rash involving the extensor surfaces of both arms and hands, as well as the anterior chest (Figure 1). The skin lesions were psoriasiform and consisted of erythematous papules coalescing into plaques with overlying scale (Figure 2). No demonstrable mucous membrane involvement, nail changes, or other skin lesions were evident. The remainder of the physical examination was remarkable only for findings of the known breast cancer.
FIGURE 1.
The patient’s photodistributed erythematous eruption over the extensor surfaces of the upper extremities.
FIGURE 2.
The patient’s eruption, consisting of erythematous papules with overlying scale.
The current eruption was felt, clinically, to be in keeping with subacute cutaneous lupus erythematosus (scle). Upon review, the patient was not taking any medications known to be associated with scle. Her chronic medications included losartan, atorvastatin, and pantoprazole. In addition to nab-paclitaxel, she had recently used metoclopramide as needed for nausea.
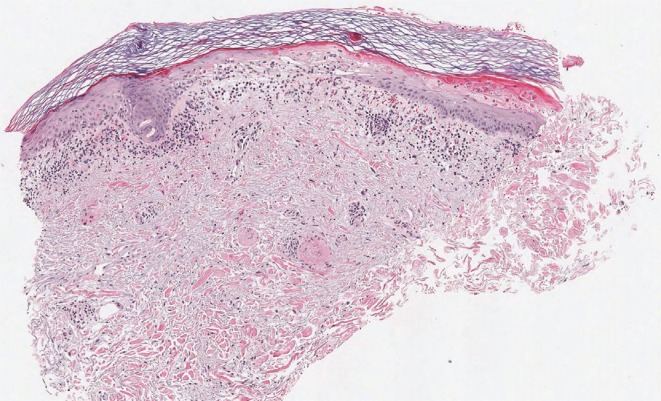
Laboratory examinations included a positive antinuclear antibody test with elevated activity index for anti-ssa (anti-Ro) of 4.7 (normal: <1.0). No other autoantibodies against extractable nuclear antigens, including anti–double-stranded dna, were detectable. Two lesional punch biopsies of affected skin on the right forearm documented the presence of vacuolar interface dermatitis with increased interstitial dermal mucin deposition (Figure 3).
FIGURE 3.
Low-power photomicrograph of skin punch biopsy, showing superficial inflammation, vacuolar epidermal change, and interstitial dermal mucinosis. Hematoxylin and eosin stain, 4×-equivalent magnification. Photomicrograph courtesy of E. Schollenberg md, Halifax, NS.
The patient was referred to a consultant dermatologist who confirmed the diagnosis of scle, papulosquamous-type, and prescribed topical glucocorticoid therapy together with sun protection.
Before her 6th cycle of systemic therapy, the patient developed locoregional cancer progression, and nab-paclitaxel was discontinued. Her eruption began to regress 4 weeks after her final nab-paclitaxel infusion, with initial decreased erythema and scaling. Ten weeks after discontinuing nab-paclitaxel, the patient’s eruption had resolved without scarring, though her anti-ssa activity index remained elevated at more than 8.0.
2. DISCUSSION
Drug-induced lupus erythematosus (dile) includes several lupus-like syndromes that develop during continuous medication exposure and resolve after the associated medication is discontinued. To date, the pathogenic mechanisms by which implicated medications induce autoimmunity and lead to dile syndromes are incompletely understood1. However, the clinicopathologic characteristics of individual dile syndromes are well described, and numerous medication associations have been documented2,3. Like idiopathic systemic lupus erythematosus, dile is associated with autoantibody formation and suggestive histopathologic findings. The specific autoantibody associations and histopathologic findings can differ among dile syndromes, and diagnosis is based on a consideration of clinical presentation, presence of autoantibodies, and supporting pathology.
Whether idiopathic, paraneoplastic, or drug-induced, scle presents with unique cutaneous morphology and autoantibody association in the absence of systemic features4. Several cutaneous variants have been described, but the skin lesions of scle are generally annular-polycyclic or papulosquamous in character and involve symmetric photodistributed regions, including the upper trunk and the extensor surfaces of the upper extremities3. The complex mechanism underlying these lesions includes increased ultraviolet light–induced apoptosis, which is associated with local inflammation and cytokine release1. Histopathologically, these lesions demonstrate interface dermatitis with vacuolar degeneration of basal keratinocytes and dermal mucinosis. This syndrome is characteristically associated with high titres of the autoantibody anti-ssa, which is documented in up to 90% of patients with idiopathic scle and 80% of patients with drug-induced scle5,6. Patients presenting with anti-ssb antibody positivity constitute a smaller percentage of cases.
First described as an adverse effect of hydrochlorothiazide treatment by Reed et al. in 1985, drug-induced scle has been noted to appear within 3 days or as long as 11 years after initiation of the causative medication6,7. After drug discontinuation, scle skin lesions improve within weeks and resolve without scarring in most patients8. Autoantibody titres generally take longer to decline and often persist6.
Given the expected resolution of skin lesions after drug withdrawal, discontinuation of the implicated medication is the cornerstone of drug-induced scle management. However, various therapies applied for idiopathic scle may also be used, including photoprotection, topical corticosteroids, and systemic antimalarials.
The list of medications reported to cause druginduced scle is extensive and includes drugs from multiple classes, most commonly antihypertensives and antifungals6. The first English-language report of taxanes causing scle was written by Adachi and Horikawa in 20079. In their article, those authors described 2 patients with pre-existing Sjögren syndrome who developed scle during paclitaxel treatment for breast cancer. In each case, typical skin lesions developed during paclitaxel therapy and were associated with high anti-ssa titres and supportive histopathology. An association between scle and paclitaxel was supported by complete resolution of the skin lesions within weeks of drug withdrawal. Since that initial publication, 2 further cases of paclitaxel-related scle have been described10,11. Unlike the publication by Adachi and Horikawa, subsequent reports involved patients who had no preceding history of rheumatologic disease9–11. Other chemotherapies, including docetaxel and capecitabine, have been associated with drug-induced scle in the literature11–13. Also, scle is not the only presentation of dile caused by chemotherapeutic agents; paclitaxel itself is a described cause of other dile syndromes14,15.
Taxanes are highly lipophilic molecules that have, in the past, required the addition of biologic solvents as vehicles. The original formulation of paclitaxel was emulsified using polyethoxylated castor oil, a vehicle known to possess pharmacologic properties leading to adverse effects, including severe hypersensitivity reactions16,17. Many of paclitaxel’s idiosyncratic adverse effects—including dile reactions—have previously been attributed to polyethoxylated castor oil rather than to the parent chemotherapy molecule15–17. Nab-paclitaxel is a new-generation solvent-free formulation of paclitaxel that utilizes nab technology to increase the bioavailability of the paclitaxel molecule18. As such, nab-paclitaxel does not include polyethoxylated castor oil and, on that basis, is associated with fewer known adverse effects18.
Pham et al.15 previously described a case of dile with systemic lupus erythematosus–like features occurring during paclitaxel therapy and resolving with paclitaxel withdrawal. The clinical features of their case included malar rash and antinuclear antibody positivity, a dile syndrome distinct from scle. Interestingly, their patient went on to receive nab-paclitaxel without a recurrence of rash, prompting the authors to postulate that the causative agent of dile was polyethoxylated castor oil.
Previously, no published reports have described drug-induced scle—or any other dile syndrome—complicating nab-paclitaxel therapy. The current case is highly suggestive of an association between scle and nab-paclitaxel. Factors supporting the association include the first occurrence of a hallmark scle presentation during nab-paclitaxel therapy, rapid resolution of the rash after nab-paclitaxel withdrawal, and the similarity of this case to earlier reports of taxane-induced scle9–11. Based on this experience, we suggest that scle is a potential adverse effect of therapy with nab-paclitaxel. Importantly, this suggestion implies that paclitaxel-induced dile syndromes are caused not solely by the vehicle (polyethoxylated castor oil) used in the original formulation of paclitaxel.
3. CONCLUSIONS
Subacute cutaneous lupus erythematosus is one presentation of dile and is a described complication of chemotherapeutic agents, including taxanes. To date, all currently available taxane formulations have been associated with scle. On that basis, it appears likely that scle is induced by the parent taxane molecules rather than by the various solvents used as their vehicles.
4. ACKNOWLEDGMENTS
The authors thank Dr. Erica Schollenberg for her assistance in the production of this manuscript.
5. CONFLICT OF INTEREST DISCLOSURES
The authors have no relevant affiliations or financial involvement with any organization or entity having a financial interest in or financial conflict with the subject matter or the materials discussed in this manuscript.
6. REFERENCES
- 1.Stavropoulos PG, Goules AV, Avgerinou G, Katsambas AD. Pathogenesis of subacute cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2008;22:1281–9. doi: 10.1111/j.1468-3083.2008.02806.x. [DOI] [PubMed] [Google Scholar]
- 2.Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99–105. doi: 10.1007/s00403-008-0895-5. [DOI] [PubMed] [Google Scholar]
- 3.Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatologic aspects. Lupus. 2009;18:934–40. doi: 10.1177/0961203309106176. [DOI] [PubMed] [Google Scholar]
- 4.Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409–15. doi: 10.1001/archderm.1979.04010120007006. [DOI] [PubMed] [Google Scholar]
- 5.Lee LA, Roberts CM, Frank MB, McCubbin VR, Reichlin M. The autoantibody response to Ro/ssa in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262–8. doi: 10.1001/archderm.1994.01690100046006. [DOI] [PubMed] [Google Scholar]
- 6.Lowe G, Henderson CL, Grau RH, Hansen CB, Sontheimer RD. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465–72. doi: 10.1111/j.1365-2133.2010.10110.x. [DOI] [PubMed] [Google Scholar]
- 7.Reed BR, Huff JC, Jones SK, Orton PW, Lee LA, Norris DA. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49–51. doi: 10.7326/0003-4819-103-1-49. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava M, Rencic A, Diglio G, et al. Drug-induced, Ro/ssa positive cutaneous lupus erythematosus. Arch Dermatol. 2003;139:45–9. doi: 10.1001/archderm.139.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Adachi A, Horikawa T. Paclitaxel-induced cutaneous lupus erythematosus in patients with serum anti-ssa/Ro antibody. J Dermatol. 2007;34:473–6. doi: 10.1111/j.1346-8138.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 10.Vihinen P, Paija O, Kivisaari A, Koulu L, Aho H. Cutaneous lupus erythematosus after treatment with paclitaxel and bevacizumab for metastatic breast cancer: a case report. J Med Case Rep. 2011;5:243. doi: 10.1186/1752-1947-5-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Crowson AN, Woofter M, Luca MB, Magro CM. Docetaxel (Taxotere) induced subacute cutaneous lupus erythematosus: report of 4 cases. J Rheumatol. 2004;31:818–20. [PubMed] [Google Scholar]
- 12.Fernandes NF, Rosenbach M, Elenitsas R, Kist JM. Subacute cutaneous lupus erythematosus associated with capecitabine monotherapy. Arch Dermatol. 2009;145:340–1. doi: 10.1001/archdermatol.2008.619. [DOI] [PubMed] [Google Scholar]
- 13.Weger W, Kränke B, Gerger A, Salmhofer W, Aberer E. Occurrence of subacute cutaneous lupus erythematosus after treatment with fluorouracil and capecitabine. J Am Acad Dermatol. 2008;59(suppl 1):S4–6. doi: 10.1016/j.jaad.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Dasanu CA, Alexandrescu DT. Systemic lupus erythematosus associated with paclitaxel use in the treatment of ovarian cancer. South Med J. 2008;101:1161–2. doi: 10.1097/SMJ.0b013e31818956f6. [DOI] [PubMed] [Google Scholar]
- 15.Pham AQ, Berz D, Karwan P, Colvin GA. Cremophor-induced lupus erythematosus-like reaction with Taxol administration: a case report and review of the literature. Case Rep Oncol. 2011;4:526–30. doi: 10.1159/000334233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–8. doi: 10.1016/S0959-8049(01)00171-X. [DOI] [PubMed] [Google Scholar]
- 17.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–85. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 18.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–53. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]