Abstract
Objective:
Suggested predose plasma quetiapine target ranges for effective therapy in schizophrenia lie between 50 and 500 µg/l. We aimed to examine data from a quetiapine therapeutic drug monitoring (TDM) service to assess the plasma quetiapine concentrations attained at specified doses in clinical practice.
Method:
We studied TDM data from patients given immediate-release quetiapine in the period 2000–2011.
Results:
There were 946 samples from 487 patients (257 males, age at time of first sample, median [range] 34 [14–87] years, and 230 females, age at time of first sample, median [range] 38 [10–92] years). The plasma quetiapine concentration was <50 and <100 µg/l in 30% and 50% of samples, respectively (no quetiapine detected in 9% of samples). The relationship between dose and plasma quetiapine was poor. The mean (95% confidence interval [CI]) quetiapine dose was higher (t = 3.6, df = 446, p <0.01) in males versus females (641 [600–1240] and 548 [600–943] mg/day, respectively), although there was no difference in median dose (600 mg/day) or in the mean (95% CI) plasma quetiapine concentrations attained. Smoking habit had no discernible effect on plasma quetiapine concentration.
Conclusions:
There was a poor relationship between dose and plasma quetiapine concentration in this study, as found by others. This is probably because of the short plasma half-life of the drug, at least in part. Nevertheless, quetiapine TDM can help assess adherence and measurement of quetiapine metabolites, notably N-desalkylquetiapine, as well as quetiapine itself may enhance the value of quetiapine TDM in future.
Keywords: adherence, quetiapine, schizophrenia, therapeutic drug monitoring
Introduction
Quetiapine is a dibenzothiazepine derivative that is licensed for the treatment of schizophrenia in an immediate-release (IR) formulation. An extended-release (ER) formulation has also been licensed in the UK (March 2010) for use in depression and in bipolar disorder. Quetiapine is provided in 25, 100, 150, 200 and 300 mg quetiapine fumarate IR and ER tablets; a 400 mg ER tablet is also available. The maximum licensed daily dose of quetiapine for the treatment of schizophrenia is 750 mg/day, and for the treatment of depression and of mania in bipolar disorder 600 and 800 mg/day, respectively [BNF, 2012].
Quetiapine has a plasma half-life of some 7 hours and maximum plasma concentrations are obtained 1–2 hours postdose [Hiemke et al. 2011; Sparshatt et al. 2011]. It is metabolized to sulfoxide, 7-hydroxy, N-desalkyl, O-desalkyl, and 7-hydroxy-N-desalkyl metabolites by cytochromes P450 (CYP) 3A4 and CYP3A5, with a possible minor contribution from CYP2D6 [Sparshatt et al. 2011; Spina and de Leon, 2007; Bakken et al. 2011]. The 7-hydroxy- and 7-hydroxy-N-desalkyl- metabolites are pharmacologically active and accumulate in plasma to concentrations of less than 10% of those of quetiapine itself [DeVane and Nemeroff, 2001]. N-Desalkylquetiapine may be a major contributor to the antidepressant effect of quetiapine [Jensenet al. 2008]. Quetiapine plasma concentrations and its effectiveness in therapy may be associated with the P-glycoprotein status of the patient [Nikischet al. 2010].
Optimal efficacy of quetiapine IR in treating the positive symptoms of schizophrenia is seen at doses of 150–750 mg/day; for treating the negative symptoms of schizophrenia a dose of 300 mg/day is recommended [Arvanitis and Miller, 1997]. For quetiapine ER doses of 600 and 800 mg/day are most effective at treating both the positive and negative symptoms of schizophrenia [Sparshatt et al. 2008]. There is currently no widely accepted target range for predose plasma quetiapine concentrations associated with either optimal clinical response, or minimal adverse effects when used to treat schizophrenia. However, target ranges of 50–100 µg/l (upper limit uncertain), 100–500 µg/l and 70–170 µg/l have been suggested [Taylor et al. 2012; Hiemke et al. 2011; Baumann et al. 2004]. There are also no accepted target ranges for plasma quetiapine concentrations when used to treat depression, although quetiapine doses of 150 mg/day (quetiapine ER) have been suggested [El-Khalili et al. 2010]. Reports of toxicity are lacking with predose plasma quetiapine concentrations in the range 27–387 µg/l [Sparshatt et al. 2011].
In order to obtain information as to the range of plasma quetiapine concentrations attained in clinical practice after use of quetiapine IR, we have examined data from a quetiapine therapeutic drug monitoring (TDM) service.
Method
Patient samples
We studied results from the analysis of plasma samples submitted for quetiapine TDM from patients in the UK in the period 2000–2011. Information was obtained from the request form at the time of the analysis, and included time and date of sample, time and date of last quetiapine dose, quetiapine dose (mg/day), duration of quetiapine treatment, age (years), sex, body weight (kg), smoking habit, the clinical indication for the assay and any other relevant information that could aid interpretation of the results, such as concomitant medication or type of quetiapine formulation prescribed. It was not possible to identify whether the patients were inpatients or outpatients from the information supplied. Patient samples that had been referred during investigation of death during quetiapine treatment, because of suspected quetiapine self-poisoning or from patients prescribed ER quetiapine, were excluded as far as such samples could be identified. Samples where nonadherence was indicated on the request form as a reason for the assay request were excluded from study of the effect of sex and smoking habit on plasma quetiapine concentration.
Quetiapine assay
Plasma quetiapine was measured in 2000–2008 by high-performance liquid chromatography with ultraviolet absorption detection (HPLC-UV; 260 nm) after extraction into methyl tert-butyl ether at pH 9.2 using loxapine as internal standard (Waters Spherisorb S5SCX sulphopropyl-modified silica column; ammonium perchlorate-modified eluent). From 2009 onwards, quetiapine was measured by high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) after extraction into butyl acetate:butanol (9+1) (ammonium acetate-modified eluent, atmospheric pressure chemical ionization [APCI]: quetiapine m/z 384.1–220.9 and 252.8, quetiapine-D8 [internal standard] m/z 392.1–225.9 and 257.8, ThermoFisher TSQ Quantum Access). These methods were cross-validated by analysis of patient and external quality assessment (EQA) samples (HeathControl, now LGC Standards, Bury, UK; http://www.lgcpt.com/default.aspx), and gave comparable results. Assay implementation and validation conformed to the standards set by the US Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) guidance for bioanalytical method validation and accuracy and precision monitoring was as documented by the Clinical and Laboratory Standards Institute [FDA/CDER, 2001; Tholen et al. 2004]. Additional validation was by repeat analysis of stored samples (N = 50) using a second LC-MS/MS method. Both methods gave comparable results [Fisher et al. 2012b].
Assay calibration was by duplicate analysis of solutions (N = 7) containing quetiapine in analyte-free, pooled, mixed gender, human plasma (most recently Sera Laboratories International, Haywards Heath, UK) in the range 10–800 µg/l and plotting the ratio of peak area of analyte to that of internal standard against analyte concentration. Internal quality control samples (25, 250 and 400 µg/l, respectively) prepared in analyte-free pooled human plasma were analysed with each sample batch and between every 10 sample extracts. Samples with plasma quetiapine markedly (approximately 10%) above the calibration range were diluted in analyte-free human plasma and re-analysed. The limit of accurate measurement was 5 µg/l (200 µl sample). For both methods intra-assay precision (relative standard deviation, %) at analyte concentrations of 25, 250 and 400 µg/l was 3–5% and the corresponding inter-assay precision was 2–5%.
Statistical analysis
Descriptive and statistical analyses were performed using Microsoft Excel 2003. Quetiapine dose and plasma concentration were grouped as appropriate to facilitate data analysis.
Results
There were 946 samples from 487 patients (257 [53%] males, age at time of first sample [median (range)] 34 (14–87) years, and 230 (47%) females, aged 38 (10–92) years). Of these, 17 samples (6 males, age at time of first sample [median (range)] 17 (14–17) years, and 11 females, age at time of first sample [median (range)] 14 (10–16) years) were from patients aged less than 18 years and 56 samples (20 males, age at time of first sample [median (range)] 69 (65–87) years, and 36 females, age at time of first sample [median (range)] 68 (65–93) years) were from patients aged 65 years or greater.
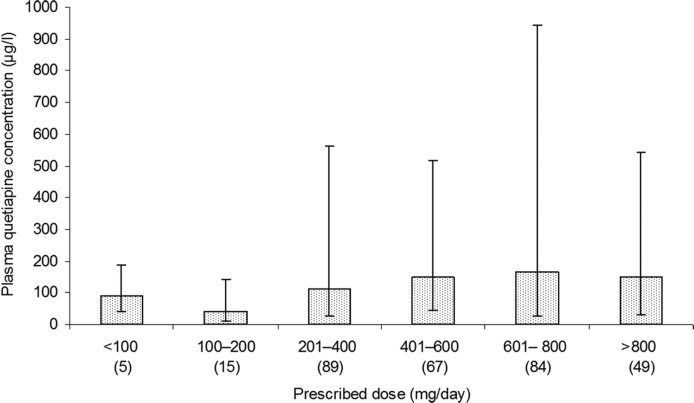
The results are summarized in Table 1 and in Figure 1. Samples per patient were: 1 (304 patients), 2–5 (163 patients), 6–10 (15 patients), 11 or more (5 patients). Information on sample timing with respect to the last dose was available for 250 samples (26% of all samples received). For 229 samples the sampling time was greater than 7 hours postdose and with 21 samples up to 7 hours postdose. Where information was available 73% of males and 50% of females were smokers at the time of sampling (male smokers, 69; nonsmokers, 26; not recorded, 402; female smokers, 20; nonsmokers, 20; not recorded, 394).
Table 1.
Summary of quetiapine TDM data 2000–2011 (946 samples, 487 patients).
| Plasma quetiapine concentration (µg/l) |
|||||
|---|---|---|---|---|---|
| <5* | 5–49 | 50–99 | 100–500 | >500 | |
| Number of samples | 83** | 206 | 171 | 373 | 113 |
| % | 9 | 22 | 18 | 39 | 12 |
Limit of accurate measurement
70 patients
Figure 1.
Median (10th and 90th percentiles) plasma quetiapine concentrations (µg/l) by dose band. Number of samples in parentheses. Samples with (i) plasma quetiapine < 5 µg/l and (ii) adherence queried on the request form excluded.
Information on coprescribed medication was available for 143 samples (15%). The 10 most mentioned coprescribed medications were (some cases more than one drug listed): sodium valproate (N = 27), procyclidine (N = 12), lorazepam (N = 11), lithium (N = 10), phenytoin (N = 10), carbamazepine (N = 9), diazepam (N = 9), lamotrigine (N = 9), sertraline (N = 9) and amisulpride (N = 8).
Clinical indication for assay request
Reasons for sending samples for analysis as documented on assay request forms (more than one reason in some cases) were: suspected nonadherence (N = 170), baseline concentration during successful therapy (N = 81), confirmation of correct dose (N = 78), suspected drug–drug interaction (N = 14), suspected adverse drug reaction (N = 3) and ‘miscellaneous’ (N = 11).
No quetiapine was detected in 14 (8%) of the ‘suspected nonadherence’ samples and in 69 (9%) of the remaining samples. Where quetiapine was detected the mean (95% CI) plasma quetiapine concentration in the ‘suspected nonadherence’ samples was significantly lower than in the remaining samples (suspected: 144 [96–535] μg/l; remaining: 234 [131–977] μg/l; t = 2.6, df = 861, p < 0.01). The mean (95% CI) quetiapine prescribed dose in samples where nonadherence was suspected (566 [600–800] mg/day) was not significantly different from those where adherence was not cited as a reason for the request (620 [600–1200] mg/day).
Plasma quetiapine and prescribed dose
Information on prescribed dose was available for 475 (50%) samples. The mean [95% CI] dose was significantly higher in males as compared with females (641 [600–1240] versus 548 [600–943] mg/day, t = 3.6, df = 446, p < 0.01), although the median dose was the same for both males and females (600 mg/day).
The mean [95% CI] plasma quetiapine concentrations in males (267 [120–962] µg/l) and females (249 [36–839] µg/l) were not significantly different. There was also no significant difference in mean (95% CI) plasma quetiapine concentration between patients aged less than 18 years (277 [38–699] µg/l) and patients aged 65 years or more (235 [11–773] µg/l) when compared with samples from patients aged 18–65 years (241 [13–935] µg/l). Similarly, although nonsmokers had higher mean [95% CI] (234 [104–570] µg/l) plasma quetiapine concentrations than smokers (180 [83–563] µg/l), this difference was not statistically significant.
For all patients the mean (95% CI) quetiapine dose was 605 (600–1200) and the median (range) 600 (25–1700) mg/day. For 58 (6%) samples (35 patients) the prescribed quetiapine dose was greater than the British National Formulary licensed limit of 800 mg/day (median dose [range] 1200 [850–1700] mg/day) [BNF, 2012]. There was a broad relationship between plasma quetiapine and prescribed dose, but there was much variation in plasma quetiapine concentration in each dose band (Table 2). Plasma quetiapine was greater than 2000 µg/l in six samples (six patients). In two samples the dose was given as 600 and 700 mg/day, respectively. Nonadherence was queried in both instances, but no further information was available.
Table 2.
Plasma quetiapine and prescribed dose (excludes samples in which no quetiapine detected, i.e. quetiapine <5 µg/l).
| Dose (mg/day) | Number of samples | Plasma quetiapine (µg/l) |
||||||
|---|---|---|---|---|---|---|---|---|
| Median | Mean | Standard deviation | Minimum | 10th percentile | 90th percentile | Maximum | ||
| <100 | 6 | 69 | 96 | 73 | 35 | 41 | 179 | 226 |
| 100–200 | 21 | 51 | 96 | 163 | 10 | 11 | 161 | 780 |
| 201–400 | 119 | 92 | 185 | 226 | 9 | 29 | 479 | 1600 |
| 401–600 | 124 | 124 | 215 | 315 | 10 | 32 | 461 | 2345 |
| 601–800 | 126 | 145 | 296 | 369 | 5 | 26 | 897 | 2100 |
| >800 | 53 | 149 | 230 | 240 | 10 | 30 | 536 | 1220 |
| Not recorded | 414 | 134 | 261 | 377 | 5 | 20 | 629 | 2711 |
| All | 863 | 123 | 242 | 339 | 5 | 24 | 589 | 2711 |
Plasma quetiapine and suggested target range
For prescribed quetiapine doses up to 800 mg/day, 71% of results were within a range not associated with reports of toxicity (27–387 µg/l), 24% of results were within a suggested target range of 50–100 μg/l and 39% within the range 100–500 μg/l (Figure 2). For prescribed doses greater than 800 mg/day, the number of samples within the ranges 27–387, 50–100 and 100–500 μg/l was 67%, 16% and 43%, respectively.
Figure 2.
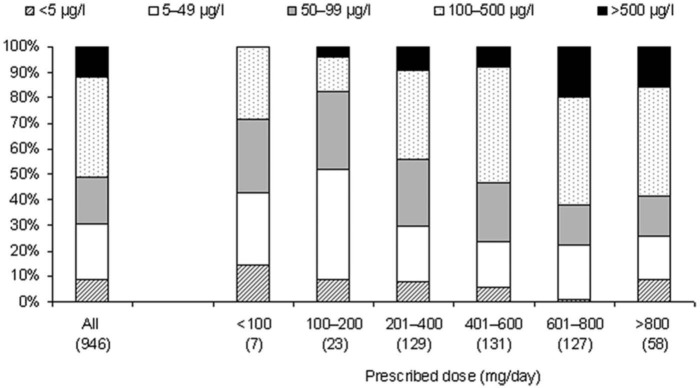
Plasma quetiapine normalized (i) for all samples and (ii) by dose band (number of samples in parentheses).
Discussion
Key findings and limitations
No quetiapine was detected in 9% of samples. The percentage of samples in which quetiapine was not detected was the same regardless of whether or not adherence was queried on the request form. Second, the magnitude of the inter-individual variation in plasma quetiapine concentration within the different quetiapine dose bands was extensive even in those patients where adherence was not queried on the request form (Figure 1). Overall, only 39% of samples had a plasma quetiapine concentration within the suggested target range of 100–500 µg/l [Hiemke et al. 2011] for prescribed doses up to 800 mg/day. It is likely that poor adherence and the relatively short plasma half-life of quetiapine as compared with other atypical antipsychotics (e.g. clozapine 6–17 h or more) were major factors in this variation. Finally, smoking status and sex had no significant effect on the plasma quetiapine concentration.
Missing information is the most significant limitation of this study. In particular, smoking status, body weight, prescribed dose, sample timing in relation to the last dose, and coprescribed medication were under-reported. Partial completion of assay request forms is common, however, and serves to limit not only the information that can sometimes be provided to clinicians in individual cases, but also detracts from the value of studies such as this that are aimed at placing individual results in a wider context. Second, quetiapine dosage may be divided throughout the day to reduce the impact of side effects such as sedation, but the effect of this potential variable on plasma quetiapine concentrations could not be investigated. Finally, no attempt was made to assess diagnosis, clinical efficacy or side effects within this study as this would have required an intrusive design incompatible with offering a routine service.
Plasma quetiapine and dose
The variability seen in plasma quetiapine concentrations at a given IR quetiapine dose has been reported by others [Bakken et al. 2011; Hasselstrøm and Linnet, 2004; Wittman et al. 2010]. A possible factor here may be changes in quetiapine metabolism when drugs are coprescribed such as sodium valproate, which is said to inhibit the CYP450 enzyme system [Aichhorn et al. 2006], and lamotrigine that is associated with reduced plasma quetiapine concentrations due to enhanced glucuronidation [Andersson et al. 2011]. Variations in sampling time since the last quetiapine dose may also contribute to the poor relationship between dose and plasma quetiapine concentration observed here (where information was available 92% of samples were taken more than 7 h postdose). The availability of quetiapine ER may reduce the impact of sample timing in relation to the last dose on the quetiapine plasma concentration:dose relationship, although it is not as yet clear whether the variability in plasma quetiapine at a given dose is less for the ER preparation than for the IR preparation.
Quetiapine has loose in vivo binding to D2 receptors, and at therapeutic doses striatal dopamine receptor occupancy is <65%, the threshold generally accepted as necessary for drugs to exert an antipsychotic effect. In addition, dopamine occupancy drops to 20–30% 12 hours postdose. In samples taken 10 h postdose, plasma quetiapine concentrations have been reported to be (once corrected for dose) 1.5-fold higher (95% CI 1.2–1.8) than those taken 14 h postdose [Bakken et al. 2011]. Indeed, it has been suggested that ‘peak’ quetiapine concentrations may show a greater correlation with dose, dopamine occupancy and hence response than ‘trough’ samples [Sparshatt et al. 2011]. The difficulty here of course is catching the peak, which will inevitably vary between patients, especially outpatients. This topic has been debated for many years in relation to cyclosporin, for example, and there is still no convincing evidence that 2 h postdose sampling (peak, C2) has any advantage over predose (trough, C0) sampling [Marin et al. 2006]. Further investigation is needed into the relationship between peak plasma quetiapine concentrations and clinical response.
The range of plasma quetiapine concentrations measured in the present study are broadly comparable with those reported by others: median (range) 101 (<4–1816) μg/l [Castberg et al. 2007] and 103 (7–1190) μg/l [Bakken et al. 2011], although the highest concentrations measured here were above these ranges for all doses above 400 mg/day. By way of comparison, of 14 patients presenting after quetiapine self-poisoning (suspected ingested dose range 1.2–18 g), serum quetiapine concentrations were in the range 1100–8800 µg/l (time between ingestion and sampling 1–26 h) [Hunfeld et al. 2006]. In this report there was no relationship between the amount of quetiapine said to have been ingested and the serum quetiapine concentration, but this may have been due at least in part to the large variation in sampling time postdose and the relatively short plasma half-life of quetiapine, as discussed above.
Where dose information was provided, most samples (79%) received in this study were from patients prescribed doses of quetiapine in the range associated with optimal treatment of the positive symptoms of schizophrenia (150–750 mg/day) and only 10% were given doses associated with optimum treatment of the negative symptoms (300 mg/day). However, since no clinical information was available it is not possible to draw any conclusions from this finding. It has been reported that the licensed dose of quetiapine is often exceeded [Citrome et al. 2005], and indeed prescribed quetiapine doses of up to 2700 and 2600 mg/day, respectively, have been recorded [Bakken et al. 2011; Castberg et al. 2007]. In the present study the prescribed dose ranged up to 1700 mg/day, and overall 6% of samples were from patients who were prescribed doses exceeding the British National Formulary recommended limit (800 mg/day) [BNF, 2012]. However, these higher doses were not associated with a particularly high proportion of high plasma quetiapine concentrations.
Target range and adherence
The clear impression gained when offering the service was that the assay was usually requested in order to assess possible reasons for treatment failure. Thus, it is not surprising that no quetiapine was detected in 9% of samples; the percentage of samples where nonadherence was indicated (plasma quetiapine <5 µg/l) was the same regardless of whether or not nonadherence was specifically queried on request forms. Overall the plasma quetiapine concentration was <50 µg/l and <100 µg/l, suggested thresholds for clinical response [Hiemke et al. 2011; Taylor et al. 2012] in some 30% and 50% of samples, respectively. Gerlach and colleagues reported similar findings (41% of serum quetiapine concentrations in adolescents [mean age 15.9 ± 1.5 years] below 70 µg/l) [Gerlach et al. 2007].
Partial adherence could not be accurately identified in this study. This may have been possible if repeat sampling was conducted over time [Reis et al. 2004]. In addition, it should be borne in mind that a patient coprescribed medication known to induce CYP3A4 may have a predose plasma quetiapine concentration that is below 5 µg/l even if (partially) adherent. Moreover, failure to detect quetiapine in a single sample does not confirm long-term nonadherence.
There is no widely accepted target range for plasma quetiapine in the treatment of schizophrenia or in depression. The target ranges that have been suggested for the treatment of schizophrenia are broad, i.e. 50–100 µg/l (upper limit uncertain), 100–500 µg/l and 70–170 µg/l [Taylor et al. 2012; Hiemke et al. 2011; Baumann et al. 2004] and have changed in the years since quetiapine was first licensed. A metabolite of quetiapine, N-desalkylquetiapine (that has a longer plasma half-life than quetiapine, i.e. 11–12 h), has been implicated in the antidepressant effect of quetiapine, and plasma N-desalkylquetiapine is more strongly related to dose than plasma quetiapine itself [Fisher et al. 2012a]. Quetiapine metabolites were not measured in this study, but measurement of N-desalkylquetiapine may be helpful in future for quetiapine dose optimization when quetiapine is used primarily as an antidepressant and in assessing adherence.
Effect of smoking status, sex and age
Although we found age to have no significant effect on plasma quetiapine concentration, others have reported plasma quetiapine (once corrected for dose) to be 39% lower in patients under 18 years and 67% higher in those patients aged 70 years or greater [Castberg et al. 2007] as compared with those of intermediate age, and 50% higher in patients aged 65 years or more [Bakken et al. 2011] compared with younger patients. There were not enough patients in the present study aged <18 years and 65 years and over to be able to study the effect of sex and smoking habit separately in these age groups.
Quetiapine is metabolized by CYP3A4, CYP3A5, and CYP2D6. CYP3A4 and CYP2D6 are not induced by the polycyclic aromatic hydrocarbons present in cigarette smoke, although CYP3A5 activity in human lung cells may be depressed by smoking [Hukkanen et al. 2003]. Cigarette smoking did not appear to have an influence on the plasma quetiapine concentration attained on a given dose in our study, as reported by others [DeVane and Nemeroff, 2001].
Even though we found that males received a significantly higher mean quetiapine dose than females, the median dose was the same (600 mg/day). Castberg and colleagues and Wittman and coworkers also reported males to be prescribed a higher mean quetiapine dose than females [Castberg et al. 2007; Wittman et al. 2010], whilst others have reported higher dosage in females when corrected for body weight [Mauri et al. 2007]. A lack of a sex difference in mean plasma quetiapine concentration, as found in this study, has also been reported [Hasselstrøm and Linnet, 2004], although again others have reported higher plasma concentrations in females than in males when corrected for dose and body weight [Aichhorn et al. 2006; Mauri et al. 2007]. In any event, if there is a sex difference in quetiapine disposition it would seem unlikely to be clinically relevant.
Conclusions
There was a poor relationship between dose and pre-dose plasma quetiapine concentration in patients given IR quetiapine, as found by others. This is probably because of the short plasma half-life of the drug, although variable adherence is also likely to be a factor. Nevertheless, quetiapine TDM can sometimes help assess adherence and measurement of quetiapine metabolites, notably N-desalkylquetiapine, which has a much longer plasma half-life than quetiapine itself, may enhance the value of quetiapine TDM in future. Similarly quetiapine TDM in patients prescribed the ER formulation may be of more value in dose adjustment than in those given IR quetiapine.
Acknowledgments
With grateful thanks to Professor Robert Zipursky for helpful comments on an earlier draft of this manuscript. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: M.X. Patel holds a Clinician Scientist Award supported by the National Institute for Health Research and has also received consultancy fees, lecturing honoraria, and/or research funding from Janssen-Cilag, Eli Lilly, Endo, and Wyeth and has previously worked on 2 clinical drug trials for Janssen-Cilag. R.J. Flanagan has received lecturing honoraria from Novartis and from Lilly.
Contributor Information
Simon A. Handley, Toxicology Unit, Department of Clinical Biochemistry, King’s College Hospital NHS Foundation Trust, Denmark Hill, London SE5 9RS, UK
Sally V.J. Bowskill, Toxicology Unit, Department of Clinical Biochemistry, King’s College Hospital NHS Foundation Trust, London, UK
Maxine X. Patel, Institute of Psychiatry, King’s College London, Department of Psychosis Studies, London, UK
Robert J. Flanagan, Toxicology Unit, Department of Clinical Biochemistry, King’s College Hospital NHS Foundation Trust, London, UK
References
- Aichhorn W., Marksteiner J., Walch T., Zernig G., Saria A., Kemmler G. (2006) Influence of age, gender, body weight and valproate comedication on quetiapine plasma concentrations. Int Clin Psychopharmacol 21: 81–85 [DOI] [PubMed] [Google Scholar]
- Andersson M., Björkhem-Bergman L., Lindh J. (2011) Possible drug–drug interaction between quetiapine and lamotrigine - evidence from a Swedish TDM database. Br J Clin Pharmacol 72: 153–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis L., Miller G. (1997) Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry 42: 233–246 [DOI] [PubMed] [Google Scholar]
- Bakken G., Rudberg I., Molden E., Refsum H., Hermann M. (2011) Pharmacokinetic variability of quetiapine and the active metabolite N-desalkylquetiapine in psychiatric patients. Ther Drug Monit 33: 222–226 [DOI] [PubMed] [Google Scholar]
- Baumann P., Hiemke C., Ulrich S., Eckermann G., Gaertner I., Gerlach M., et al. (2004) The AGNP-TDM Expert Group Consensus Guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry 37: 243–265 [DOI] [PubMed] [Google Scholar]
- BNF (2012) British National Formulary, 63rd edition. London: BMJ Group/Pharmaceutical Press [Google Scholar]
- Castberg I., Skogvoll E., Spigset O. (2007) Quetiapine and drug interactions: evidence from a routine therapeutic drug monitoring service. J Clin Psychiatr 68: 1540–1545 [DOI] [PubMed] [Google Scholar]
- Citrome L., Jaffe A., Levine J., Lindenmayer J.(2005) Dosing of quetiapine in schizophrenia: how clinical practice differs from registration studies. J Clin Psychiatry 66: 1512–1516 [DOI] [PubMed] [Google Scholar]
- DeVane C., Nemeroff C. (2001) Clinical pharmacokinetics of quetiapine, an atypical antipsychotic. Clin Pharmacokinet 40: 509–522 [DOI] [PubMed] [Google Scholar]
- El-Khalili N., Joyce M., Atkinson S., Buynak R., Datto C., Lindgren P., et al. (2010) Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol 13: 917–932 [DOI] [PubMed] [Google Scholar]
- FDA/CDER (2001) Guidance for industry. Bioanalytical method validation. Food and Drug Administration/Center for Drug Evaluation and Research. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf
- Fisher D., Handley S., Flangan R., Taylor D. (2012a) Plasma concentrations of quetiapine, N-desalkylquetiapine, O-desalkylquetiapine, 7-hydroxyquetiapine and quetiapine sulfoxide in relation to dose, formulation and other factors. Ther Drug Monit 34: 415–421 [DOI] [PubMed] [Google Scholar]
- Fisher D., Handley S., Taylor D., Flanagan R. (2012b) Measurement of quetiapine and four quetiapine metabolites in human plasma by LC-MS/MS. Biomed Chromatogr 26: 1125–1132 [DOI] [PubMed] [Google Scholar]
- Gerlach M., Hünnerkopf R., Rothenhöfer S., Libal G., Burger R., Clement H. (2007) Therapeutic drug monitoring of quetiapine in adolescents with psychotic disorders. Pharmacopsychiatry 40:72–6 [DOI] [PubMed] [Google Scholar]
- Hasselstrøm J., Linnet K. (2004) Quetiapine serum concentrations in psychiatric patients, the influence of comedication. Ther Drug Monit 26: 486–491 [DOI] [PubMed] [Google Scholar]
- Hiemke C., Baumann P., Bergemann N., Conca A., Dietmaier O., Egberts K., et al. (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44: 195–235 [DOI] [PubMed] [Google Scholar]
- Hukkanen J., Väisänen T., Lassila A., Piipari R., Anttila S., Pelkonen O., et al. (2003) Regulation of CYP3A5 by glucocorticoids and cigarette smoke in human lung-derived cells. J Pharamcol Exp Ther 304: 745–752 [DOI] [PubMed] [Google Scholar]
- Hunfeld N., Westerman E., Boswijk D., de Haas J., van Putten M., Touw D. (2006) Quetiapine in overdosage: a clinical and pharmacokinetic analysis of 14 cases. Ther Drug Monit 28: 185–189 [DOI] [PubMed] [Google Scholar]
- Jensen N., Rodriguiz R., Caron M., Wetsel W., Rothman R., Roth B. (2008) N-Desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-htia agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology 33: 2303–2312 [DOI] [PubMed] [Google Scholar]
- Marin J., Levine M., Ensom M. (2006) Is C2 monitoring or another limited sampling strategy superior to C0 monitoring in improving clinical outcomes in adult liver transplant recipients? Ther Drug Monit 28: 637–642 [DOI] [PubMed] [Google Scholar]
- Mauri M., Volonteri L., Fiorentini A., Pirola R., Bareggi S. (2007) Two weeks’ quetiapine treatment for schizophrenia, drug-induced psychosis and borderline personality disorder: a naturalistic study with drug plasma levels. Expert Opin Pharmacother 8: 2207–13 [DOI] [PubMed] [Google Scholar]
- Nikisch G., Baumann P., Kießling B., Reinert M., Wiedemann G., Kehr J. (2010) Cytochrome P450 and ABCB1 genetics: association with quetiapine and norquetiapine plasma and cerebrospinal fluid concentrations and with clinical response in patients suffering from schizophrenia. A pilot study. J Psychopharmacol 30: 496–503 [DOI] [PubMed] [Google Scholar]
- Reis M., Aberg-Wistedt A., Agren H., Akerblad A., Bengtsson F. (2008) Compliance with SSRI medication during 6 months of treatment for major depression: an evaluation by determination of repeated serum drug concentrations. J Affective Disorders 82: 443–446 [DOI] [PubMed] [Google Scholar]
- Sparshatt A., Jones S., Taylor D. (2008) Quetiapine, dose–response relationship in schizophrenia. CNS Drugs 22: 49–68 [DOI] [PubMed] [Google Scholar]
- Sparshatt A., Taylor D., Patel M., Kapur S. (2011) Relationship between daily dose, plasma concentrations, dopamine receptor occupancy, and clinical response to quetiapine: A review. J Clin Psychiatr 72: 1108–23 [DOI] [PubMed] [Google Scholar]
- Spina E., de Leon J. (2007) Metabolic drug interactions with newer antipsychotics: A comparative review. Basic Clin Pharmacol Toxicol 100: 4–22 [DOI] [PubMed] [Google Scholar]
- Taylor D., Paton C., Kapur S. (2012) The Maudsley Prescribing Guidelines, 11th edition. London: Informa Healthcare [Google Scholar]
- Tholen D., Kallner A., Kennedy J., Krouwer J., Meier K. (2004) Evaluation of Precision Performance of Quantitative Measurement Methods; Approved Guideline – Second Edition. Available at: http://webstore.ansi.org/ansidocstore/product.asp?sku=EP05%2DA2
- Wittmann M., Hausner H., Köstlbacher A., Hajak G., Haen E. (2010) Individual clearance and therapeutic drug monitoring of quetiapine in clinical practice. Neuro-Endocrin Lett 31: 203–207 [PubMed] [Google Scholar]