Abstract
Lithium has been used for the treatment of mood disorders for over 60 years, yet the exact mechanisms by which it exerts its therapeutic effects remain unclear. Two enzymatic chains or pathways emerge as targets for lithium: inositol monophosphatase within the phosphatidylinositol signalling pathway and the protein kinase glycogen synthase kinase 3. Lithium inhibits these enzymes through displacing the normal cofactor magnesium, a vital regulator of numerous signalling pathways. Here we provide an overview of evidence, supporting a role for the inhibition of glycogen synthase kinase 3 and inositol monophosphatase in the pharmacodynamic actions of lithium. We also explore how inhibition of these enzymes by lithium can lead to downstream effects of clinical relevance, both for mood disorders and neurodegenerative diseases. Establishing a better understanding of lithium’s mechanisms of action may allow the development of more effective and more tolerable pharmacological agents for the treatment of a range of mental illnesses, and provide clearer insight into the pathophysiology of such disorders.
Keywords: glycogen synthase kinase 3, inositol monophosphatase, lithium, pharmacology
Introduction
It has been over 50 years since Cade first described this simple cation’s therapeutic role in manic illness [Cade, 1949]. Since this discovery, our understanding of its mechanisms of action have developed significantly [Lenox and Hahn, 2000], although the full nature of its effects are yet to be elucidated.
Lithium is primarily used in the recovery of bipolar affective disorder, with evidence supporting its role in both acute mania [Gershon and Soares, 1997; Soares and Gershon, 1998, 2000; Bowden, 2000; Poolsup et al. 2000; Shafti, 2010] and prophylactic treatment [Soares and Gershon, 1998, 2000; Burgess et al. 2001; Geddes et al. 2004, 2010]. It is also efficacious in the treatment of unipolar depression [Coppen, 2000], particularly as augmentation therapy in severe refractory depression [Bauer et al. 2003b; Kennedy and Paykel, 2004], and bipolar depression [Lloyd et al. 2011]. In addition, it has been noted to effect aggressiveness [Jones et al. 2011], reduce suicide rates in affective disorders [Tondo and Baldessarini, 2000; Cipriani et al. 2005] and, more speculatively, has been considered as a possible therapeutic agent for treating chronic neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s diseases [Marmol, 2008; Dudev and Lim, 2011].
Yet, despite lithium’s extensive clinical applications and its ability to provide potentially lifesaving treatment to patients [Nemeroff, 2000; Jope, 2003], the exact mechanisms by which it exerts its therapeutic effects remain incompletely understood [Phiel and Klein, 2001; Gould and Manji, 2005; Haimovich et al. 2012]. Further exploration is required, to determine more fully why lithium provides effective mood stabilisation for patients and allow clearer insight into mood disorder pathophysiology. In addition, lithium’s usage is limited by its narrow therapeutic window and significant range of adverse side effects [Livingstone and Rampes, 2006; McKnight et al. 2012]; better understanding its mechanism of action may allow the development of more effective and more tolerable pharmacological agents for the treatment of mood disorders.
The principal actions of lithium
Lithium’s pharmacodynamic actions are multifaceted and complex [Pasquali et al. 2010]. Unlike many other psychopharmacological agents, such as traditional antidepressants and antipsychotics, it does not bind to cellular receptors; instead, lithium appears to exert therapeutic actions through modification of intracellular second messenger systems, downstream of metabotropic neurotransmitter receptors, via enzyme inhibition [Stahl, 2008], with subsequent alteration of a complex and interconnected intracellular enzymatic cascade.
Two distinct enzymatic chains or pathways emerge as targets for lithium: inositol monophosphatase (IMPase) within the phosphatidylinositol (PI) signalling pathway [Berridge et al. 1989] and the protein kinase glycogen synthase kinase 3β (GSK-3β) [Ryves and Harwood, 2001], although therapeutic effects may be due to further downstream effects [Pasquali et al. 2010].
Lithium and the phosphatidylinositol signalling pathway
Over the last 40 years, a key theory regarding lithium’s pharmacodynamic actions, which has evolved from biochemical data, involves the depletion of free myo-inositol concentrations [Berridge et al. 1989; Harwood, 2005]. Myo-inositol is a prominent form of the six-carbon sugar inositol, and an essential component of the PI intracellular signalling pathway [Hallcher and Sherman, 1980]. It plays an important role in the formation of numerous intracellular chemical compounds, including those acting as signal molecules, and depletion of myo-inositol can have significant effects on the cell, including alteration of cell signalling [Harwood, 2005].
Within the PI signalling pathway (Figure 1), the enzyme IMPase typically regenerates myo-inositol from inositol monophosphates, which in turn leads to the resynthesis of phosphatidylinositol [Silverstone et al. 2005]. At therapeutically relevant doses, lithium is a potent inhibitor of various phosphoinositol phosphates involved in inositol phosphate metabolism, including the intracellular enzymes IMPase and inositol polyphosphatase 1-phosphatase (IPP 1) [Allison and Stewart, 1971; Berridge et al. 1989; Phiel and Klein, 2001]; this inhibition leads to inositol depletion, a consequential reduction in the resynthesis of phosphatidylinositol bisphosphate (PIP2) and prevents regeneration of the second messenger inositol-1,4,5, triphosphate (IP3) [Phiel and Klein, 2001], with subsequent effects on signal transduction [Haimovich et al. 2012].
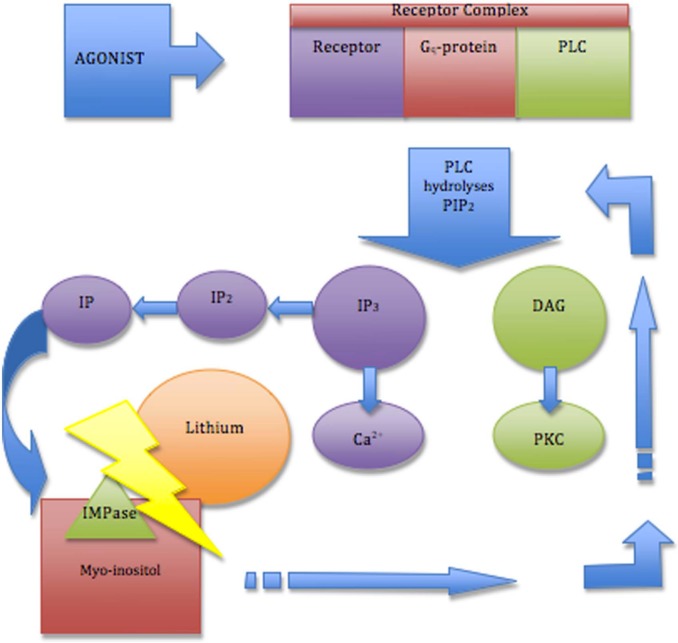
Figure 1.
Inositol depletion within the PI signalling pathway. An agonist binds to a receptor complex, consisting of a receptor, Gq-protein and phospholipase (PLC). PLC hydrolyses the phospholipid phosphatidylinositol 4,5-biophosphate (PIP2) to form two second messengers: inositol-1,4,5 triphosphate (IP3) and 1,2-diacylglycerol (DAG). IP3 binds to specific receptors to help open the calcium (Ca2+) channel and DAG initiates activation of protein kinase C (PKC). IP3 is sequentially broken down into inositol bisphosphates (IP2) and then inositol monophosphates (IP). IP is finally broken down into myo-inositol by the enzyme inositol monophosphatase (IMPase). Lithium inhibits IMPase, leading to myo-inositol depletion. Myo-inositol is also the substrate for synthesis of phosphatidylinositol (PI), which is phosphorylated to form mono-, bis- and tris- phosphatidylinositol. Lithium induced myo-inositol depletion therefore prevents the resynthesis of PIP2 and subsequent regeneration of IP3 and DAG, affecting cell signalling.
Allison and Stewart’s widely replicated research provided initial support for this hypothesis [Allison and Stewart, 1971], reporting that acute lithium administration in rats led to a depletion of myo-inositol. Studies have since demonstrated the validity of this hypothesis as an explanation for the therapeutic action of lithium; magnetic resonance spectroscopy (MRS) findings have shown abnormal PI-cycle activity and elevated myo-inositol concentrations in patients with bipolar disorders [Silverstone et al. 2005], thus it follows that lithium potentially exerts a therapeutic effect by affecting cell signalling as a result of IMPase inhibition, and subsequent reduction of elevated inositol and phosphatidylinositol levels [Haimovich et al. 2012].
This notion is further supported by the fact that lithium is an uncompetitive inhibitor of IMPase [Berridge and Irvine, 1989], thus the level of inhibition increases at high substrate concentrations; since myo-inositol levels are higher in bipolar patients [Silverstone et al. 2005], the level of inhibition is increased in these individuals, potentially explaining why lithium treatment is effective in bipolar disorders but not in comparative normal subjects [Berridge and Irvine, 1989].
Despite the extensive evidence in support of inositol depletion as a viable explanation of lithium’s pharmacodynamic actions, other observations have been inconsistent and often contradictory [Marmol, 2008]. Shaltiel and colleagues, for example, found reduced IMPase activity in lymphocyte-derived cell lines of bipolar patients [Shaltiel et al. 2001]. A lack of novel blood–brain barrier penetrant IMPase inhibitors currently limits evaluating the precise biochemical and therapeutic effects of lithium-induced inositol depletion [Gould and Manji, 2005]. The mechanism by which lithium exerts its effects on the PI signalling pathway is still unclear, and it remains possible, for example, that a decrease in intracellular myo-inositol is only the first stage of action, initiating a cascade of secondary changes in the PKC signalling pathway and gene expression [Agam et al. 2002; Manji and Chen, 2002], that are ultimately associated with lithium’s therapeutic efficacy.
Further research, and the development of appropriate pharmacological agents, are therefore still required, to enable results of greater consistency, and to determine the exact mechanism by which lithium-induced inositol depletion has a therapeutic effect in patients with mood disorders.
Glycogen synthase kinase 3
The ubiquitous serine/threonine protein kinase glycogen synthase kinase 3 (GSK3), offers another potential target for lithium. GSK3 is a critical downstream regulator of diverse signalling pathways [Zhang et al. 2003; Chiu and Chuang, 2010], and has a key role in the regulation of a number of cell functions, including insulin receptor signalling, the specification of cell fates during embryonic development, immunity and inflammation responses and neurotransmission [Cohen and Frame, 2001; Kaidanovich-Beilin and Woodgett, 2011]. Although composed of two isoforms, GSK-3α and GSK-3β, which generally have similar biochemical properties [Gould and Manji, 2005], much of the research in this area has focused on GSK-3β [Phiel and Klein, 2001].
GSK3 displays high activity in cells under resting conditions [Sutherland et al. 1993; Stambolic and Woodgett, 1994; Lochhead et al. 2006] and is primarily regulated through inhibition of its activity via a combination of factors [Kaidanovick-Beilin and Woodgett, 2011], including phosphorylation, intracellular localisation and sequestration by binding proteins [Doble and Woodgett, 2003; Jope and Johnson, 2004; Kockeritz et al. 2006]. Its activity is positively regulated by phosphorylation on tyrosine residues (Thy 279 for GSK-3α and 216 for GSK-3β) [Hughes et al. 1993; Lochhead et al. 2006] and negatively regulated by inhibitory phosphorylation of the N-terminal serines 21 and 9 (Ser 21 for GSK-3α and Ser 9 for GSK-3β) [Sutherland et al. 1993; Stambolic and Woodgett, 1994; Sutherland and Cohen, 1994; Cross et al. 1995].
The phosphorylation state of this site is dynamic [Kaidanovich-Beilin and Woodgett, 2011] and regulated by a variety of kinases, including protein kinase B (Akt) [Cross et al. 1995], cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) [Fang et al. 2000] and PKC [Fang et al. 2002], although activation of Akt kinases provide the most prevalent negative regulation of GSK3 [Freland and Beaulieu, 2012].
Activation of Akt involves phosphorylation of a regulatory threonine residue (Thr 308) by phosphatidylinositol-dependent kinase 1 (PDK1) and additional phosphorylation of the Ser 473 residue by the PDK2/TORC2 kinase [Alessi and Cohen, 1998; Jacinto et al. 2006], in response to phosphatidylinositol kinase (PI3K)-mediated signalling [Beaulieu et al. 2008; Freland and Beaulieu, 2012], leading to GSK3 inhibition. The protein phosphatase 2A (PP2A) participates in the inhibition of Akt [Beaulieu et al. 2005], leading to the opposing effect of GSK3 activation; thus, Akt phosphorylation and GSK3 phosphorylation result from equilibrium between Akt activation and inactivation [Pasquali et al. 2010].
Direct and indirect inhibition of GSK3 by lithium
In 1996, two independent studies demonstrated lithium’s effects as a direct inhibitor of GSK3 in vitro and in cells [Klein and Melton, 1996; Stambolic et al. 1996]. Studies have since found that this is due to a competitive binding for magnesium, leading to disrupted catalytic functioning of GSK3 [Ryves and Harwood, 2001; Pasquali et al. 2010]. The clinical relevance of these findings has remained unclear however, as the high Ki values of lithium for both GSK3 isoforms are greater than therapeutic doses of lithium [Phiel and Klein, 2001], although these values can be affected by the availability of magnesium ions [Ryves and Harwood, 2001].
In addition to direct inhibition, lithium indirectly inhibits GSK3, through enhanced phosphorylation of N-terminal serine residues of GSK3 [Chiu and Chuang, 2010; Pasquali et al. 2010], either due to inhibition of the protein phosphatases [Mora et al. 2002; Zhang et al. 2003] or because of increased activity of PKC and Akt activation [Chalecka-Franaszek and Chuang, 1999; De Sarno et al. 2002].
The activation of Akt by lithium is explained, in part, by its effects on a signalling complex comprised of Akt, beta-arrestin 2(βArr2) and protein phosphatase 2A (Akt;βArr2;PP2A), as a result of competition with magnesium for Akt/ βArr2 interaction [Beaulieu et al. 2008]. The formation of the Akt;βArr2;PP2A signalling complex, typically triggered by stimulation of the dopamine (DA) D2 receptor (D2R) by DA, normally promotes Akt dephosphorylation/inactivation [Beaulieu et al. 2005], leading to activation of GSK3 by dopamine.
Rodent studies have found that lithium disrupts this signalling complex, affecting the regulation of Akt/GSK3 signalling and related behaviours, leading to enhanced Akt activity and increased inhibition of GSK3 (Figure 2) [Beaulieu et al. 2008]. This disruption occurs within therapeutically relevant lithium concentrations (0.5–1.0 mM) [Beaulieu et al. 2008], indicating the potential clinical relevance of these findings.
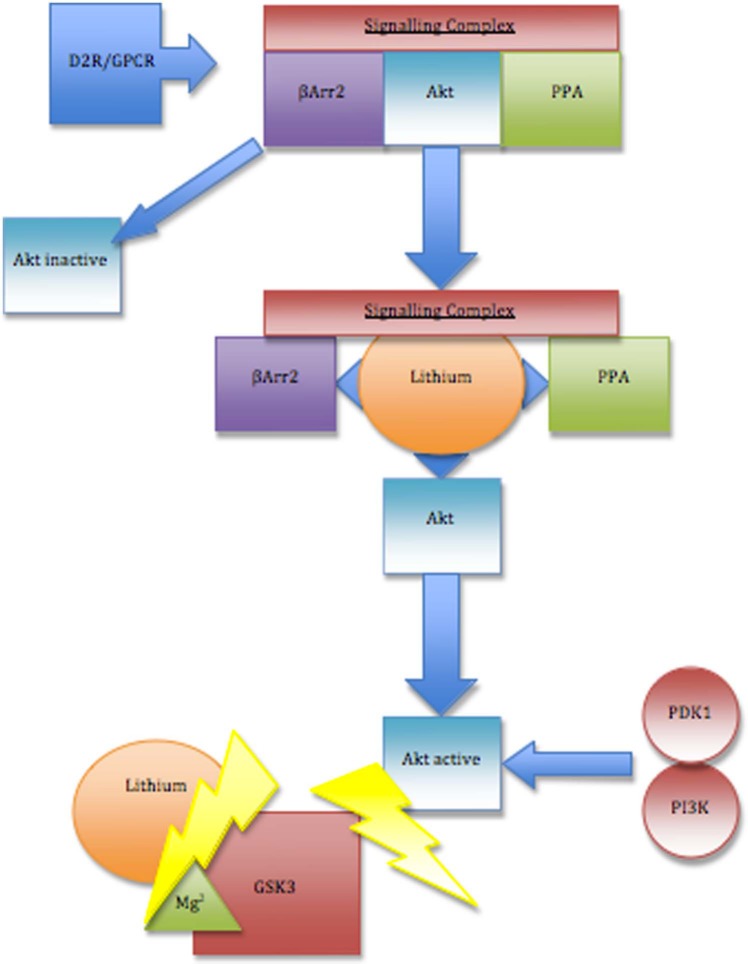
Figure 2.
Inhibition of glycogen synthase kinase 3 (GSK3) by lithium. Lithium directly inhibits GSK3 by competitive binding for magnesium (Mg2+), disrupting the catalytic functioning of GSK3. Lithium also indirectly inhibits GSK3 by increasing serine phosphorylation, through P13K-mediated phosphorylation/activation of Akt. Lithium is able to activate Akt by disrupting the formation of a protein kinase B (Akt), beta-arrestin 2(βArr2) and protein phosphatase 2A (Akt;βArr2;PP2A) comprised signalling complex, triggered by activation of the dopamine 2 receptor (D2R) and potentially other G-protein coupled receptors (GPCR). The Akt;βArr2;PP2A signalling complex typically leads to inactivation of Akt, preventing GSK3 inhibition; the destabilisation of this signalling complex by lithium reduces Akt dephosphorylation, enhancing Akt activity, thus indirectly inhibiting GSK3.
Interestingly, recent evidence suggests that GSK3 is also able to promote its own activation, by enhancing activation of a phosphatase that removes N-terminal inhibitory phosphate groups on GSK3 [Zhang et al. 2003] and by stabilising the Akt;βArr2;PP2A signalling complex, leading to Akt dephosphorylation [O’Brien et al. 2011]. Thus, direct inhibition of GSK3 by lithium would block both mechanisms of auto-activation, providing at least two additional mechanisms by which lithium’s existing therapeutic effects can be strengthened [Freland and Beaulieu, 2012]. Furthermore, recent findings have shown that GSK-3β transcription can be decreased by lithium treatment in vitro and in vivo [Mendes et al. 2009], highlighting the wide-ranging effects of lithium on GSK3 regulation.
GSK3 inhibition is therefore an attractive hypothesis, providing a further explanation for lithium’s pharmacodynamic actions. Evidence of its therapeutic relevance is emerging from animal studies, which link GSK3 and manic- or depressive-like behaviours [Beaulieu et al. 2004; Gould et al. 2004; Kaidanovich-Beilin et al. 2004; O’Brien et al. 2004, Prickaerts et al. 2006; Polter et al. 2010], potentially due to lithium-induced Akt activation [Pan et al. 2011]. Furthermore, abnormal GSK3 activity appears to occur in humans with depression [Karege et al. 2007; Inkster et al. 2009] and bipolar disorder [Polter et al. 2010].
Extensive evidence supports the role of GSK3 inhibition in lithium’s mechanism of action. Given the complexities of lithium pharmacodynamics, however, it is unlikely to be the sole therapeutic target of lithium’s mechanism of action. Evidence has tended to explore IMPase and GSK3 inhibition as distinct targets; however, it may be that the therapeutic effect of lithium arises from a combination of both [Harwood, 2005]. Further research is now needed, to clarify the clinical relevance of these findings and determine the mood stabilising effects of GSK3 and IMPase inhibition in patients with mood disorders [Beaulieu et al. 2008].
Magnesium: the common cofactor
One key hypothesis for the inhibitory effects of lithium on enzymatic targets such as GSK3 and IMPase postulates the competition between lithium and the native enzymatic cofactor magnesium for metal-binding sites [Dudev and Lim, 2011]. Lithium and magnesium (group IIA) possess similar ionic radii (0.60 and 0.65 Å, respectively) and similar physicochemical properties [Dudev and Lim, 2011]. As a result, lithium is able to compete with magnesium and successfully bind to metal-binding sites in several magnesium-dependent enzymes including GSK3 [Ryves and Harwood, 2001] and IMPase [Leech et al. 1993; Haimovich et al. 2012]. Lithium also competes with magnesium for Akt/beta-arrestin-2 interaction, thus providing an explanation for lithium’s ability to destabilise the Akt;βArr2;PP2A signalling complex [Beaulieu et al. 2008].
Although magnesium possesses three binding sites, lithium ions reside in the low-affinity magnesium binding site II and preferentially bind to solvent-exposed magnesium sites with a positive charge density [Haimovich et al. 2012]. This specificity explains why lithium displaces magnesium only in certain enzymes that are key targets of lithium therapy, not in magnesium enzymes that are essential to cells [Dudev and Lim, 2011].
The downstream effects of lithium
The therapeutic effects of lithium typically require long-term treatment and its beneficial actions are not immediately reversed following discontinuation of treatment [Chiu and Chuang, 2010]. This has led to the hypothesis that the effects of lithium on aberrant signalling pathways trigger long-term changes in neuronal intracellular signalling patterns [Lenox and Hahn, 2000], leading to downstream effects of clinical relevance.
Accumulating evidence suggests that the therapeutic effects of mood stabilisers are realised through neurotrophic/neuroprotective effects, offering an explanation for the clinical efficacy of lithium in mood disorders and implicating lithium as a potential therapeutic agent in the treatment of neurodegenerative diseases [Hunsberger et al. 2009].
Cytoskeletal growth stabilisation and plasticity
Lithium alters the level of phosphorylation of cytoskeletal proteins, leading to neuroplastic changes [Lenox and Hahn, 2000]. GSK3 phosphorylates various proteins, including microtubule-associated proteins (MAPs), such as tau and MAP-1B, which regulate the neuronal cytoskeletal network. Inhibition of GSK3 by lithium [Klein and Melton, 1996; Stambolic et al. 1996; Chalecka-Franaszek and Chuang, 1999; De Sarno et al. 2002; Beaulieu et al. 2004] decreases the phosphorylation of tau protein and of MAP-1B [Hong et al. 1997; Munoz-Montano et al. 1997; Lovestone et al. 1999; Engel et al. 2006; Leroy et al. 2010], reducing their ability to bind to microtubules, leading to the promotion of microtubule assembly [Hong et al. 1997; Munoz-Montano et al. 1997] and increased axonal spreading and increases in the growth cone area and perimeter [Garcia-Perez et al. 1998], respectively. Thus, lithium-induced GSK3 inhibition can disrupt microtubule assembly, with effects on cytoskeletal protein association dynamics mediating neuroplastic changes [Lenox and Hahn, 2000].
Downstream effects on cytoskeletal growth stabilisation and plasticity also occur following disruption of the PKC signalling pathway, a secondary effect of lithium-induced IMPase inhibition [Manji and Chen, 2002]. Chronic lithium treatment downregulates the expression of the PKC substrate ‘myristoylated alanine-rich C kinase substrate’ (MARCKS), a protein associated with long-term neuroplastic events in the developing and adult brain [Manji and Lenox, 1999].
Induction of autophagy
Autophagy is a physiological process for the bulk degradation of cytoplasmic proteins or organelles [Sarkar et al. 2005] and an important regulator of cellular (including neuronal) survival and function [Chiu and Chuang, 2010]. Lithium alters rates of autophagy through both the GSK-3β and IMPase pathways, with dose-dependent effects. Lithium-induced IMPase inhibition at lower doses (Ki ≈ 0.8 mM) can enhance autophagy [Sarkar et al. 2005], whilst inhibition of GSK-3β by higher doses of lithium (Ki ≈ 2 mM) suppresses autophagy, by varying activation of the negative regulator mTOR [Sarkar et al. 2008; Chiu and Chuang, 2010].
Glutamate receptor functions
The Akt/GSK3 signalling pathway has been implicated in the downstream regulation of ionotropic glutamate receptor functions [Beaulieu et al. 2009]. Notably, activation of GSK3 has been shown to inhibit the development of glutamatergic N-methyl-D-aspartate (NMDA) receptor-dependent long-term potentiation (LTP), causing changes to neuronal synaptic plasticity and contributing to learning and memory deficits [Zhu et al. 2007]. In addition, GSK3 inhibition has been shown to prevent the development of long-term depression (LTD) in rat hippocampal slices [Peineau et al. 2007], reducing the efficacy of neuronal synapses.
Control of intracellular calcium concentration
There is a general consensus that chronic lithium treatment may modify one or more calcium signalling pathways in the brain [Sourial-Bassillious et al. 2009]. The effects of lithium on the PI signalling pathway, for example [Berridge et al. 1989], leads to a reduction in levels of IP3, an important stimulator for intracellular calcium (Ca2+) levels [Sourial-Bassillious et al. 2009].
IP3 typically mediates calcium release from the endoplasmic reticulum (ER), the primary site for protein synthesis, folding, trafficking, with additional roles in calcium signalling regulation [Chiu and Chuang, 2010]. Lithium’s reduction of IP3 levels therefore inhibits calcium release from the ER, with effects on neuronal functioning [Harwood, 2005]; this includes reduction in the activity of the calcium-dependent protein calpain and calpain-mediated activation of pro-apoptotic cyclin-dependent kinase 5 (Cdk5), leading to reduced cellular death [Crespo-Biel et al. 2009].
Circadian patterns
Circadian rhythmicity, an evolutionarily highly conserved molecular autoregulatory feedback loop of protein translation and transcription, regulates numerous physiological processes, including neurotransmitter expression, on an approximately 24-hour cycle. GSK3 (and in drosophila studies, its homologue Sgg) has been shown to regulate circadian period length through the translation and transcription of ‘clock’ genes such as mPer2. Animal models [Martinek et al. 2001; Mohawk et al. 2009; Kaladchibachi et al. 2007] have demonstrated that lithium lengthens the circadian period in a dose-dependent manner. Clock-mutant mice have been shown to display ‘manic’ behaviours that is reversed by the administration of lithium [Roybal et al. 2007] posited to be GSK3 inhibition, although lithium has also been noted to affect the free firing rate of mouse suprachiasmatic nucleus neurons [Abe et al. 2000; Li et al. 2012].
Altering metabotropic monoaminergic signalling
Dopaminergic and serotonergic (dys)functioning in mental illness has been heavily researched, although much of this research has focused on the neurotransmitter–receptor complexes, not least because these are the sites for antipsychotic and antidepressant medication functioning, and less on the more complex downstream effects.
Animal models have demonstrated that clinically relevant doses of lithium lead to neurobiological changes in DA function, with lithium administration in rodents over a 1-month period affecting presynaptic DA function and attenuating potassium-evoked DA release [Ferrie et al. 2006]. Lithium-induced attenuation of presynaptic DA function can affect the phosphorylation and activity of GSK3 [McQuade and Robinson, 2012], and further contribute to its inhibition by lithium treatment. Dopamine D2 receptor activation leads to formation of an intracellular complex of β-arrestin2, Akt and protein phosphatase 2A (PP2A) that deactivates the Akt and subsequently stimulates GSK3 signalling, as well as accumulation of β-arrestins that both terminate the signalling and internalise the receptor complex [Beaulieu et al. 2009]. Lithium treatment can acutely affect this postsynaptic DA function by interfering with the effect of DA on the Akt/GSK3 signalling cascade [Beaulieu et al. 2004].
Serotonin can differentially inhibit or promote GSK3 activity through 5HT1A and 5HT2A agonism respectively, although the relative contribution of each to normal and pathological functioning is uncertain [Li et al. 2004]. However, whilst lithium is clearly implicated in altering GSK3/Akt, effects on normal dopaminergic and serotonergic functioning, and in the presence of other pathway-modifying medications, remain to be fully elucidated.
Neuroprotection
Considerable interest has been shown in putative neuroprotective actions of lithium, particularly with regards to dementing illnesses, although the epidemiological evidence remains challengeable [Young, 2011]. Several of the previously described mechanisms, independently or synergistically, may be protective of brain cell functioning [Chiu and Chuang 2010]: inhibition of glutamatergic excitotoxicity via NMDA receptor-mediated calcium influx; inhibition of autophagy, including in the presence of the insult of β-amyloid [Alvarez et al. 2002]; increasing neuronal growth cones, via IMPase inhibition and inositol depletion; and induction and upregulation of the cortical developmental neurotrophins brain-derived neurotrophic factor (BDNF) [Yasuda et al. 2009] and vascular endothelial growth factor (VEGF) [Guo et al. 2009].
Grey and white matter volume
Magnetic resonance imaging (MRI) studies have demonstrated increased grey matter volume in bipolar patients, following administration of lithium [Moore et al. 2000b; Sassi et al. 2002; Bearden et al. 2007]. Studies have generally failed to identify any effects in white matter, although Monkul and colleagues found increased dorsolateral prefrontal cortex and cingulate grey matter volume and increased white matter volume in healthy subjects following lithium administration [Monkul et al. 2007], potentially highlighting the different generalised effects of lithium in healthy and diseased brains.
The regional specificity of these findings makes it unlikely that these findings are due to the osmotic effects of lithium; instead, the neurotrophic effect of lithium seems a more viable explanation [Moore et al. 2000b; Sassi et al. 2002; Bearden et al. 2007; Monkul et al. 2007]. Notably, lithium’s ability to robustly increase expression of the cytoprotective protein B-cell lymphoma/leukaemia 2 (bcl-2) [Chen et al. 1999; Manji et al. 2000a; Moore et al. 2000b], as well as its effects on GSK3 [Klein and Melton, 1996; Stambolic et al. 1996; Chalecka-Franaszek and Chuang, 1999; De Sarno et al. 2002; Beaulieu et al. 2004], is thought to exert major neurotrophic effects, resulting in neuropil increases, increased N-acetyl-aspartate (NAA) levels (a postulated marker of neuronal viability and function), with significant effects on grey matter volume [Manji et al. 2000b; Moore et al. 2000a].
Conclusion: pulling the evidence together
Lithium is chemically remarkably simple and, in human neuronal tissue, biochemically remarkably complex. Its clinical efficacy in mood disorders is well established and there is growing epidemiological evidence to support broader effects including positively altering aggression and suicide rates, and potentially being protective against neurodegenerative disorders.
Its actions are incompletely understood at best, but there are recognised intracellular targets and plausible biochemical actions to account for at least some of its effects. Two major enzymatic pathways, the PI and GSK3, are altered by lithium, and magnesium displacement might be a common mechanism of action, although the precise contribution of each to clinical effects is uncertain.
The effects of these pathways and their downstream effects offer tantalising, incomplete, evidence for how lithium might exert clinical effects. Altered autophagy could provide cognitive [Caccamo et al. 2010], neuroprotective [Chiu and Chuang 2010] and mood stabilisation [Cleary et al. 2008]. Cytoskeletal growth stabilisation and plasticity appear to lead to stabilisation of aberrant neuronal activity, a conceptually attractive target for a mood-stabilising drug [Lenox and Watson, 1994]. Decreased dopamine release following chronic treatment has been suggested as a viable, at least partial, explanation for lithium’s mood-stabilising action [Ferrie et al. 2006] and effects on glutamate receptor functions through GSK3 inhibition may preserve and enhance both LTP and LTD [Peineau et al. 2007], with potential effects on learning and memory, which can be adversely affected in affective disorders, as well as neurodegenerative disorders.
Alteration of intracellular calcium homeostasis has been identified in mood disorders, and downstream regulation of calcium signalling following lithium [Sourial-Bassillious et al. 2009] offers another potential therapeutic mechanism [Bauer et al. 2003a; Wasserman et al. 2004; Perova et al. 2008]. Similarly alterations in sleep patterns are a clinically long recognised precipitant and symptom of mood disturbances: most therapeutic work in this regard has explored monoaminergic effects on the thalamus, but there is now evidence for lithium modulating circadian patterns and suprachiasmatic nuclear functioning and expression of clock genes. Lithium’s evidenced ability to prevent or partly reverse the grey-matter deficits seen in bipolar patients [Sassi et al. 2002] has also been linked to specific therapeutic effects, including its effects on suicidality, potentially through impacting on goal directed behaviour and affecting cognitive distortions [Benedetti et al. 2011].
Further work is required to better elucidate the mechanism of action of this effective, but potentially toxic, drug. Understanding which processes contribute to clinical improvement, and how, affords at least the potential to develop targeted compounds, although undoubtedly the challenges faced in so doing are enormous. Better knowledge of the neuropathophysiology will also enhance our understanding of the neuroscience of affective disorders and potentially neurodegenerative conditions.
Acknowledgments
The authors are grateful to Dr Richard McQuade, Psychobiology Research Group, Institute of Neuroscience, Newcastle University, for email correspondence and discussion of his on-going work in this field that helped with the preparation of the final draft of this manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: DKT has received honoraria from Lilly UK and Roche for educational talks.
Contributor Information
Kayleigh M. Brown, Institute of Psychiatry, King’s College London, PO Box 63, De Crespigny Park, Denmark Hill, London SE5 8AF, UK
Derek K. Tracy, The Institute of Psychiatry, King’s College, London, UK and Oxleas NHS Foundation Trust, UK
References
- Abe M., Herzog E., Block G. (2000) Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport 11: 3261–3264 [DOI] [PubMed] [Google Scholar]
- Agam G., Shamir A., Shaltiel G., Greenberg M. (2002) Myo-inositol-1-phosphate (MIP) synthase: a possible new target for antibipolar drugs. Bipolar Disord 4: 15–20 [DOI] [PubMed] [Google Scholar]
- Alessi D., Cohen P. (1998) Mechanism of activation and function of protein kinase B. Curr Opin Genetics Dev 8: 55–62 [DOI] [PubMed] [Google Scholar]
- Allison J., Stewart M. (1971) Reduced brain inositol in lithium-treated rats. Nat New Biol 233: 267–268 [DOI] [PubMed] [Google Scholar]
- Alvarez G., Munoz-Montano J., Satrustegui J., Avila J., Bogonez E., Diaz-Nido J. (2002) Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord 4: 153–165 [DOI] [PubMed] [Google Scholar]
- Bauer M., Alda M., Priller J., Young L. (2003a) Implications of the neuroprotective effects of lithium for the treatment of bipolar and neurodegenerative disorders. Pharmacopsychiatry 36: S250–S254 [DOI] [PubMed] [Google Scholar]
- Bauer M., Forsthoff A., Baethge C., Adli M., Berghofer A., Dopfmer S., et al. (2003b) Lithium augmentation therapy in refractory depression - Update 2002. Eur Arch Psychiatry Clin Neurosci 253: 132–139 [DOI] [PubMed] [Google Scholar]
- Bearden C., Thompson P., Dalwani M., Hayashi K., Lee A., Nicoletti M., et al. (2007) Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry 62: 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J., Gainetdinov R., Caron M. (2009) Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol 49: 327–347 [DOI] [PubMed] [Google Scholar]
- Beaulieu J., Marion S., Rodriguiz R., Medvedev I., Sotnikova T., Ghisi V., et al. (2008) A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132: 125–136 [DOI] [PubMed] [Google Scholar]
- Beaulieu J., Sotnikova T., Marion S., Lefkowitz R., Gainetdinov R., Caron M. (2005) An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122: 261–273 [DOI] [PubMed] [Google Scholar]
- Beaulieu J., Sotnikova T., Yao W., Kockeritz L., Woodgett J., Gainetdinov R., et al. (2004) Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A 101: 5099–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Radaelli D., Poletti S., Locatelli C., Falini A., Colombo C., et al. (2011) Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J Affect Disord 135: 139–147 [DOI] [PubMed] [Google Scholar]
- Berridge M., Downes C., Hanley M. (1989) Neural and developmental actions of lithium - a unifying hypothesis. Cell 59: 411–419 [DOI] [PubMed] [Google Scholar]
- Berridge M., Irvine R. (1989) Inositol phosphates and cell signaling. Nature 341: 197–205 [DOI] [PubMed] [Google Scholar]
- Bowden C. (2000) Efficacy of lithium in mania and maintenance therapy of bipolar disorder. J Clin Psychiatry 61: 35–40 [PubMed] [Google Scholar]
- Burgess S., Geddes J., Hawton K., Townsend E., Jamison K., Goodwin G. (2001) Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev: CD003013. [DOI] [PubMed] [Google Scholar]
- Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. (2010) Molecular Interplay between mTOR, A (beta), and tau: effects on cognitive impairments. J Biol Chem 285: 13107–13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade J. (1949) Lithium salts in the treatment of psychotic excitement. Med J Austral 2: 349–352 [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E., Chuang D. (1999) Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A 96: 8745–8750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Zeng W., Yuan P., Huang L., Jiang Y., Zhao Z., et al. (1999) The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem 72: 879–882 [DOI] [PubMed] [Google Scholar]
- Chiu C., Chuang D. (2010) Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther 128: 281–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A., Pretty H., Hawton K., Geddes J. (2005) Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: A systematic review of randomized trials. Am J Psychiatry 162: 1805–1819 [DOI] [PubMed] [Google Scholar]
- Cleary C., Linde J., Hiscock K., Hadas I., Belmaker R., Agam G., et al. (2008) Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res Bull 76: 469–473 [DOI] [PubMed] [Google Scholar]
- Cohen P., Frame S. (2001) The renaissance of GSK3. Nat Rev Mol Cell Biol 2: 769–776 [DOI] [PubMed] [Google Scholar]
- Coppen A. (2000) Lithium in unipolar depression and the prevention of suicide. J Clin Psychiatry 61: 52–56 [PubMed] [Google Scholar]
- Crespo-Biel N., Camins A., Pallas M., Canudas A. (2009) Evidence of calpain/cdk5 pathway inhibition by lithium in 3-nitropropionic acid toxicity in vivo and in vitro. Neuropharmacology 56: 422–428 [DOI] [PubMed] [Google Scholar]
- Cross D., Alessi D., Cohen P., Andjelkovich M., Hemmings B. (1995) Inhibition of glycogen-synthase kinase-3 by insulin-mediated by protein-kinase-B. Nature 378: 785–789 [DOI] [PubMed] [Google Scholar]
- De Sarno P., Li X., Jope R. (2002) Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology 43: 1158–1164 [DOI] [PubMed] [Google Scholar]
- Doble B., Woodgett J. (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116: 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudev T., Lim C. (2011) Competition between Li+ and Mg2+ in metalloproteins. Implications for lithium therapy. J Am Chem Soc 133: 9506–9515 [DOI] [PubMed] [Google Scholar]
- Engel T., Goni-Oliver P., Lucas J., Avila J., Hernandez F. (2006) Chronic lithium administration to FTDP-17 tau and GSK-3 beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J Neurochem 99: 1445–1455 [DOI] [PubMed] [Google Scholar]
- Fang X., Yu S., Lu Y., Bast R., Woodgett J., Mills G. (2000) Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A 97: 11960–11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Yu S., Tanyi J., Lu Y., Woodgett J., Mills G. (2002) Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol 22: 2099–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie L., Young A., McQuade R. (2006) Effect of lithium and lithium withdrawal on potassium-evoked dopamine release and tyrosine hydroxylase expression in the rat. Int J Neuropsychopharmacol 9: 729–735 [DOI] [PubMed] [Google Scholar]
- Freland L., Beaulieu J. (2012) Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front Mol Neurosci 5: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez J., Avila J., Diaz-Nido J. (1998) Implication of cyclin-dependent kinases and glycogen synthase kinase 3 in the phosphorylation of microtubule-associated protein 1B in developing neuronal cells. J Neurosci Res 52: 445–452 [DOI] [PubMed] [Google Scholar]
- Geddes J., Burgess S., Hawton K., Jamison K., Goodwin G. (2004) Long-term lithium therapy for bipolar disorder: Systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry 161: 217–222 [DOI] [PubMed] [Google Scholar]
- Geddes J., Goodwin G., Rendell J., Azorin J., Cipriani A., Ostacher M., et al. (2010) Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet 375: 385–395 [DOI] [PubMed] [Google Scholar]
- Gershon S., Soares J. (1997) Current therapeutic profile of lithium. Arch Gen Psychiatry 54: 16–20 [DOI] [PubMed] [Google Scholar]
- Gould T., Einat H., Bhat R., Manji H. (2004) AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol 7: 387–390 [DOI] [PubMed] [Google Scholar]
- Gould T., Manji H. (2005) Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology 30: 1223–1237 [DOI] [PubMed] [Google Scholar]
- Guo S., Arai K., Stins M., Chuang D., Lo E. (2009) Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes. Stroke 40: 652–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich A., Eliav U., Goldbourt A. (2012) Determination of the lithium binding site in inositol monophosphatase, the putative target for lithium therapy, by magic-angle-spinning solid-state NMR. J Am Chem Soc 134: 5647–5651 [DOI] [PubMed] [Google Scholar]
- Hallcher L., Sherman W. (1980) The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem 255: 896–901 [PubMed] [Google Scholar]
- Harwood A. (2005) Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry 10: 117–126 [DOI] [PubMed] [Google Scholar]
- Hong M., Chen D., Klein P., Lee V. (1997) Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem 272: 25326–25332 [DOI] [PubMed] [Google Scholar]
- Hughes K., Nikolakaki E., Plyte S., Totty N., Woodgett J. (1993) Modulation of the glycogen-synthase kinase-3 family by tyrosine phosphorylation. EMBO J 12: 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger J., Austin D., Henter I., Chen G. (2009) The neurotrophic and neuroprotective effects of psychotropic agents. Dialogues Clin Neurosci 11: 333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkster B., Nichols T., Saemann P., Auer D., Holsboer F., Muglia P., et al. (2009) Association of GSK3 beta polymorphisms with brain structural changes in major depressive disorder. Arch Gen Psychiatry 66: 721–728 [DOI] [PubMed] [Google Scholar]
- Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S., et al. (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127: 125–137 [DOI] [PubMed] [Google Scholar]
- Jones R., Arlidge J., Gillham R., Reagu S., van den Bree M., Taylor P. (2011) Efficacy of mood stabilisers in the treatment of impulsive or repetitive aggression: systematic review and meta-analysis. Br J Psychiatry 198: 93–98 [DOI] [PubMed] [Google Scholar]
- Jope R. (2003) Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci 24: 441–443 [DOI] [PubMed] [Google Scholar]
- Jope R., Johnson G. (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29: 95–102 [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O., Milman A., Weizman A., Pick C., Eldar-Finkelman H. (2004) Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry 55: 781–784 [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O., Woodgett J. (2011) GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci 4: 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladchibachi S., Doble B., Anthopoulos N., Woodgett J., Manoukian A. (2007) Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. J Circadian Rhythms 5: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F., Perroud N., Burkhardt S., Schwald M., Ballmann E., La Harpe R., et al. (2007) Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3 beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry 61: 240–245 [DOI] [PubMed] [Google Scholar]
- Kennedy N., Paykel E. (2004) Treatment and response in refractory depression: results from a specialist affective disorders service. J Affect Disord 81: 49–53 [DOI] [PubMed] [Google Scholar]
- Klein P., Melton D. (1996) A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A 93: 8455–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockeritz L., Doble B., Patel S., Woodgett J. (2006) Glycogen synthase kinase-3 - An overview of an over-achieving protein kinase. Curr Drug Targets 7: 1377–1388 [DOI] [PubMed] [Google Scholar]
- Leech A., Baker G., Shute J., Cohen M., Gani D. (1993) Chemical and kinetic mechanism of the inositol monophosphatase reaction and its inhibition by Li+. Eur J Biochem 212: 693–704 [DOI] [PubMed] [Google Scholar]
- Lenox R., Hahn C. (2000) Overview of the mechanism of action of lithium in the brain: fifty-year update. J Clin Psychiatry 61: 5–15 [PubMed] [Google Scholar]
- Lenox R., Watson D. (1994) Lithium and the brain – a psychopharmacological strategy to a molecular-basis for manic-depressive illness. Clin Chem 40: 309–314 [PubMed] [Google Scholar]
- Leroy K., Ando K., Heraud C., Yilmaz Z., Authelet M., Boeynaems J., et al. (2010) Lithium treatment arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathology. J Alzheimers Dis 19: 705–719 [DOI] [PubMed] [Google Scholar]
- Li J., Lu W., Beesley S., Loudon A., Meng Q. (2012) Lithium impacts on the amplitude and period of the molecular circadian clockwork. Plos One 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhu W., Roh M., Friedman A., Rosborough K., Jope R. (2004) In vivo regulation of glycogen synthase kinase-3 beta (GSK3 beta) by serotonergic activity in mouse brain. Neuropsychopharmacology 29: 1426–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone C., Rampes H. (2006) Lithium: a review of its metabolic adverse effects. J Psychopharmacol 20: 347–355 [DOI] [PubMed] [Google Scholar]
- Lloyd L., Giaroli G., Taylor D., Tracy D. (2011) Bipolar depression: clinically missed, pharmacologically mismanaged. Ther Adv Psychopharmacol 1: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead P., Kinstrie R., Sibbet G., Rawjee T., Morrice N., Cleghon V. (2006) A chaperone-dependent GSK3 beta transitional intermediate mediates activation-loop autophosphorylation. Mol Cell 24: 627–633 [DOI] [PubMed] [Google Scholar]
- Lovestone S., Davis D., Webster M., Kaech S., Brion J., Matus A., et al. (1999) Lithium reduces tau phosphorylation: Effects in living cells and in neurons at therapeutic concentrations. Biol Psychiatry 45: 995–1003 [DOI] [PubMed] [Google Scholar]
- Manji H., Chen G. (2002) PKC, MAP kinases and the bcl-2 family of proteins as long-term targets for mood stabilizers. Mol Psychiatry 7: S46–S56 [DOI] [PubMed] [Google Scholar]
- Manji H., Lenox R. (1999) Protein kinase C signaling in the brain: Molecular transduction of mood stabilization in the treatment of manic-depressive Illness. Biol Psychiatry 46: 1328–1351 [DOI] [PubMed] [Google Scholar]
- Manji H., Moore G., Chen G. (2000a) Lithium up-regulates the cytoprotective protein bcl-2 in the CNS in vivo: A role for neurotrophic and neuroprotective effects in manic depressive illness. J Clin Psychiatry 61: 82–96 [PubMed] [Google Scholar]
- Manji H., Moore G., Chen G. (2000b) Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: Implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry 48: 740–754 [DOI] [PubMed] [Google Scholar]
- Marmol F. (2008) Lithium: bipolar disorder and neurodegenerative diseases. Possible cellular mechanisms of the therapeutic effects of lithium. Prog NeuroPsychopharmacol Biol Psych 32: 1761–1771 [DOI] [PubMed] [Google Scholar]
- Martinek S., Inonog S., Manoukian A., Young M. (2001) A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105: 769–779 [DOI] [PubMed] [Google Scholar]
- McKnight R., Adida M., Budge K., Stockton S., Goodwin G., Geddes J. (2012) Lithium toxicity profile: a systematic review and meta-analysis. Lancet 379: 721–728 [DOI] [PubMed] [Google Scholar]
- McQuade R., Robinson S. (2012) Attenuation of presynaptic dopamine function contributes to the effects of lithium on GSK-3β phosphorylation, in preparation. [Google Scholar]
- Mendes C., Mury F., Moreira E., Alberto F., Forlenza O., Dias-Neto E., et al. (2009) Lithium reduces Gsk3b mRNA levels: implications for Alzheimer disease. Eur Arch Psychiatry Clin Neurosci 259: 16–22 [DOI] [PubMed] [Google Scholar]
- Mohawk J., Miranda-Anaya M., Tataroglu O., Menaker M. (2009) Lithium and genetic inhibition of GSK3 beta enhance the effect of methamphetamine on circadian rhythms in the mouse. Behav Pharmacol 20: 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkul E., Matsuo K., Nicoletti M., Dierschke N., Hatch J., Dalwani M., et al. (2007) Prefrontal gray matter increases in healthy individuals after lithium treatment: A voxel-based morphometry study. Neurosci Lett 429: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G., Bebchuk J., Hasanat K., Chen G., Seraji-Bozorgzad N., Wilds I., et al. (2000a) Lithium increases N-acetyl-aspartate in the human brain: In vivo evidence in support of bcl-2s neurotrophic effects? Biol Psychiatry 48: 1–8 [DOI] [PubMed] [Google Scholar]
- Moore G., Bebchuk J., Wilds I., Chen G., Menji H. (2000b) Lithium-induced increase in human brain grey matter. Lancet 356: 1241–1242 [DOI] [PubMed] [Google Scholar]
- Mora A., Sabio G., Risco A., Cuenda A., Alonso J., Soler G., et al. (2002) Lithium blocks the PKB and GSK3 dephosphorylation induced by ceramide through protein phosphatase-2A. Cell Signal 14: 557–562 [DOI] [PubMed] [Google Scholar]
- Munoz-Montano J., Moreno F., Avila J., DiazNido J. (1997) Lithium inhibits Alzheimers disease-like tau protein phosphorylation in neurons. FEBS Lett 411: 183–188 [DOI] [PubMed] [Google Scholar]
- Nemeroff C. (2000) Introduction - Fifty years of lithium use in the treatment of bipolar disorder. J Clin Psychiatry 61: 3–410910011 [Google Scholar]
- O’Brien W., Harper A., Jove F., Woodgett J., Maretto S., Piccolo S., et al. (2004) Glycogen synthase kinase-3 beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci 24: 6791–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien W., Huang J., Buccafusca R., Garskof J., Valvezan A., Berry G., et al. (2011) Glycogen synthase kinase-3 is essential for beta-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J Clin Invest 121: 3756–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Lewis M., Ketterman J., Clore E., Riley M., Richards K., et al. (2011) AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology 36: 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali L., Busceti C., Fulceri F., Paparelli A., Fornai F. (2010) Intracellular pathways underlying the effects of lithium. Behav Pharmacol 21: 473–492 [DOI] [PubMed] [Google Scholar]
- Peineau S., Taghibiglou C., Bradley C., Wong T., Liu L., Lu J., et al. (2007) UP inhibits LTD in the hippocampus via regulation of GSK3 beta. Neuron 53: 703–717 [DOI] [PubMed] [Google Scholar]
- Perova T., Wasserman M., Li P., Warsh J. (2008) Hyperactive intracellular calcium dynamics in B lymphoblasts from patients with bipolar I disorder. Int J Neuropsychopharmacol 11: 185–196 [DOI] [PubMed] [Google Scholar]
- Phiel C., Klein P. (2001) Molecular targets of lithium action. Annu Rev Pharmacol Toxicol 41: 789–813 [DOI] [PubMed] [Google Scholar]
- Polter A., Beurel E., Yang S., Garner R., Song L., Miller C., et al. (2010) Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology 35: 1761–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolsup N., Po A., de Oliveira I. (2000) Systematic overview of lithium treatment in acute mania. J Clin Pharm Ther 25: 139–156 [DOI] [PubMed] [Google Scholar]
- Prickaerts J., Moechars D., Cryns K., Lenaerts I., van Craenendonck H., Goris I., et al. (2006) Transgenic mice overexpressing glycogen synthase kinase 3 beta: A putative model of hyperactivity and mania. J Neurosci 26: 9022–9029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal K., Theobold D., Graham A., DiNieri J., Russo S., Krishnan V., et al. (2007) Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A 104: 6406–6411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryves W., Harwood A. (2001) Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun 280: 720–725 [DOI] [PubMed] [Google Scholar]
- Sarkar S., Floto R., Berger Z., Imarisio S., Cordenier A., Pasco M., et al. (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170: 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Krishna G., Imarisio S., Saiki S., OKane C., Rubinsztein D. (2008) A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum Mol Genet 17: 170–178 [DOI] [PubMed] [Google Scholar]
- Sassi R., Nicoletti M., Brambilla P., Mallinger A., Frank E., Kupfer D., et al. (2002) Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett 329: 243–245 [DOI] [PubMed] [Google Scholar]
- Shafti S. (2010) Olanzapine vs. lithium in management of acute mania. J Affect Disord 122: 273–276 [DOI] [PubMed] [Google Scholar]
- Shaltiel G., Shamir A., Nemanov L., Yaroslavsky Y., Nemets B., Ebstein R., et al. (2001) Inositol monophosphatase activity in brain and lymphocyte-derived cell lines of bipolar patients. World J Biol Psychiatry 2: 95–8 [DOI] [PubMed] [Google Scholar]
- Silverstone P., McGrath B., Kim H. (2005) Bipolar disorder and myo-inositol: a review of the magnetic resonance spectroscopy findings. Bipolar Disord 7: 1–10 [DOI] [PubMed] [Google Scholar]
- Soares J., Gershon S. (1998) The lithium ion: A foundation for psychopharmacological specificity. Neuropsychopharmacology 19: 167–182 [DOI] [PubMed] [Google Scholar]
- Soares J., Gershon S. (2000) The psychopharmacologic specificity of the lithium ion: Origins and trajectory. J Clin Psychiatry 61:16–22 [PubMed] [Google Scholar]
- Sourial-Bassillious N., Rydelius P., Aperia A., Aizman O. (2009) Glutamate-mediated calcium signaling: a potential target for lithium action. Neuroscience 161: 1126–1134 [DOI] [PubMed] [Google Scholar]
- Stahl S. (2008) Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 3rd edn (Essential Psychopharmacology Series). Cambridge: Cambridge University Press [Google Scholar]
- Stambolic V., Ruel L., Woodgett J. (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics Wingless signalling in intact cells. Curr Biol 6: 1664–1668 [DOI] [PubMed] [Google Scholar]
- Stambolic V., Woodgett J. (1994) Mitogen inactivation of glycogen-synthase kinase-3-beta in intact-cells via serine-9 phosphorylation. Biochem J 303: 701–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C., Cohen P. (1994) The alpha-isoform of glycogen-synthase-kinase-3 from rabbit skeletal-muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in-vitro. FEBS Lett 338: 37–42 [DOI] [PubMed] [Google Scholar]
- Sutherland C., Leighton I., Cohen P. (1993) Inactivation of glycogen-synthase-kinase-3-beta by phosphorylation - new kinase connections in insulin and growth-factor signaling. Biochem J 296: 15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondo L., Baldessarini R. (2000) Reduced suicide risk during lithium maintenance treatment.J Clin Psychiatry 61: 97–104 [PubMed] [Google Scholar]
- Wasserman M., Corson T., Sibony D., Cooke R., Parikh S., Pennefather P., et al. (2004) Chronic lithium treatment attenuates intracellular calcium mobilization. Neuropsychopharmacology 29:759–769 [DOI] [PubMed] [Google Scholar]
- Yasuda S., Liang M., Marinova Z., Yahyavi A., Chuang D. (2009) The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 14: 51–59 [DOI] [PubMed] [Google Scholar]
- Young A. (2011) More good news about the magic ion: lithium May prevent dementia. Br J Psychiatry 198: 336–337 [DOI] [PubMed] [Google Scholar]
- Zhang F., Phiel C., Spece L., Gurvich N., Klein P. (2003) Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium – evidence for autoregulation of GSK-3. J Biol Chem 278: 33067–33077 [DOI] [PubMed] [Google Scholar]
- Zhu L., Wang S., Liu D., Yin Y., Tian Q., Wang X., et al. (2007) Activation of glycogen synthase kinase-3 inhibits long term potentiation with synapse-associated impairments. J Neurosci 27: 12211–12220 [DOI] [PMC free article] [PubMed] [Google Scholar]