Abstract
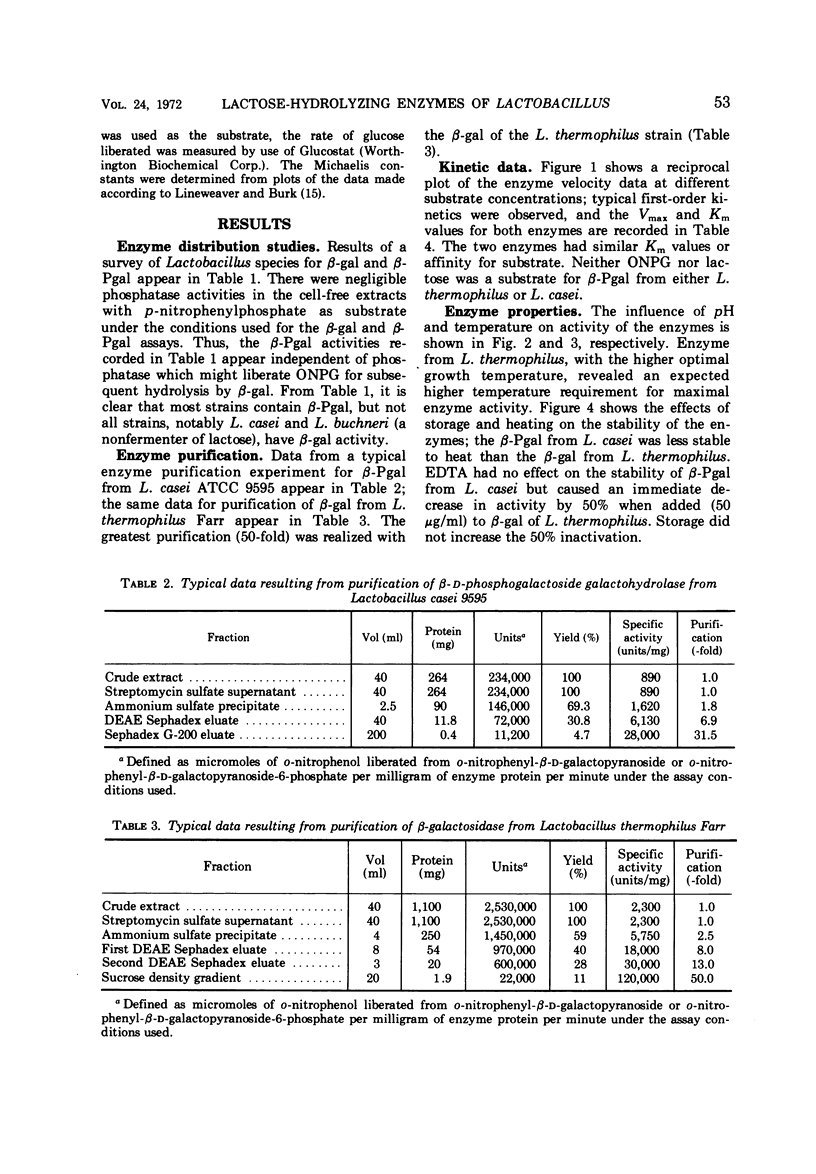
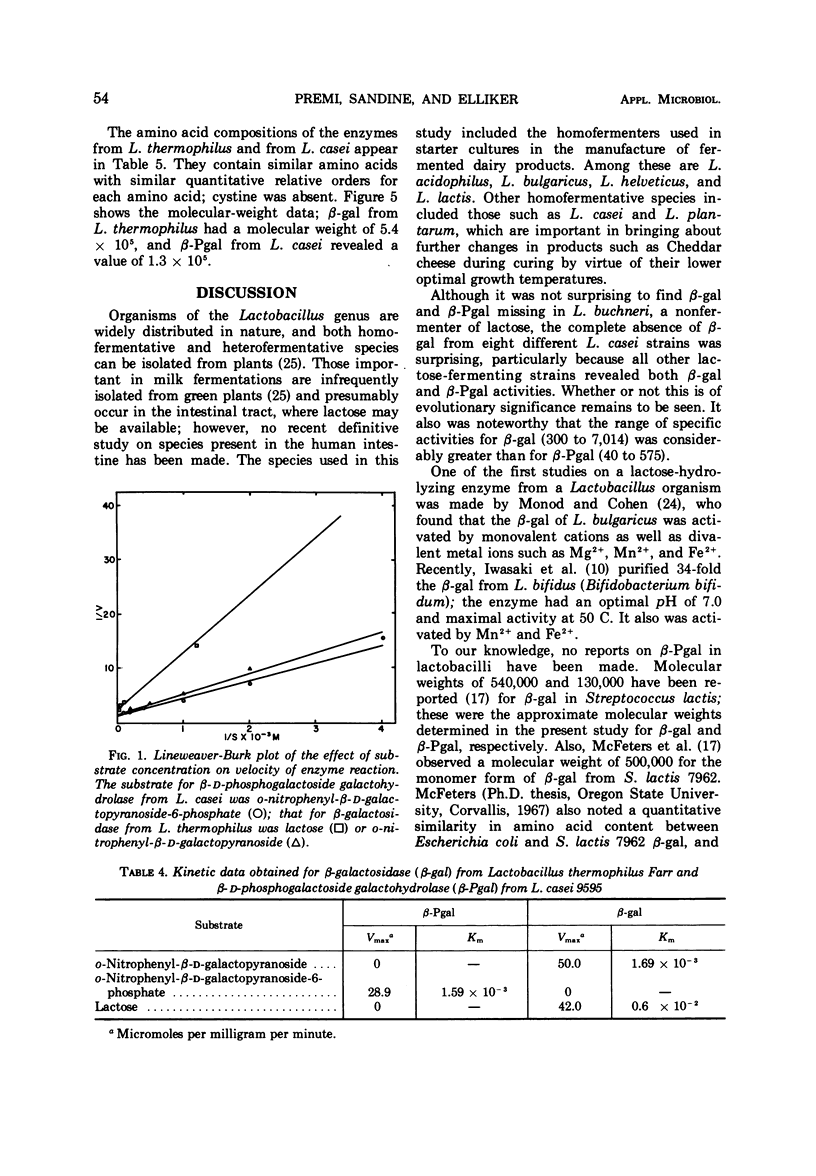
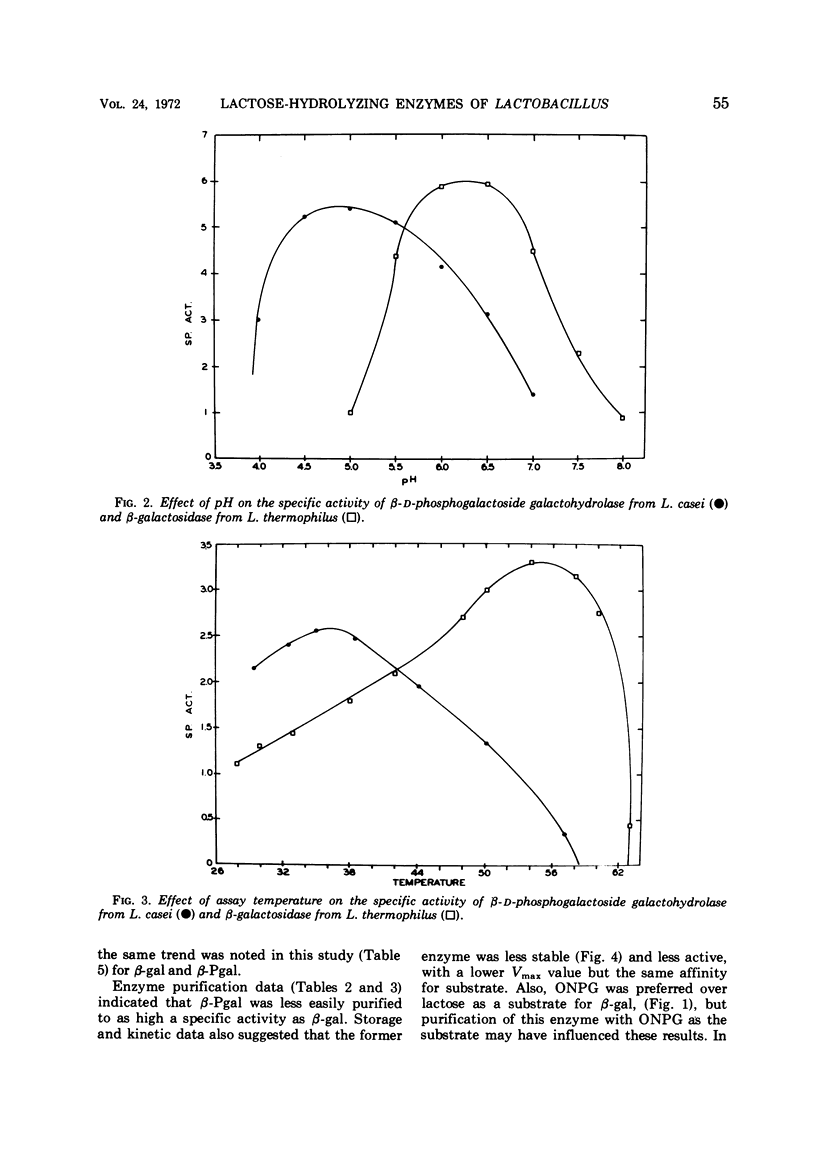
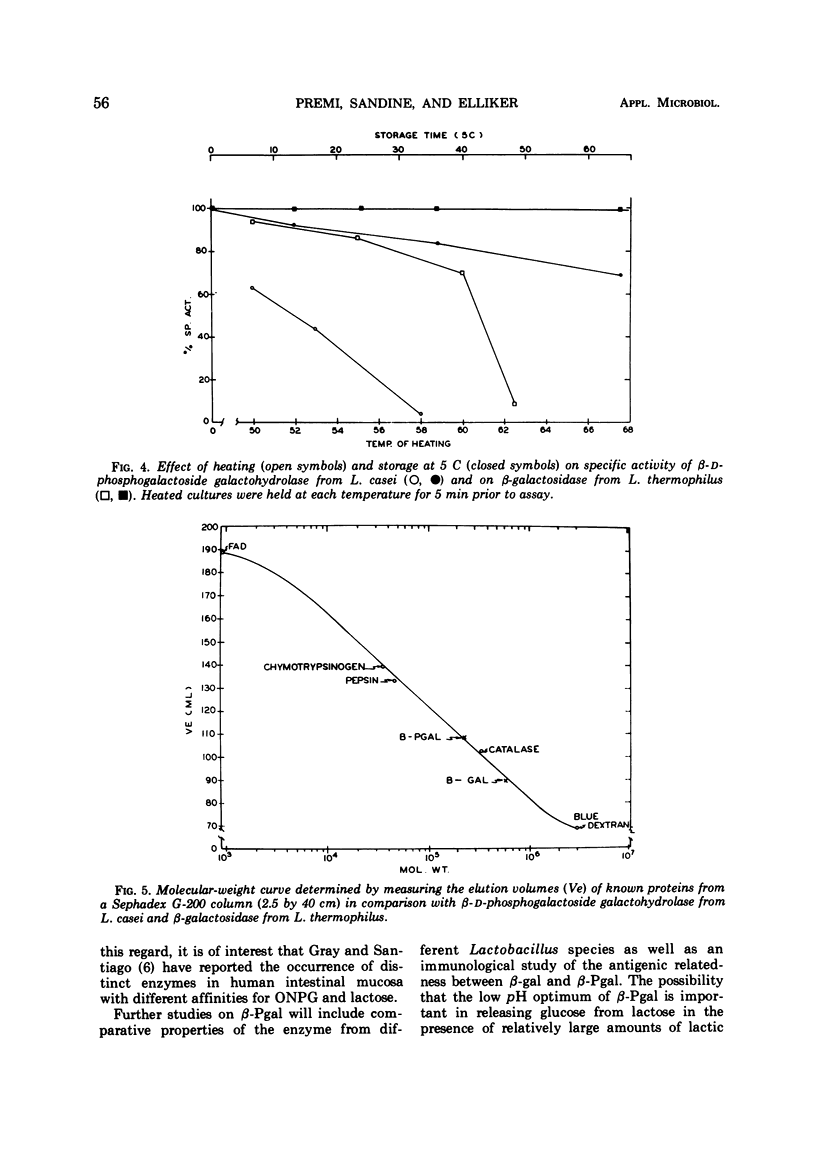
β-Galactosidase (β-gal, EC 3.2.1.23) and β-D-phosphogalactoside galactohydrolase (β-Pgal) activities were observed in all of 13 Lactobacillus species studied except L. casei and L. buchneri. Only the latter enzyme was detected in nine strains of L. casei. The β-gal from L. thermophilus and the β-Pgal from L. casei were purified and characterized. In comparison with β-gal, the β-Pal was slightly less active (Vmax values were 28.9 and 50.0 μmoles per mg per min, respectively), but the substrate affinitives were similar (Km values were 1.69 × 10-3 M and 1.59 × 10-3 M, respectively). Although the two enzymes had similar amino acid compositions, the molecular weight of β-gal was 5.4 × 105 and that of β-Pgal was 1.3 × 105. The β-gal from L. thermophilus and the β-Pgal from L. casei had optimal temperature and pH activity values of 55 C at pH 6.2 and 37 C at pH 5.0, respectively. The complete absence of β-gal from a homofermentative Lactobacillus species of industrial importance is further evidence of the heterogeneity of this genus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITTI J. E., SANDINE W. E., ELLIKER P. R. BETA-GALACTOSIDASE OF STREPTOCOCCUS LACTIS. J Bacteriol. 1965 Apr;89:937–942. doi: 10.1128/jb.89.4.937-942.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B. R., Boroff D. A., Rothstein E. Chromatographic fractionation of the crystalline toxin of Clostridium botulinum type A. Biochem Biophys Res Commun. 1966 Mar 22;22(6):750–756. doi: 10.1016/0006-291x(66)90212-9. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. 3. Studies of the transport process. Biochim Biophys Acta. 1966 Jan 4;112(1):63–73. doi: 10.1016/s0926-6585(96)90009-6. [DOI] [PubMed] [Google Scholar]
- Gray G. M., Santiago N. A. Intestinal beta-galactosidases. I. Separation and characterization of three enzymes in normal human intestine. J Clin Invest. 1969 Apr;48(4):716–728. doi: 10.1172/JCI106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. V. The accumulation of phosphorylated carbohydrate derivatives, and evidence for a new enzyme-splitting lactose phosphate. Proc Natl Acad Sci U S A. 1967 Jul;58(1):274–279. doi: 10.1073/pnas.58.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. VI. The nature of the derivatives accumulated. J Biol Chem. 1968 Apr 25;243(8):1881–1885. [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Metabolism of lactose by Staphylococcus aureus. J Bacteriol. 1968 Dec;96(6):2187–2188. doi: 10.1128/jb.96.6.2187-2188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Scarborough G. A. Mechanism of hydrolysis of O-nitrophenyl-beta-galactoside in Staphylococcus aureus and its significance for theories of sugar transport. Proc Natl Acad Sci U S A. 1967 Jul;58(1):225–228. doi: 10.1073/pnas.58.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laue P., MacDonald R. E. Identification of thiomethyl-beta-D-galactoside 6-phosphate accumulated by Staphylococcus aureus. J Biol Chem. 1968 Feb 10;243(3):680–682. [PubMed] [Google Scholar]
- MONOD J., COHN M. La biosynthèse induite des enzymes; adaptation enzymatique. Adv Enzymol Relat Subj Biochem. 1952;13:67–119. [PubMed] [Google Scholar]
- McFeters G. A., Sandine W. E., Becker R. R., Elliker P. R. Some factors affecting association-dissociation of beta-galactosidase from Streptococcus lactis 7962. Can J Microbiol. 1969 Jan;15(1):105–110. doi: 10.1139/m69-016. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Sandine W. E., Elliker P. R. Involvement of sulfhydryl groups in the -galactosidase of Streptococcus lactis. J Bacteriol. 1971 Oct;108(1):599–600. doi: 10.1128/jb.108.1.599-600.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Sandine W. E., Elliker P. R. Purification and properties of Streptococcus lactis beta-galactosidase. J Bacteriol. 1967 Mar;93(3):914–919. doi: 10.1128/jb.93.3.914-919.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., 3rd, Sandine W. E., Elliker P. R. Deoxyribonucleic acid base composition of lactobacilli determined by thermal denaturation. J Bacteriol. 1970 Apr;102(1):278–280. doi: 10.1128/jb.102.1.278-280.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt J. O., Hammer J. L. Lactobacilli on plants. Appl Microbiol. 1968 Sep;16(9):1326–1330. doi: 10.1128/am.16.9.1326-1330.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


