SUMMARY
Objectives
The epidermal growth factor receptor (EGFR) is a validated target in head and neck squamous cell carcinoma (HNSCC). In recurrent and/or metastatic (R/M) HNSCC, resistance to anti-EGFR therapy inevitably occurs. Downstream activation of the PI3K/Akt/mTOR pathway is an established resistance mechanism. Concurrent mTOR blockade may improve efficacy of anti-EGFR therapy.
Materials and methods
Erlotinib 150 mg daily and temsirolimus 15 mg weekly were administered to patients with platinum-refractory R/M HNSCC and ECOG performance status 0–2. The primary endpoint was progression-free survival (PFS). Correlative studies determined PIK3CA and HRAS mutation status; p16, EGFR, pS6K, pAkt and PTEN expression; and pre- and post-treatment plasma levels of 20 immunomodulatory cytokines.
Results
Twelve patients enrolled; six withdrew within 6 weeks due to toxicity or death, prompting early closure of the trial. Grade ≥3 toxicities included fatigue, diarrhea, gastrostomy tube infection, peritonitis, pneumonia, dyspnea, and HN edema. Median PFS was 1.9 months. Median overall survival was 4.0 months. Six/12 tumors were p16(+), 9/11 lacked measurable PTEN expression, and 1/12 harbored a PIK3CA mutation. On exploratory analysis, high baseline plasma VEGF and interferon-gamma levels marginally associated with tumor progression.
Conclusions
The combination of erlotinib and temsirolimus was poorly tolerated. Low prevalence of PTEN expression and 8% incidence of PIK3CA mutations indicate biological relevance of this pathway in R/M disease. Investigation of more tolerable combinations of EGFR and PI3K/Akt/mTOR pathway inhibitors in selected HNSCC patients is warranted.
Keywords: Temsirolimus, Erlotinib, Platinum-refractory, Head and neck squamous cell carcinoma, mTOR, EGFR, PIK3CA
Introduction
Head and neck cancer (HNC) is the sixth leading incident cancer worldwide.1 In the United States, the estimated disease burden for 2012 is 52,600 new cases and 11,500 deaths.2 Head and neck squamous cell carcinoma (HNSCC) comprises the majority of HNC. Despite advances in multimodality therapy, 5-year overall survival (OS) is 40–50%, with modest increase over the past two decades.3 Improved prognosis is largely attributable to changing epidemiology; an increasing proportion of oropharyngeal HNSCC is infected with high-risk human papillomavirus (HPV), thought to confer sensitivity to conventional treatments.4,5 Patients with recurrent and/or metastatic (R/M) HNSCC have a particularly poor prognosis, with a median OS of 6–10 months. Options for palliation are limited. For nearly three decades, the cornerstone of first line chemotherapy has been cisplatin.6 Recently, EGFR has been validated as a therapeutic target. Cetuximab, a monoclonal antibody against EGFR, increased progression-free survival (PFS) and OS in R/M disease when combined with platinum/5-fluorouracil.7 Cetuximab is also indicated as monotherapy in patients with R/M, platinum-refractory HNSCC where the response rate (RR) is 10–13% and PFS is 2.2–2.8 months.8 Small molecule inhibitors of EGFR, erlotinib and gefitinib, also have activity in this setting with RR 4–11% and PFS 1.9–2.2 months.9,10
EGFR is a member of the ErbB/HER family of transmembrane receptor tyrosine kinases (RTK). Activated EGFR initiates proliferative signaling cascades through downstream effectors including Ras/MAPK, PI3K/Akt/mTOR (mammalian target of rapamycin), and STAT.11 Forced overexpression of EGFR is sufficient to transform oral epithelial cells in vitro, suggesting EGFR is a bone fide oncogene in HNSCC.12 EGFR overexpression and/or amplification occur in the majority of HNSCC, correlating with increased stage and reduced survival.13,14
Despite the documented role of EGFR as an oncogene and prognostic biomarker in HNSCC, de novo or acquired resistance to anti-EGFR therapy is common. One established resistance mechanism is downstream activation of the PI3K/Akt/mTOR pathway.15 Independent activation of Akt predicts resistance to EGFR inhibitors in EGFR-overexpressing cancer cell lines.16 Although Akt activation is observed in most HNSCC tumors, it correlates poorly with phosphorylated EGFR, suggesting EGFR-independent signaling mechanisms are involved.17 Constitutive Akt signaling may be initiated by post-EGFR alterations including PIK3CA activating mutations, or disrupted negative regulation by phosphatase and tensin homolog (PTEN) through mutation or epigenetic silencing.18–20 The net result of PI3K/Akt/mTOR activation is the translation of pro-growth, pro-angiogenic, and anti-apoptotic proteins. Akt/mTOR activation is an early event in HNSCC carcinogenesis, is implicated in progression from dysplasia to invasive carcinoma, and predicts recurrence when identified at the surgical margin.21,22
In preclinical models, cancers with Akt activation are growth-inhibited by mTOR blockade.23,24 In HNSCC, dual targeting of EGFR and mTOR with erlotinib and temsirolimus demonstrated synergistic tumor inhibition in a xenograft model.25 Consequently, we hypothesized that concurrent blockade of EGFR and mTOR may overcome resistance to EGFR inhibition, prolonging PFS compared to historical cetuximab or erlotinib monotherapy. We evaluated the combination of erlotinib and temsirolimus in patients with R/M, platinum-refractory HNSCC.
Patients and methods
Clinical trial eligibility criteria
This study was approved in November 2009 by the Human Research Review Committee at the University of New Mexico (UNM). Eligibility criteria included: age ≥18 years; histologic/cytologic diagnosis of HNSCC from any primary site, including unknown primary; distant metastases or locoregional recurrence unsuitable for surgical salvage; platinum-refractory disease defined as progressing during/after first line platinum-based chemotherapy for R/M disease or progression within 6 months of platinum-based chemoradiotherapy for localized disease; measurable disease by RECIST criteria version 1.126; Eastern Cooperative Oncology Group Performance Status (ECOG-PS) 0–2; adequate hematologic reserve and end organ function. Exclusion criteria included: prior treatment with anti-EGFR therapy for R/M disease; prior treatment with anti-EGFR therapy for localized disease if delivered within the previous 3 months; serious medical comorbidities. All patients provided written, informed consent. Patient safety and data quality were monitored by UNM’s Data Safety and Monitoring Committee (DSMC).
Study treatment
Patients were treated with continuous 28-day cycles of erlotinib 150 mg by mouth daily and temsirolimus 15 mg intravenous weekly, per the phase I maximum tolerated dose established in glioblastoma multiforme (GBM).27 Toxicity was described according to NCI Common Terminology Criteria for Adverse Events, version 3.28 Optional dose escalation of temsirolimus to 20 mg weekly was permitted for patients without Grade ≥3 toxicity during cycle 1. A single dose reduction to erlotinib 100 mg daily and temsirolimus 12 mg weekly was permitted for Grade ≥3 toxicities. Tumor measurements were conducted every 8 weeks.
Statistical design
The study incorporated a single-stage, phase II design. The primary efficacy endpoint was median PFS. Kaplan Meier method was used to describe the PFS and OS of the study population, and the exact test would determine if median PFS significantly differed from the null. The original sample size of 35 evaluable patients had 80% power at the 5% significance level to detect improvement in median PFS from 2.2 to 4.4 months. PFS was defined as the interval from first study treatment to RECIST progression or death. Patients who withdrew for toxicity without documented progression, and had no subsequent response assessment, were censored for PFS on the date of dropout. Secondary efficacy endpoints included RR and OS.
Correlative studies
PIK3CA and HRAS mutations were analyzed using DNA isolated from paraffin-embedded tissues as previously described.29,30 The association of each mutation with RECIST was quantified with a Fisher’s exact test that defined groups as (1) wildtype vs. mutant and (2) RECIST percent change ≤0 vs. >0.
Immunohistochemistry was performed to detect: phospho-Akt (1:50 dilution, overnight incubation, Cell Signaling Tech); EGFR (1:100 dilution, 2 h incubation, Cell Signaling Tech); phosphop-70S6K (1:100 dilution, overnight incubation, Cell Signaling Tech); PTEN (prediluted, Biocare Medical, PM278AA); p16 (prediluted, MTM (CINtech), 9518). Tumors were considered positive for p16 if demonstrating ≥70% diffuse cytoplasmic and nuclear staining, and positive for other antigens if demonstrating ≥5% reactivity in tumor cells.
A panel of immunomodulatory cytokines (TGF-beta 1–3, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-15, IFN-alpha and -gamma, TNF-alpha, VEGF, Eotaxin, GCSF, GRO, OPN and CXCL12) was measured in 13 pre- and 11-post temsirolimus plasma samples (48 h after first dose) using multiplex Luminex bead assays.31 Cytokine values below the detection threshold were replaced with zero. We selected the pre-treatment sample closest to treatment initiation for the patient with duplicate samples. The relative cytokine response in individuals experiencing treatment-emergent infection vs. none was quantified through a t-test comparing pre and post-treatment change in cytokine levels between the two groups, assuming equal variance in both groups to account for small sample size. Cytokine levels between patients with any measurable response and no response were assessed with t-statistics on pretreatment data.
Results
Temsirolimus and erlotinib combination therapy demonstrated significant toxicity
Twelve patients enrolled from December 2009 through March 2011. Six patients withdrew within the first 6 weeks of treatment, due to toxicity (5/6) and treatment-unrelated death (1/6), prompting referral to the DSMC and early study termination.
Baseline characteristics are reported in Table 1. The median number of 28-day cycles of erlotinib–temsirolimus was 1.75 (range 0.25–8). Three patients were escalated to temsirolimus 20 mg IV weekly after cycle 1. Five patients required dose reduction for intolerable toxicity, including asthenia (4/5) and facial edema (1/ 5).
Table 1.
Baseline characteristics.
| Characteristic | Number (%)a |
|---|---|
| Age (Years) | |
| Median | 61.5 |
| Range | 45–87 |
| Sex | |
| Male | 11 (92%) |
| Female | 1 (8%) |
| Primary site | |
| Oral cavity | 4 (33%) |
| Oropharynx | 6 (50%) |
| Paranasal sinus | 1 (8%) |
| Unknown primary | 1 (8%) |
| Prior anti-EGFR therapyb | |
| Cetuximab | 5 (42%) |
| Erlotinib | 2 (17%) |
| None | 5 (42%) |
| ECOG performance status | |
| 0 | 5 (42%) |
| 1 | 5 (42%) |
| 2 | 2 (17%) |
| Tobacco use | |
| Current | 3 (25%) |
| Former | 7 (58%) |
| None | 2 (17%) |
| p16 Status | |
| Positive | 6 (50%) |
| Negative | 6 (50%) |
Number (percent) unless units otherwise specified.
Prior anti-EGFR therapy delivered with concurrent chemoradiotherapy for localized disease.
Toxicities are summarized in Table 2. Overall, seven patients (58%) experienced Grade ≥3 toxicity. Two patients withdrew due to severe diarrhea (non-responsive to loperamide), gastrostomy tube infection, and peritonitis; one of these patients presented with simultaneous aspiration pneumonia. In both cases, peritonitis resolved with antibiotics and non-operative management. An additional two patients withdrew for profound asthenia. The fifth patient developed grade 4 laryngeal edema requiring emergent tracheostomy, the day following first temsirolimus administration. The event was initially attributed to pre-existing, near-obstructing tumor recurrence for which elective tracheostomy had been declined. However, as the study accrued a unique edema pattern emerged. Six (50%) patients experienced treatment- emergent Grade ≥1 edema of the face, neck or larynx within 1–35 days of starting protocol therapy. In five cases, facial and/ or neck edema was manageable with manual lymphatic drainage; temsirolimus dose reduction was also necessary in one patient. One treatment-unrelated death occurred, from hypoxic arrest after failure of portable oxygen equipment.
Table 2.
Toxicities.
| Nonhematologic toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total Grade ≥3 |
|---|---|---|---|---|---|---|
| Dermatologic | ||||||
| Acneiform rash | 4 (33%) | 6 (50%) | 0 | 0 | 0 | 0 |
| Edema | ||||||
| Facial/neck | 3 (25%) | 1 (8%) | 1 (8%) | 0 | 0 | 1 (8%) |
| Laryngeal | 0 | 0 | 0 | 1 (8%) | 0 | 1 (8%) |
| Constitutional | ||||||
| Asthenia | 2 (17%) | 3 (25%) | 4 (33%) | 1 (8%) | 0 | 5 (42%) |
| Gastrointestinal | ||||||
| Oral mucositis | 3 (25%) | 4 (33%) | 0 | 0 | 0 | 0 |
| Diarrhea | 5 (42%) | 0 | 2 (17%) | 0 | 0 | 2 (17%) |
| Peritonitis/infection | 0 | 0 | 2 (17%) | 0 | 0 | 2 (17%) |
| Anorexia | 0 | 2 (17%) | 1 (8%) | 0 | 0 | 1 (8%) |
| Nausea/vomiting | 3 (25%) | 1 (8%) | 0 | 0 | 0 | 0 |
| Elevated triglycerides | 1 (8%) | 0 | 1 (8%) | 0 | 0 | 1 (8%) |
| Pulmonary | ||||||
| Aspiration pneumonia | 0 | 0 | 1 (8%) | 0 | 0 | 1 (8%) |
| Dyspnea | 1 (8%) | 0 | 1 (8%) | 2 (17%) | 0 | 3 (25%) |
| Pneumonitis | 1 (8%) | 0 | 0 | 0 | 0 | 0 |
| Hypoxia | 0 | 0 | 0 | 0 | 1 (8%) | 1 (8%) |
Limited response assessment
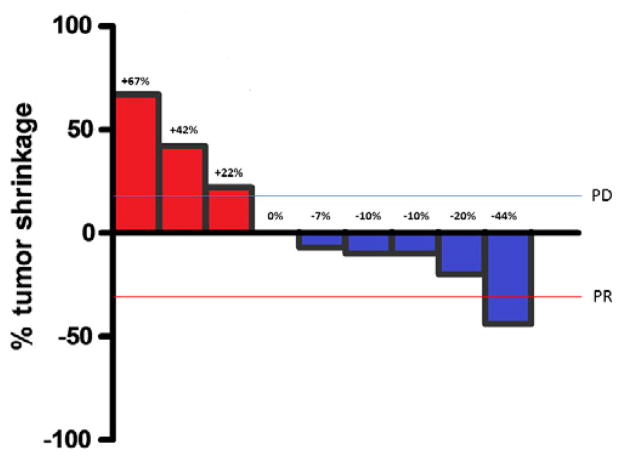
Four patients were censored for progression on the date of study withdrawal for toxicity, as no subsequent response assessment was conducted. Median PFS was 1.9 months. Median OS was 4.0 months. Survival curves are presented in Fig. 1. The best RECIST response in nine patients undergoing formal response assessment is presented in Fig. 2. One patient had PR by caliper assessment; the response was not confirmed due to the patient’s withdrawal from study.
Figure 1.
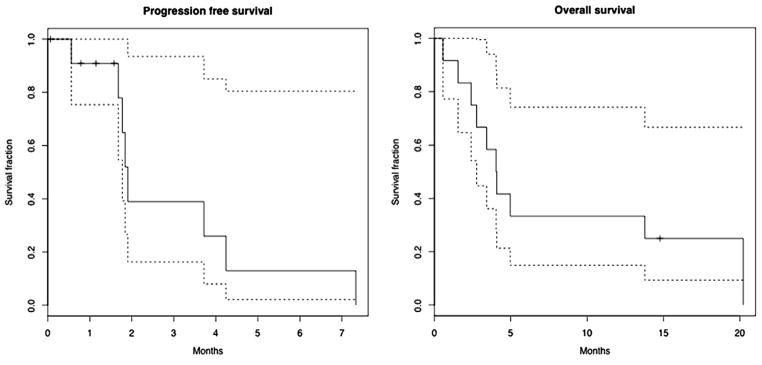
Progression-free and overall survival.
Figure 2.
Waterfall plot of best RECIST response.
Correlative studies
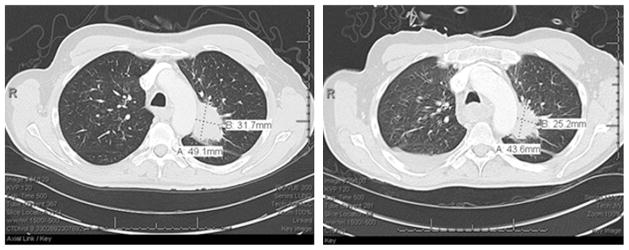
Twelve archived tumors were obtained for correlative analyses. Patient biomarker data is presented in Table 3. Tumor expression levels of EGFR, pAKT, p-p70S6K, PTEN or p16 did not significantly associate with patient outcome. Nine tumors (9/11, 82%) lacked measurable PTEN expression. Six tumors (6/12, 50%) were p16(+) including five oropharyngeal tumors from three former and two never-smokers. One patient with p16(+) disease harbored an activating PIK3CA mutation (E545K). The patient was hospitalized for toxicities after 3 weeks of protocol therapy and withdrew from study. A CT scan conducted during hospitalization demonstrated disease regression not meeting criteria for PR (Fig. 3). No HRAS mutations were identified.
Table 3.
Biomarkers by patient (N = 12).
| Patient | PIK3CA mut | HRAS mut | p16 | EGFR | PTEN | p-S6K | p-AKT | RECIST | PFS, mos |
|---|---|---|---|---|---|---|---|---|---|
| 1 | WT | WT | Neg | Pos | Neg | Pos | Pos | 0% | NA |
| 2 | WT | WT | Pos | Pos | Neg | Pos | Pos | −10% | 3.8 |
| 3 | WT | WT | Pos | Pos | Neg | Pos | Pos | NA | 0.6 |
| 4 | WT | WT | Pos | Pos | Neg | Pos | Neg | +67% | 1.9 |
| 5 | WT | WT | Pos | Neg | Neg | Neg | Pos | +42% | 1.7 |
| 6 | WT | WT | Neg | Neg | Neg | Pos | Neg | +22% | 1.8 |
| 7 | WT | WT | Neg | Neg | Pos | Pos | Pos | NA | NA |
| 8 | WT | WT | Neg | Neg | Pos | Pos | Neg | NA | NA |
| 9 | WT | WT | Neg | Neg | Neg | Pos | Pos | −44% | NA |
| 10 | WT | WT | Neg | Neg | Neg | Pos | Neg | −20% | 4.3 |
| 11 | E545K | WT | Pos | NA | NA | NA | NA | −10% | 1.9a |
| 12 | WT | WT | Pos | Pos | Neg | Pos | Pos | −7% | 7.4 |
Patient progressed at 1.9 months, however withdrew for toxicity after 3 weeks of therapy.
Figure 3.
Plasma VEGF or IFN-gamma and best RECIST response.
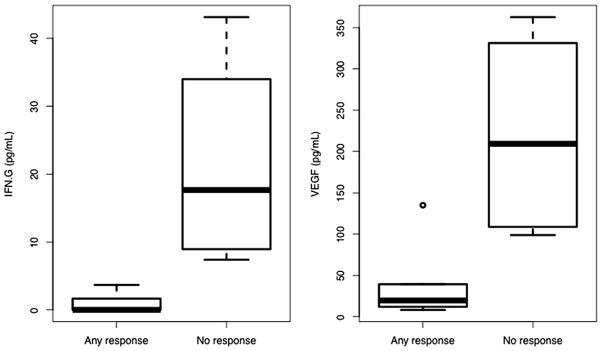
We examined twenty immunomodulatory cytokines in pre- and post-treatment plasma samples. Cytokine changes did not significantly associate with treatment-emergent infection. No cytokine level in pre-treatment plasma significantly associated with survival outcome. However, higher baseline IFN-γ (p = 0.09) and VEGF (p = 0.07) levels marginally associated with tumor growth vs. any response (Fig. 4).
Figure 4.
Patient with PIK3CA mutation. (a) Baseline and (b) After 3 weeks of protocol therapy.
Discussion
This study was designed to evaluate the efficacy of erlotinib and temsirolimus in patients with platinum-refractory, R/M HNSCC. Unfortunately, the combination was poorly tolerated precluding estimate of efficacy. Six of 12 patients withdrew early due to toxicity or death, and the study was terminated. The observed toxicity rate exceeded the <30% dose-limiting toxicity accepted in conventional phase I design, highlighting an important concern for the clinical translation of promising regimens into R/M HNSCC. Here, the recommended for phase II dose (RP2D) was defined exclusively in patients with recurrent GBM. R/M HNSCC patients have unique disease- and treatment-related comorbidities which may have potentiated toxicities not observed in GBM patients. Specifically relevant are prior surgery and/or radiation to the neck associated with lymphatic obliteration and lymphedema32; frequent feeding tube dependence33; and inherent immunosuppression.34 In this trial, the unique toxicity of head and neck edema was seen in six patients (6/12, 50%). Temsirolimus was previously associated with peripheral edema in patients with renal cell carcinoma, however head and neck edema was not reported.35 Two patients developed severe diarrhea and peritonitis; both had concurrent gastrostomy tube infection. Although both erlotinib and temsirolimus cause diarrhea, neither is associated with peritonitis which may have developed due to tracking of fecal bacteria along a foreign body. In kidney transplantation, high-dose mTOR inhibitors are associated with impaired wound healing ascribed to decreased fibroblast proliferation and angiogenesis; however, not with wound infection. 36 Overall, the baseline immunosuppression associated with HNSCC did not clearly impact regimen toxicity as the rate of infection was numerically similar to temsirolimus monotherapy in RCC (27% all grades; 5% grade 3–4).35 Overall, our unexpected toxicity experience suggests that a two-stage design, with formal stopping rules for toxicity, would be preferable in R/M HNSCC when adapting RP2D from a non-HNSCC setting.
The inability to combine mTOR inhibition with full dose cytotoxic chemotherapy in R/M cancer, due to myelosuppression, has been consistent in phase I studies for multiple solid tumors.37–39 In R/M HNSCC, a phase I trial of cisplatin, cetuximab and everolimus closed early for toxicities including infection and mucositis.40 However, adding mTOR inhibition to induction chemotherapy for previously-untreated, locally advanced HNSCC has been feasible; two phase II trials are proceeding (NCT01133678, NCT00935961). The present study in R/M HNSCC combined temsirolimus with a non-myelosuppressive biologic and demonstrated hematologic tolerability. However, excessive non-hematologic toxicity prohibits further regimen development.
Given the magnitude of early withdrawals, evaluation of the efficacy of this combination was inadequate. The median PFS of 1.9 months was uninspiring, approximating the null rate; however, the sample size was admittedly very small. The waterfall plot of best RECIST response, including a PR in a patient withdrawing early for toxicity, implies potential for meaningful clinical activity if a more tolerable regimen of EGFR-mTOR inhibition can be established.
Although the curtailed sample size reduced power to find predictive relationships between biomarkers and clinical outcomes, we gained important descriptive insights into R/M disease and identified biomarker candidates for future study. Of note, 50% of enrolled patients had p16(+) tumors; 5 were oropharyngeal primaries thus HPV-associated. This proportion of HPV-associated disease appears substantially higher than the 5% described in a R/M study conducted 5 years earlier.41 Although HPV is associated with favorable survival, the absolute number of HPV-positive treatment failures will increase simply due to rising incidence of oropharynx cases.4 R/M trials will likely enroll an increasing proportion of p16(+) cases. As in locally advanced disease, differential response to treatment on the basis of favorable biology or immunology should be a focus of future translational research.
One PIK3CA and no HRAS mutations were identified in this R/M cohort, numerically similar to rates in localized HNSCC42, generating no signal that either mutation contributes to relapse. Although anecdotal, the disease regression observed in the patient harboring a PIK3CA mutation parallels a phase I program’s report that retrospective identification of a PIK3CA mutation predicted response to PI3K/Akt/mTOR axis blockade.43 Moreover, 9 (9/11, 82%) tumors lacked PTEN expression further supporting deregulation of the PI3K/Akt/mTOR pathway in R/M HNSCC. A reasonable question is whether vertical blockade of EGFR-mTOR is important in the setting of PIK3CA mutation or absent PTEN expression, or whether mTOR inhibition alone would elicit similar activity. Of interest, a phase II trial of everolimus in R/M HNSCC closed after the first enrollment stage when no responses were seen; however, PIK3CA mutation and PTEN expression were not assessed.44
Both EGFR and mTOR signaling are important for immune competence. 45,46 Thus we analyzed a panel of immunomodulatory cytokines in plasma to explore the relationship to oncologic or toxicity outcomes. We observed higher VEGF and IFN-γ expression in the pre-treatment plasma of study patients experiencing tumor growth vs. any response. This association parallels the established relationship between VEGF expression and poor prognosis in HNSCC.47 IFN-γ is expressed by T cells and natural killer cells; higher IFN-γ may signify deregulated immune function in non-responders.
The importance of Akt/mTOR signaling in HNSCC oncogenesis and recurrence justifies evaluation of mTOR inhibitors across the disease spectrum. The present study evaluated dual EGFR-mTOR inhibition to improve efficacy of anti-EGFR therapy, given the frequency of EGFR-independent Akt/mTOR activation. This compelling question remains unanswered due to regimen intolerability. However, the conclusion that dual mTOR-EGFR blockade is unsafe in R/M HNSCC would be premature, as a phase II trial evaluating temsirolimus with or without cetuximab is successfully enrolling without prohibitive toxicity (NCT01256385). Multiple trials of mTOR inhibitors in HNSCC are ongoing (Table 4). Early reports, such as this one, are biased toward studies halted for toxicity or non-efficacy. A comprehensive picture of the value of mTOR inhibition in HNSCC awaits maturation of successfully populated clinical trials. Our preliminary results suggest that PIK3CA mutation status, PTEN expression, and plasma levels of VEGF and IFN-gamma warrant additional study as candidate biomarkers in the setting of mTOR blockade.
Table 4.
Clinical trials testing mTOR inhibition in HNSCC.
| Trial number | Stage | Phase | Regimen | Recruitment | Results |
|---|---|---|---|---|---|
| NCT00858663 | Locally advanced, definitive radiotherapy | I | Cisplatin, IMRT, everolimus | Complete | RP2D: Everolimus 5 mg daily, cisplatin 30 mg/m2 weekly. DLT mucositis |
| NCT01333085 | Locally advanced, induction | I/II | Carboplatin, paclitaxel, everolimus | Ongoing | NRa |
| NCT01133678 | Locally advanced, induction | I/II | Cisplatin, paclitaxel, cetuximab, everolimus | Ongoing | NR |
| NCT01111058 | Locally advanced, adjuvant | II | Everolimus vs. placebo | Not recruiting | NR |
| NCT01172769 | R/M | II | Temsirolimus | Ongoing | NR |
| NCT01051791 | R/M | II | Everolimus | Terminated | Terminated after stage I, no objective responses |
| NCT00942734 | R/M | II | Everolimus, erlotinib | Ongoing | NR |
| NCT01256385 | R/M | II | Temsirolimus ± cetuximab | Ongoing | NR |
| NCT01009203 | R/M | II | Temsirolimus, erlotinib | Terminated | Terminated early for toxicities |
| NCT01009346 | R/M | I | Cisplatin, cetuximab, everolimus | Terminated | Terminated early for toxicities |
| NCT01015664 | R/M | I/II | Cisplatin, cetuximab, temsirolimus | Ongoing | NR |
| NCT01283334 | R/M | I/II | Carboplatin, cetuximab, everolimus | Ongoing | NR |
| NCT01016769 | R/M | I/II | Carboplatin, paclitaxel, temsirolimus | Complete | RP2D: Carboplatin AUC 1.5, paclitaxel 80 mg/m2, temsirolimus 25 mg IV on D1, 8 of 21 day cycle |
Not Reported.
Acknowledgments
Supported by grants from the investigator-initiated trials programs of Genentech, Inc. and Pfizer, Inc. Trial design and conduct supported by UNM Cancer Center Shared Resources, 2P30CA118100. Biomarker experiments and analysis supported by R01 DE017982. HRAS mutation analysis performed in the Vanderbilt Innovative Translational Research Shared Resource supported by the Vanderbilt-Ingram Cancer Center and the TJ Martell Foundation.
Footnotes
Conflict of interest statement
Dr. Jones formerly served as a scientific consultant and member of the speaker’s bureau for Genentech, Inc. No other competing interest is declared.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong WK, Schaefer S, Issell B, et al. A prospective randomized trial of methotrexate versus cisplatin in the treatment of recurrent squamous cell carcinoma of the head and neck. Cancer. 1983;52:206–10. doi: 10.1002/1097-0142(19830715)52:2<206::aid-cncr2820520204>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 8.Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112:2710–9. doi: 10.1002/cncr.23442. [DOI] [PubMed] [Google Scholar]
- 9.Perez CA, Song H, Raez LE, et al. Phase II study of gefitinib adaptive dose escalation to skin toxicity in recurrent or metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2012 doi: 10.1016/j.oraloncology.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 11.Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003;39:1348–54. doi: 10.1016/s0959-8049(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 12.Goessel G, Quante M, Hahn WC, et al. Creating oral squamous cancer cells: a cellular model of oral–esophageal carcinogenesis. Proc Natl Acad Sci USA. 2005;102:15599–604. doi: 10.1073/pnas.0409730102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–84. [PubMed] [Google Scholar]
- 14.Chung CH, Ely K, McGavran L, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–6. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–56. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianco R, Shin I, Ritter CA, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–22. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 17.Molinolo AA, Hewitt SM, Amornphimoltham P, et al. Dissecting the Akt/ mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13:4964–73. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 18.Qiu W, Schonleben F, Li X, et al. PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:1441–6. doi: 10.1158/1078-0432.CCR-05-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011 doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurasawa Y, Shiiba M, Nakamura M, et al. PTEN expression and methylation status in oral squamous cell carcinoma. Oncol Rep. 2008;19:1429–34. [PubMed] [Google Scholar]
- 21.Amornphimoltham P, Sriuranpong V, Patel V, et al. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res. 2004;10:4029–37. doi: 10.1158/1078-0432.CCR-03-0249. [DOI] [PubMed] [Google Scholar]
- 22.Nathan CO, Amirghahri N, Rice C, Abreo FW, Shi R, Stucker FJ. Molecular analysis of surgical margins in head and neck squamous cell carcinoma patients. Laryngoscope. 2002;112:2129–40. doi: 10.1097/00005537-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 23.DeGraffenried LA, Fulcher L, Friedrichs WE, Grunwald V, Ray RB, Hidalgo M. Reduced PTEN expression in breast cancer cells confers susceptibility to inhibitors of the PI3 kinase/Akt pathway. Ann Oncol. 2004;15:1510–6. doi: 10.1093/annonc/mdh388. [DOI] [PubMed] [Google Scholar]
- 24.Gera JF, Mellinghoff IK, Shi Y, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–46. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 25.Jimeno A, Kulesza P, Wheelhouse J, et al. Dual EGFR and mTOR targeting in squamous cell carcinoma models, and development of early markers of efficacy. Br J Cancer. 2007;96:952–9. doi: 10.1038/sj.bjc.6603656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz LH, Bogaerts J, Ford R, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–7. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Robins HI, Wen PY, Chang SM, et al. Phase I study of erlotinib and CCI-779 (temsirolimus) for patients with recurrent malignant gliomas 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007:2057. [Google Scholar]
- 28.National Cancer Institute Common Terminology Criteria for Adverse Events. Version 3: Cancer Therapy Evaluation, Program. Aug 9, 2006. [Google Scholar]
- 29.Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Z, Dias-Santagata D, Duke M, et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J Mol Diagn. 2011;13:74–84. doi: 10.1016/j.jmoldx.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byers LA, Holsinger FC, Kies MS, et al. Serum signature of hypoxia-regulated factors is associated with progression after induction therapy in head and neck squamous cell cancer. Mol Cancer Ther. 2010;9:1755–63. doi: 10.1158/1535-7163.MCT-09-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43:244–52. doi: 10.1016/j.jpainsymman.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–7. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 34.Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 36.Nashan B, Citterio F. Wound healing complications and the use of Mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94:547–61. doi: 10.1097/TP.0b013e3182551021. [DOI] [PubMed] [Google Scholar]
- 37.Kollmannsberger C, Hirte H, Siu LL, et al. Temsirolimus in combination with carboplatin and paclitaxel in patients with advanced solid tumors: a NCIC-CTG, phase I, open-label dose-escalation study (IND 179) Ann Oncol. 2012;23:238–44. doi: 10.1093/annonc/mdr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramalingam SS, Harvey RD, Saba N, et al. Phase 1 and pharmacokinetic study of everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel for recurrent/refractory nonsmall cell lung cancer. Cancer. 2010;116:3903–9. doi: 10.1002/cncr.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fury MG, Sherman E, Ho A, et al. A phase I study of temsirolimus plus carboplatin plus paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC) Cancer Chemother Pharmacol. 2012 doi: 10.1007/s00280-012-1894-y. [DOI] [PubMed] [Google Scholar]
- 40.Chung CH, Wang H, Tsottles N, et al. A phase I study of everolimus in combination with cetuximab and cisplatin as first-line therapy in recurrent and metastatic head and neck squamous cell carcinoma. 2012 ASCO Annual Meeting Proceedings. J Clin Oncol. 2012 [suppl; abstr e16061] [Google Scholar]
- 41.Chung CH, Aulino J, Muldowney NJ, et al. Nuclear factor-kappa B pathway and response in a phase II trial of bortezomib and docetaxel in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2010;21:864–70. doi: 10.1093/annonc/mdp390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janku F, Tsimberidou AM, Garrido-Laguna I, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–65. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varadarajan P, Kotsakis AP, Martin D, Gutkind JS, Gibson MK, Argiris A. Phase II trial of everolimus in patients with previously treated recurrent or metastatic squamous cell carcinoma of the head and neck. 2012 ASCO Annual Meeting. J Clin Oncol. 2012 [suppl; abstr 5541]
- 45.Yamashita M, Chattopadhyay S, Fensterl V, Saikia P, Wetzel JL, Sen GC. Epidermal growth factor receptor is essential for toll-like receptor 3 signaling. Sci Signal. 2012;5:ra50. doi: 10.1126/scisignal.2002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bi Y, Liu G, Yang R. MTOR regulates T-cell differentiation and activation in immunity and autoimmunity. Crit Rev Eukaryot Gene Expr. 2011;21:313–22. doi: 10.1615/critreveukargeneexpr.v21.i4.20. [DOI] [PubMed] [Google Scholar]
- 47.Tse GM, Chan AW, Yu KH, et al. Strong immunohistochemical expression of vascular endothelial growth factor predicts overall survival in head and neck squamous cell carcinoma. Ann Surg Oncol. 2007;14:3558–65. doi: 10.1245/s10434-007-9632-0. [DOI] [PubMed] [Google Scholar]