Abstract
Objective
To determine the anaerobic power and muscle strength of preadolescents with human immunodeficiency virus (HIV).
Design
Cross-sectional design.
Setting
Human performance laboratory at the University District Hospital at the Puerto Rico Medical Center.
Participants
Fifteen preadolescents (8 girls and 7 boys) with a classification of HIV A and B attending an investigational treatment program at the University Pediatric Hospital. Fifteen seronegative control subjects matched by age and gender also were included.
Main Outcome Measures
The power of the lower extremities was measured with use of the Wingate Anaerobic Power Test on a MONARK cycle ergometer (mean power in watts). Local muscle strength of the dominant knee extensors (peak torque/body weight × 100) was tested with an isokinetic dynamometer set at 60 deg/s. Statistical analysis was performed with the Wilcoxon signed-rank test, and statistical significance was accepted at an α level of <.05.
Results
No significant differences between the control group and study group were detected on muscle strength testing. The study group presented a lower anaerobic power (mean power) compared with control subjects (P = .04).
Conclusions
This exploratory study suggests that HIV-infected preadolescents present lower anaerobic power compared with uninfected control subjects. Our findings of impaired anaerobic capacity can have clinical implications in this population because most of the activities of daily living, such as play, leisure, and sport activities, are short term and high intensity (anaerobic) in nature.
Introduction
As the result of recent therapeutic advances, human immunodeficiency virus (HIV) has become a chronic disease [1,2]. As children with HIV grow older, it is likely they will have to face the long-term sequelae of the infection and its treatment. The authors of previous studies have examined the physical fitness profile and efficacy of aerobic exercise training in adult patients diagnosed with HIV. However, few studies have addressed physical fitness profiles and effects of exercise programs in pediatric patients with HIV infection. In one study [3], authors found low maximal aerobic power (V02 max) in adolescents seropositive for HIV compared with control subjects. In another study conducted by the same group [4], reduced aerobic capacity in late adolescents infected with HIV also was reported.
In recent years, interest in the study of the effects of exercise programs on children with sedentary lifestyles and chronic illnesses has increased. Miller [5] found that progressive resistance exercise training of 2 adolescents with perinatally acquired HIV infection resulted in an improvement in muscle strength and body composition, as well as a decrease in visceral and subcutaneous adipose tissue.
In a recent clinical trial with a larger sample size, Miller et al [6] demonstrated the safety and effectiveness of a hospital-based fitness program for improving general fitness, strength, and lean body mass in children with HIV [6].
The level of anaerobic power and muscle strength determines, in pan, the ease and effectiveness of performance of many daily living, recreational, and sports activities. To the best of our knowledge, no investigators have measured both anaerobic power and muscle strength in children with HIV. Therefore the objective of the present study was to measure muscle strength and anaerobic power in preadolescenls with HIV and seronegative control subjects matched by age and gender. Because the infection and associated complications could affect multiple body systems and most of these children have sedentary lifestyles, we hypothesized that preadolescents with HIV have lower anaerobic power and muscle strength compared with an age- and gender-matched control group.
Methods
Subjects
Fifteen sedentary preadolescent patients (mean age, 11 years; gender, 8 girls and 7 boys) who were seropositive for HIV-1 classification A and B (confirmed by Western blot) and who attended an investigational treatment protocol program at the University Pediatric Hospital volunteered for the study. All patients were infected perinatally (in utero or at birth). The patients represented a convenience sample selected on the basis of the attendance at the clinic (the most recent 15 patients at the clinic) and the mild nature of their clinical signs and symptoms. The mean value of the CD3/CD4 ratio, an indicator of immunologic status, was 25%, which is equivalent to a moderate level of compromise. No evidence was found of involvement of the central nervous system. Fifteen seronegative age- and gender-matched control subjects with the same socioeconomic status also were selected from among relatives of the HIV-1–seropositive patients. Informed consent was obtained from all parents after a detailed explanation of the proposed study program was provided. All control subjects were tested according to the same protocol as die patients. The study was approved by the Institutional Review Board of the University of Puerto Rico Medical Sciences Campus.
Anthropometry
The patients' weight and height were determined with use of a scale and a measuring stick. Body mass index was calculated as weight/height2 (kg/m2). Sexual maturity was determined through the use of Tanner staging.
Anaerobic Power
Power was defined as the ability to generate force in relation to time (force × distance/time) and was measured with the Wingate Anaerobic Power Test [7,8]. The test/retest reliability of this test has been reported to be 0.95-0.97 [9]. The test protocol involved pedaling at maximal velocity for 30 seconds on a MONARK cycle ergometer with a computer interface (Power Pack Ultra TM, SMI, Inc, Minneapolis, MN). The resistance was set according to the body weight. The seat height was adjusted for each subject, and his or her feet were firmly attached to the pedals with toe clips and elastic straps.
Pedal revolutions were continuously measured and displayed by optical sensors (SMI OptoSensor 2000 TM) attached to the cycle-ergometer's flywheel and connected to a computer's serial port. After a warm-up of 2 minutes, with a rest interval of 3-5 minutes, subjects were instructed to begin the test at the command of “Ready—go” and to pedal as fast as possible. The subjects were advised to maintain a maximal pedal rate throughout the test. At the end of the test, the resistance was lowered, and the subjects were allowed to pedal until sufficiently recovered. Peak and mean power were calculated by computer built-in software (Power TM) for each 5-second period. Anaerobic capacity (total power output) was calculated with use of the total number of counted revolutions.
Muscle Strength
Subjects were tested with a Cybex isokinetic dynamometer Model 6000. The knee extensors of the dominant knee were tested at angular velocities of 60 and 180 deg/s. The dynamometer's lever arm was attached to the tibia. Its axis of rotation was aligned with the anatomic axis of rotation of the joint. The subjects were seated with back support and restrained with straps at the level of the chest, pelvis, and thigh. The hip joint angle was between 90° and 100° of flexion. The fully extended knee was considered equal to 0° of flexion. The subjects were verbally encouraged to exert maximal force. Each subject was allowed 3 warm-up repetitions, followed by 3 maximal repetitions.
Statistical Analysis
Medians were used as measure of central tendency for the description of continuous variables because the distributions were not normal. A test of the hypothesis for the comparison of continuous variables was performed with the Wilcoxon signed-rank test. Statistical significance was accepted at an α level <.05. This analysis was performed with SYSTAT 11 statistical software (Richmond, CA).
Results
The subjects' general characteristics are shown in Table 1. No significant differences were found between the 2 groups with respect to average age, body weight, body height, body mass index, sexual maturity, or duration of HIV infection. For the comparisons of muscle strength and muscle power, box plots were generated with SYSTAT 11 statistical software (Richmond, CA). A box plot provides an excellent visual summary of many aspects of a distribution. This figure allows the user to explore maximum, minimum, median, upper quartile, lower quartile, and outliers.
Table 1. General characteristics.
| Control | Patients | P Value | |
|---|---|---|---|
| Age, y | 11 (7-14) | 11 (7-14) | |
| Gender, female/male | 8/7 | 8/7 | |
| Body weight, kg | 36.8 | 35.0 | .396 |
| Body height, cm | 143 | 140 | .177 |
| Body mass index weight/height2 | 17 | 18 | .374 |
| Tanner genital development | 1.93 | 1.87 | .157 |
Ranges are shown parentheses.
Anaerobic Power
HIV subjects had significantly lower mean power than did control subjects (P = .041; Figure 1).
Figure 1.
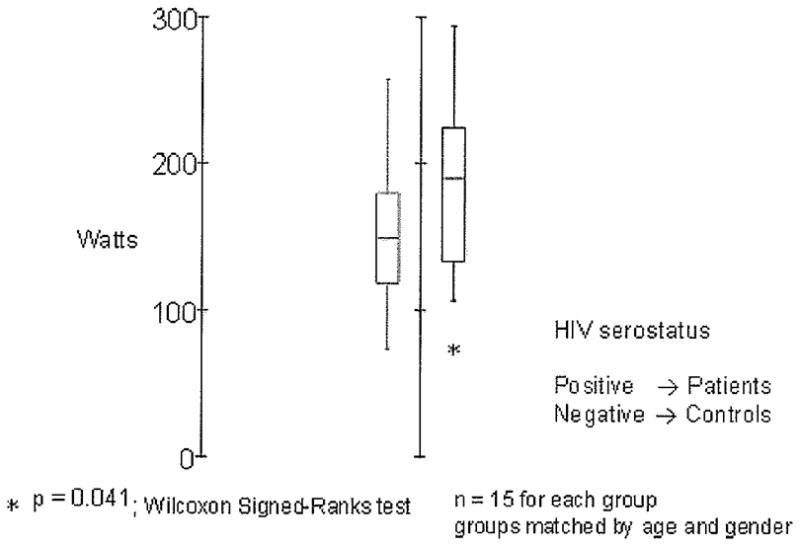
Mean peak power in N/s (watts). HIV = human immunodeficiency virus.
Muscle Strength
The values of isokinetic peak torques of the knee extensors at 60 deg/s are presented in Figure 2. No significant differences were noted between groups.
Figure 2.
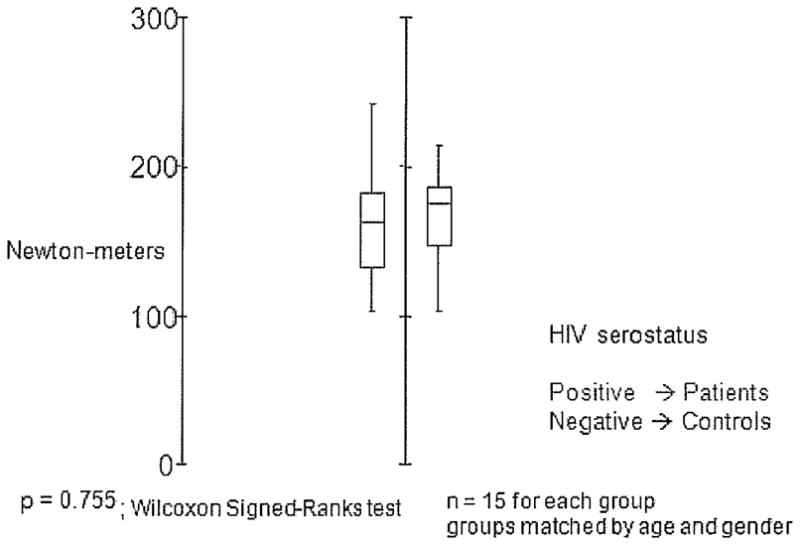
Peak torque extensors of the knee at 60° per second correct for weight. HIV = human immunodeficiency virus.
Discussion
In this pilot study, our initial hypothesis was partially proven. Although no significant differences in muscle strength were found, significant differences in anaerobic power were evident (P = .04). The authors of previous studies have addressed the cardiopulmonary status and effect of an exercise program in this population. To the best of our knowledge, this is the first study that has investigated both anaerobic power and muscle strength in preadolescents with HIV compared with age- and gender-matched control subjects.
Possible explanations for the reduction in anaerobic power in preadolescents with HIV include smaller muscle mass, lower adenosine triphosphate–phosphocreatine (ATP-PCR) stores, impaired glycolytic capacity, sedentary lifestyle, and deficient neuromuscular coordination. Hack et al [10] demonstrated an abnormally low glutathione and PCR level in the skeletal muscle tissue of simian immunodeficiency virus–infected animals but essentially normal ATP, creatinine, and adenosine diphosphate levels. An impairment in the creatine kinase reaction is known to compromise the oxidative energy metabolism of mitochondria [10], which in turn indirectly affects the glycolytic pathway.
Furthermore, studies performed in a different population of patients with juvenile-onset idiopathic inflammatory myopathy have shown lower peak and mean power using a Wingate Anaerobic Exercise Test [11,12]. The authors suggested that the decrease in exercise capacity in persons with juvenile-onset idiopathic inflammatory myopathy can be explained by lower ATP and PCR concentrations in the studied population. This energy source is important for very short duration muscle actions, such as those used during strength testing. This explanation is inconsistent with our findings of no significant differences in muscle strength. Nevertheless, it is worth noting that these authors did not measure muscle strength in their patients.
In a similar study performed in patients with hemophilia, Falk et al [13] reported that adolescents with severe hemophilia A have lower muscle strength and anaerobic power than do age-matched control subjects. In addition, this same study was able to demonstrate a direct relationship between the findings in strength and anaerobic power to the patient's level of reported physical activity by using the Godin Leisure-Time Questionnaire [13,14]. This factor was not evaluated in our study but could be considered in future studies.
Another reason for our findings could be a deficiency in neuromuscular coordination, which is an important determinant of the performance of an anaerobic test, such as the one used in the present study. One of the clinical presentations of children with HIV is central nervous system involvement [15,16]. Decreased central activation may contribute to weakness, and reduced strength has been reported in other populations, such as in children with cerebral palsy (CP). In the particular case of anaerobic capacity, the authors of studies involving children with CP have shown marked differences between the anaerobic power of children with CP [17,18] and their healthy peers. It is fair to speculate that in our population, early central nervous system involvement may result in subclinical spasticity and less energy-efficient mechanical performance during pedaling [19,20].
Our findings of impaired anaerobic capacity can have clinical implications in this population because most of the activities of daily living, such as recreational play, leisure, and sport activities, are short term and high intensity (anaerobic) in nature. A study in a different pediatric population with juvenile arthritis [21] reported that patients with a lower anaerobic power tend to have a lower functional status. In the adult population, this relationship has been studied more extensively [22-24]. In these studies, a positive correlation has been found to exist between levels of physical function and levels of anaerobic power. The role of physical training in this population should require further investigation.
The present study has some limitations. First, the sample size of this cross-sectional study is small. To improve sample size, a multicenter approach is needed. With a larger patient sample, physical and physiological factors contributing to anaerobic exercise capacity could be further evaluated. Second, subjects in our study were limited to preadolescents with HIV classification A or B. In future studies, the inclusion of all classifications should be considered. In addition, the possible contribution of the immunologic status could be evaluated further with a larger sample size. Finally, with the current study design, we could not discriminate between the effects of the infection and adverse effects of drugs taken to treat the infection. Several medications have been implicated in altered mitochondrial function, including indinavir, stavudine, and zidovudine [25,26]. The authors of several studies have reported that myopathic symptoms can occur as a complication of HIV type 1 infection or from its treatment [27-29]. Future studies should include the medication management of participants in an attempt to compare any differences related to medication use.
Conclusion
In conclusion, this study demonstrated that although preadolescents with HIV classification A or B presented with normal muscle strength, their anaerobic power was affected when compared with age- and gender-matched control subjects. This study is the first to evaluate the effects of HIV on muscle strength and anaerobic power in this population. Further studies should be performed to evaluate both aerobic and anaerobic power to determine an appropriate therapy program for these children. The goal is to ensure that their function in short-term, high-intensity activities is not compromised so they can live an active life and prevent further complications.
Acknowledgments
This project was partially supported by grants 1 U 54 RR 026139-01A1, RCMI G12 RR 03051, National Center for Research Resources, National Institutes of Health, RCMI Division of Clinical Research Infrastructure initiative RCR 1 P 20 RR 11 126, and Committee on the Integration of Scientific Research Development (CIDIC) 2 D 34 MB 02054.
Footnotes
Peer reviewers and oil others who control content have no relevant financial relationships to disclose.
Disclosure Key can be found on the Table of Contents and at www.pmrjournal.org
Contributor Information
Edwardo Ramos, Physical Medicine, Rehabilitation and Sports Medicine Department, University of Puerto Rico, School of Medicine, Office A-204, PO Box 365067, San Juan, PR 00936-5067. Disclosure: nothing to disclose.
Suzanne Guttierrez-Teissoonniere, Department of Physical Medicine and Rehabilitation, New York University and Rusk Institute ot Rehabilitation Medicine, New York, NY Disclosure: nothing to disclose.
Jose G. Conde, Division of Graduate Studies, University of Puerto Rico, School of Medicine, Son Juan, PR Disclosure: nothing to disclose.
Jose A. Baez-Cordova, Physical Medicine, Rehabilitation and Sports Medicine Department, University of Puerto Rico, School of Medicine, San Juan, PR Disclosure: nothing to disclose.
Brenda Guzman-Villar, Physical Medicine, Rehabilitation and Sports Medicine Department, University of Puerto Rico, School of Medicine, San Juan, PR Disclosure: nothing to disclose.
Edgar Lopafegui-Corsino, Inter American University of Puerto Rico, Metropoliton Campus, San Juan, PR Disclosure: nothing to disclose.
Walter R. Frontera, Puerto Rico Clinical and Translational Research Consortium and Departments of Physical Medicine and Rehabilitation and Physiology, School of Medicine, MSC, University of Puerto Rico, San Juan, PR Disclosure: 8B, NIH grant support.
References
- 1.Lee GM, Gonmaker SL, Mcintosh K, Hughes MD, Oleske JM. Quality of life for children and adolescents: Impact of HIV infection and antiretroviral treatment. Pediatrics. 2006;117:273–283. doi: 10.1542/peds.2005-0323. [DOI] [PubMed] [Google Scholar]
- 2.Brown LK, Lourie KJ, Pao M. Children and adolescents living with HIV and AIDS: A review. J Child Psychol Psychiatry. 2000;41:81–96. [PubMed] [Google Scholar]
- 3.Keyser RE, Peralta L, Cade WT, Miller S, Anixt J. Functional aerobic impairment in adolescents seropositive for HIV: A quasiexperimental analysis. Arch Phys Med Rchabil. 2000;81:1479–1484. doi: 10.1053/apmr.2000.17810. [DOI] [PubMed] [Google Scholar]
- 4.Cade WT, Peralta L, Keyser RE. Aerobic capacity in late adolescents infected with HIV and control. Pediatr Rehab. 2002;5:161–169. doi: 10.1080/1363849021000039362. [DOI] [PubMed] [Google Scholar]
- 5.Miller TL. A hospital-based exercise program to improve body composition, strength, and abdominal adiposity in 2 HIV-infected children. AIDS Read. 2007;17:450–452. [PubMed] [Google Scholar]
- 6.Miller TL, Somarriba G, Kinnamon DD, Weinberg GA, Friedman LB, Scott GB. The effect of a structures exercise program on nutrition and fitness outcomes in human immunodeficiency virus-infected children. AIDS Res Hum Retroviruses. 2010;26:313–319. doi: 10.1089/aid.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar-Or O, Rowland TW. Pediatric Exercise Medicine: From Physiologic Principles to Health Care Application. Champaign, IL: Human Kinetics; 2004. pp. 12–18. [Google Scholar]
- 8.Beneke R, Hullcr M, Leithauser RM. Anaerobic performance and metabolism in boys and male adolescents. Eur J Appl Physiol. 2007;101:671–677. doi: 10.1007/s00421-007-0546-0. [DOI] [PubMed] [Google Scholar]
- 9.Bar-Or O. The Wingate anaerobic test: An update on methodology, reliability and validity. Sports Med. 1987;4:381–394. doi: 10.2165/00007256-198704060-00001. [DOI] [PubMed] [Google Scholar]
- 10.Hack V, Gross A, Bohm A, Stahl-Hennig C, Droge W. Decrease in phosphocrcatine level in skeletal muscle of SlV-infectcd rhesus macaques correlates with decrease in intracellular glutathione. AIDS Res Human Retroviruses. 1997;13:1089–1092. doi: 10.1089/aid.1997.13.1089. [DOI] [PubMed] [Google Scholar]
- 11.Takken T, van der Net J, Hclders PJM. Anaerobic exercise capacity in patients with juvenile-onset idiopathic inflammatory myopathies. Arthritis Rheum. 2005;53:173–177. doi: 10.1002/art.21066. [DOI] [PubMed] [Google Scholar]
- 12.Takken T, Elst EF, van der Net J. Pathophysiology factors which determine the exercise intolerance in patients with juvenile dermatomyositis. Curr Rheumatol Rev. 2005;1:91–99. [Google Scholar]
- 13.Falk B, Tiktinsky R, Weinstein Y, Constantini N, Martinowitz U. Anaerobic power and muscle strength in young hemophilia patients. Med Sci Sports Exerc. 2000;32:52–57. doi: 10.1097/00005768-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Kaczor JJ, Ziolkowski W, Popinigis J, Tarnopolsky MA. Anaerobic and aerobic enzyme activities in human skeletal muscle from children and adults. Pediatr Res. 2005;57:331–335. doi: 10.1203/01.PDR.0000150799.77094.DE. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez RS, Canto-Nogues C, Munoz-Fernandez A. Reconstructing the course of HIV-1-associated progressive encephalopathy in children. Med Sci Monit. 2002;8:249–252. [PubMed] [Google Scholar]
- 16.Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4:319–327. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- 17.Parker DL, Carriere L, Hebestreit H, Bar-Or O. Anaerobic exercise and peak muscle power in children with spastic cerebral palsy. Am J Dis Child. 1992;146:1069–1073. doi: 10.1001/archpedi.1992.02160210071024. [DOI] [PubMed] [Google Scholar]
- 18.Parker DL, Carriere L, Hebestreit H, Salsberg A, Bar-Or O. Muscle performance and gross motor function of children with cerebral palsy. Dev Med Child Neurol. 1993;35:17–23. doi: 10.1111/j.1469-8749.1993.tb11547.x. [DOI] [PubMed] [Google Scholar]
- 19.Berbrayer D, Ashby P. Reciprocal inhibition in cerebral palsy. Neurology. 1990;40:653–656. doi: 10.1212/wnl.40.4.653. [DOI] [PubMed] [Google Scholar]
- 20.Unnithan VB, Dowling G, Frost B, Volpe Ayub, Bar-Or O. Cocontraction and phasic activity during gait in children with cerebral palsy. Elcciromyogr Clin Neurophysiol. 1996;36:487–494. [PubMed] [Google Scholar]
- 21.Stephens S, Singh-Grewal D, Bar-OR O, et al. Reliability of exercise testing and functional activity questionnaires in children with juvenile arthritis. Arthritis Rheum. 2007;57:1446–1452. doi: 10.1002/art.23089. [DOI] [PubMed] [Google Scholar]
- 22.Cress ME, Buchner DM, Questad KA, Esselman PC, deLateur BJ, Schwartz RS. Continuous-scale physical functional performance in healthy older adults: A validation study. Arch Phys Med Rehabil. 1996;77:1243–1250. doi: 10.1016/s0003-9993(96)90187-2. [DOI] [PubMed] [Google Scholar]
- 23.Slade JM, Miszko TA, Laity JH, Agrawal SK, Cress ME. Anaerobic power and physical function in strength in strength-trained and non-strength-trained older adults. J Gerentol. 2002;57A:M168–M172. doi: 10.1093/gerona/57.3.m168. [DOI] [PubMed] [Google Scholar]
- 24.Miszko TA, Cress ME, Slade JM, Covey CJ, Agrawal SK, Doerr CE. Effect of strength and power training on physical function in community-dwelling older adults. J Gerentol. 2003;58A:171–175. doi: 10.1093/gerona/58.2.m171. [DOI] [PubMed] [Google Scholar]
- 25.Desai VG, Lee T, Moland CL, et al. Effect of short-term exposure to zidovudine (AZT) on the expression of mitochondria-related genes in skeletal muscle of neonatal mice. Mitochondrion. 2009;9:9–16. doi: 10.1016/j.mito.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Viengchareun S, Caron M, Auclair M, et al. Mitochondrial toxicity of indinavir, slavudme and zidovudine involves multiple cellular targets in while and brown adipocytes. Antivir Thcr. 2007;12:919–929. [PubMed] [Google Scholar]
- 27.Cupler EJ, Danon MJ, Jay C, Hench K, Ropka M, Dalakas MC. Early features of zidovudine-associated myopathy: Hisiopathological findings and clinical correlations. Acta Neuropathol. 1995;90:1–6. doi: 10.1007/BF00294452. [DOI] [PubMed] [Google Scholar]
- 28.Simpson DM, Citak KA, Godfrey E, Godbold J, Wolfe DE. Myopathies associated with human immunodeficiency virus and zidovudine: Can their effects be distinguished? Neurology. 1993;43:971–976. doi: 10.1212/wnl.43.5.971. [DOI] [PubMed] [Google Scholar]
- 29.Chalmers AC, Greco CM, Miller RG. Prognosis in AZT myopathy. Neurology. 1991;41:1181–1184. doi: 10.1212/wnl.41.8.1181. [DOI] [PubMed] [Google Scholar]


