Abstract
Objective To assess the role of nasal continuous positive airway pressure (CPAP) initiated at birth for prevention of death and bronchopulmonary dysplasia in very preterm infants.
Design Systematic review.
Data sources PubMed, Embase, the Cochrane Central Register of Controlled Trials, and online Pediatric Academic Society abstracts from the year of inception to June 2013.
Eligibility criteria for selecting studies Randomised controlled trials evaluating the effect of nasal CPAP compared with intubation in preterm infants born at less than 32 weeks’ gestation and presenting the outcomes of either death or bronchopulmonary dysplasia, or both (defined as the need for oxygen support or mechanical ventilation at 36 weeks corrected gestation), during hospital stay.
Results Four randomised controlled trials (2782 participants) met the inclusion criteria, with 1296 infants in the nasal CPAP group and 1486 in the intubation group. All the trials reported bronchopulmonary dysplasia independently at 36 weeks corrected gestation, with borderline significance in favour of the nasal CPAP group (relative risk 0.91, 95% confidence interval 0.82 to 1.01, risk difference −0.03, 95% confidence interval −0.07 to 0.01). No difference in death was observed (relative risk 0.88, 0.68 to 1.14, risk difference −0.02, −0.04 to 0.01, respectively). Pooled analysis showed a significant benefit for the combined outcome of death or bronchopulmonary dysplasia, or both, at 36 weeks corrected gestation for babies treated with nasal CPAP (relative risk 0.91, 0.84 to 0.99, risk difference −0.04, -0.07 to 0.00), number needed to treat of 25).
Conclusion One additional infant could survive to 36 weeks without bronchopulmonary dysplasia for every 25 babies treated with nasal CPAP in the delivery room rather than being intubated.
Introduction
Despite recent advances in perinatal-neonatal care, there is a trend of increased incidence of bronchopulmonary dysplasia among survivors of prematurity.1 Most infants who develop bronchopulmonary dysplasia are born prematurely, and 75% of affected babies weigh less than 1000 g at birth.2 3 The risk of developing bronchopulmonary dysplasia increases with decreasing birth weight, with reported incidence as high as 85% in neonates weighing between 500 g and 699g, but only 5% in infants with birth weights over 1500 g.2 3 In the most immature infants, even minimal exposure to supplemental oxygen and mechanical ventilation could be enough to contribute to bronchopulmonary dysplasia.2 3 This puts a heavy burden on health resources because these infants are at risk of frequent hospital readmissions in the first two years after birth and, even as adolescents, have lung function abnormalities and persistent respiratory symptoms.4 5
The lungs of very preterm infants are uniquely susceptible to injury because they are structurally immature, deficient in surfactant, and not supported by a stiff chest wall. Hence the lung of very preterm infants is easily damaged by mechanical ventilation.6 To maintain functional residual capacity and improve lung compliance and oxygenation, nasal continuous positive airway pressure (CPAP) has been advocated at the initiation of respiratory support.7 8 9 10 Observational studies in both the era before the widespread use of antenatal steroids and after the introduction of surfactant have documented an association between lower rates of bronchopulmonary dysplasia and increased use of nasal CPAP shortly after birth—that is, “primary” continuous positive airway pressure as a means of avoiding endotracheal intubation and mechanical ventilation.11 12 13 An observational study reported a lower rate of bronchopulmonary dysplasia with much greater use of nasal CPAP in one centre compared with seven other centres.11 Another study reported higher bronchopulmonary dysplasia rates in two Boston centres when compared with a single centre in New York (22% v 4%), with the higher rates in Boston associated with more use of mechanical ventilation (75% v 29%).12 Another retrospective study, of 261 preterm infants, that compared intubation and ventilation with primary nasal CPAP reported lower mortality and lower rates of administered surfactant, bronchopulmonary dysplasia, and intraventricular haemorrhage in infants who received nasal CPAP.13 In addition, a study compared rates of bronchopulmonary dysplasia, intubation in the delivery room, and mechanical ventilation for more than 24 hours in 14 tertiary level neonatal intensive care units in northern Italy.14 Centres with high delivery room intubation rates had higher rates of ventilation and bronchopulmonary dysplasia.14 These studies prompted the launch of large randomised controlled trials comparing nasal CPAP with endotracheal intubation soon after birth. We reviewed the available literature on the use of primary nasal CPAP soon after birth compared with intubation and mechanical ventilation for the prevention of death or bronchopulmonary dysplasia in very preterm infants.
Methods
We searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials using a predefined algorithm (see supplementary file), reviewed abstracts from annual meetings of the Pediatric Academic Society (2000-12), and performed a manual search of references in narrative and systematic reviews. Search terms included “infant”, “newborn”, “resuscitation”, “continuous positive airway pressure”, and “sustained inflation”.
Study selection
We included studies if they were randomised controlled trials, compared nasal CPAP with endotracheal intubation as the primary mode of respiratory support after birth in preterm populations at less than 32 weeks’ gestation, and presented the outcomes of either death or bronchopulmonary dysplasia (defined as the need for oxygen support or mechanical ventilation at 36 weeks corrected gestation) during hospital stay. Our primary outcome measure was death or bronchopulmonary dysplasia, or both, in a preterm population at less than 32 weeks’ gestation. Secondary outcomes included the need for any mechanical ventilation during stay on a neonatal intensive care unit, treatment with surfactant, pneumothorax, postnatal corticosteroid treatment, intraventricular haemorrhage (grade III/IV or described as severe), any necrotising enterocolitis, any patent ductus arteriosus or any patent ductus arteriosus needing ligation, and any or severe retinopathy of prematurity. The review team resolved any discrepancies in inclusion through consensus.
Data extraction
Data were recorded using a standardised data collection form to record study design and methodological characteristics, patient characteristics, interventions, and outcomes, including the relative risks and 95% confidence intervals. We also documented information on mode of randomisation, allocation concealment, blinding, and intention to treat analysis. Two investigators (GMS, MK) independently extracted data and resolved discrepancies in consultation with another member of the review team (PYC).
Assessment of methodological quality
We assessed the methodological quality of the included trials and evaluated risk of bias using elements of the Cochrane Collaboration tool.15 The domains used in the present systematic review pertained to randomisation and allocation concealment (selection bias), blinding (performance and detection bias), and adherence to the intention to treat principle (attrition bias).
Statistical analysis
The principal summary measures were the weighted mean difference for continuous outcomes and relative risk and absolute risk reduction for dichotomous outcomes. For each trial we retrieved or calculated the crude relative risk and absolute risk reduction estimates and corresponding 95% confidence intervals for the assessed outcomes. We explored heterogeneity using a χ2 test and measured the quantity of heterogeneity with the I2 statistic.16 We used random effects models to summarise relative risk and absolute risk reduction estimates.17 Analyses were performed in RevMan version 5.2 (Cochrane Collaboration, 2013). All P values are two tailed. Where the pooled estimates of relative risk were statistically significant we calculated the numbers needed to treat (NNT) for all outcomes. The study is reported according to the PRISMA checklist.18
Results
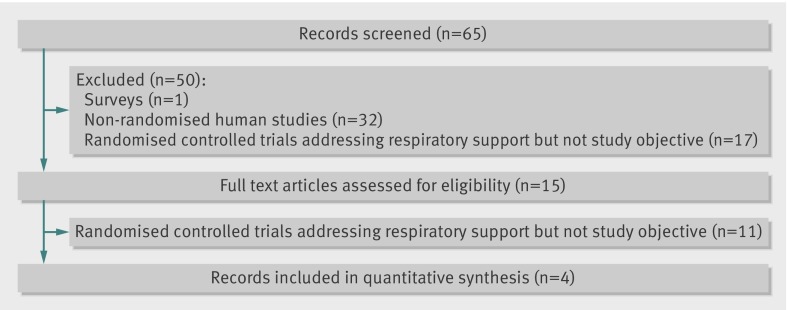
Figure 1 shows the flow of studies through the selection process. Our initial search identified 65 citations of potentially eligible studies, of which 50 were rejected based on a screening of the study title and abstract. Of the remaining 15 studies that were assessed in full text, four trials including 2780 infants7 8 9 10 fulfilled the inclusion criteria (table 1). Table 2 presents an assessment of risk of bias for the included studies. All described adequate randomisation. We assessed all the studies as being at high risk of bias for blinding of participants and caregivers, as it was not feasible to do so for the type of interventions being compared; however, the studies did provide objective criteria for defining their primary outcome and failure of treatment. All the studies provided inhospital outcome data for the randomised participants, and infants in all the studies were analysed by intention to treat. The nasal CPAP and intubation groups were well matched; birth weight and gestational age did not differ significantly (table 1). Other aspects of respiratory treatment, including resuscitation devices used and criteria for using endotracheal intubation and surfactant, were adequately described in the studies and conformed to current international guidelines. Overall, 518/1296 infants in the nasal CPAP group required intubation within the first week after birth and 643/1296 infants in the nasal CPAP group and 1402/1486 in the intubation group received surfactant.
Fig 1 Flowchart for selection of eligible studies
Table 1.
Characteristics of included randomised controlled studies. Values are means (standard deviations) unless stated otherwise
| Variables | Morley (2008)7 | SUPPORT (2010)8 | Sandri (2010)10 | Dunn (2011)9 |
|---|---|---|---|---|
| Total No of participants | 610 | 1316 | 208 | 648* |
| Gestation (weeks) | 250/7-286/7 | 240/7-276/7 | 250/7-286/7 weeks | 260/7-296/7 |
| CPAP group: | ||||
| No in group | 307 | 663 | 103 | 223 |
| Birth weight (g) | 964 (212) | 834 (188) | 967 (221) | 1053 (252) |
| Gestational age (weeks) | 26.9 (1.0) | 26.2 (1.1) | 27.0 (1.0) | 28.1 (1.1) |
| Intubation group: | ||||
| No in group | 303 | 653 | 105 | 425 |
| Birth weight (g) | 952 (217) | 825 (198) | 913 (200) | 1040 (244) |
| Gestational age (weeks) | 26.9 (1.0) | 26.2 (1.1) | 27.0 (1.0) | 28.0 (1.1) |
| Timing of randomisation | After delivery | Before delivery | After delivery | After delivery |
| Stratification (weeks) | 250/7-266/7 and 270/7-286/7 | 240/7-256/7 and 260/7-276/7 | 250/7-266/7 and 270/7-286/7 | 260/7-276/7 and 280/7-296/7 |
CPAP=continuous positive airway pressure.
*Infants randomised to prophylactic surfactant group and intubate, surfactant, or extubate group were combined in the intubation group (prophylactic surfactant group, gestational age 28 (1.1 weeks) and birth weight 1040 g (244 g), intubate, surfactant, or extubate group gestational age 28.1 (1.1) and birth weight 1066 (270 g)).
Table 2.
Assessment of risk of bias in included studies
| Study | Year | Random sequence generation | Allocation concealment | Blinding of participants and staff | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting |
|---|---|---|---|---|---|---|---|
| Morley et al7 | 2008 | Low | Low | High | Unclear | Low | Low |
| SUPPORT8 | 2010 | Low | Low | High | Unclear | Low | Low |
| Sandri et al10 | 2010 | Low | Low | High | Unclear | Low | Low |
| Dunn et al9 | 2011 | Low | Low | High | Unclear | Low | Low |
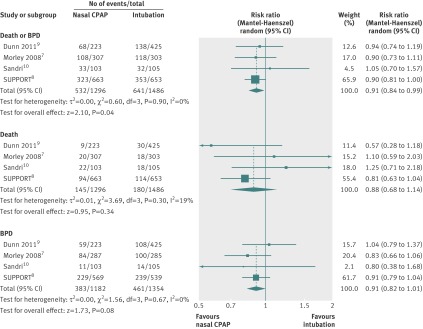
Table 3 and figure 2 show the pooled results for our primary outcome. All trials reported death or bronchopulmonary dysplasia independently at 36 weeks corrected gestation. A reduction of bronchopulmonary dysplasia occurred with borderline significance in favour of the nasal CPAP group: relative risk 0.91 (95% confidence interval 0.82 to 1.01), risk difference 0.03 (95% confidence interval −0.07 to 0.01, table 3, fig 2). Pooled analysis showed a significant benefit for the combined outcome of death or bronchopulmonary dysplasia, or both, at 36 weeks corrected gestation for babies treated with nasal CPAP: relative risk 0.91 (0.84 to 0.99), risk difference −0.04 (−0.07 to −0.00), NNT of 25 (fig 2).
Table 3.
Results of primary and secondary outcomes. Values are numbers of participants unless stated otherwise
| Variables | No of studies (references) | Nasal CPAP group | Intubation group | Relative risk (95% CI) | Risk difference (95% CI) | Number needed to treat |
|---|---|---|---|---|---|---|
| Death at 36 weeks corrected gestation | 47-10 | 145/1296 | 180/1486 | 0.88 (0.68 to 1.14) | −0.02 (−0.04 to 0.01) | |
| BPD at 36 weeks corrected gestation | 47-10 | 383/1182 | 461/1354 | 0.91 (0.82 to 1.01) | −0.03 (−0.07 to 0.01) | |
| Death or BPD, or both | 47-10 | 532/1296 | 641/1486 | 0.91 (0.84 to 0.99) | −0.04 (−0.07 to −0.00) | 25 |
| Received any mechanical ventilation | 47-10 | 839/1296 | 1441/1486 | 0.56 (0.32 to 0.97) | −0.34 (−0.68 to −0.01) | |
| Surfactant treatment | 47-10 | 643/1296 | 1402/1486 | 0.40 (0.23 to 0.70) | −0.51 (−0.79 to −0.23) | |
| Pneumothorax | 47-10 | 86/1296 | 78/1486 | 1.26 (0.51 to 3.09) | 0.01 (−0.04 to 0.05) | |
| Postnatal corticosteroid treatment | 37 8 10 | 97/1041 | 137/1024 | 0.73 (0.49 to 1.10) | −0.04 (−0.07 to 0.00) | |
| Intraventricular haemorrhage (grade III/IV) | 47-10 | 133/1270 | 126/1445 | 1.1 (0.84 to 1.44) | 0.00 (−0.03 to 0.03) | |
| Any necrotising enterocolitis | 47-10 | 122/1286 | 115/1469 | 1.19 (0.93 to 1.52) | 0.01 (−0.01 to 0.03) | |
| Any patent ductus arteriosus | 37 9 10 | 251/632 | 321/832 | 1.06 (0.87 to 1.30) | 0.03 (−0.06 to 0.11) | |
| Patent ductus arteriosus needing ligation | 27 10 | 50/410 | 60/408 | 0.83 (0.59 to 1.17) | −0.03 (−0.07 to 0.01) | |
| Any retinopathy of prematurity | 38-10 | 278/602 | 336/771 | 1.05 (0.80 to 1.36) | 0.01 (−0.09 to 0.11) | |
| Severe retinopathy of prematurity | 38-10 | 87/806 | 83/941 | 1.22 (0.71 to 2.08) | 0.01 (−0.02 to 0.05) |
CPAP=continuous positive airway pressure; BPD=bronchopulmonary dysplasia.
Fig 2 Forest plot comparison of death or bronchopulmonary dysplasia (BPD), or both, at 36 weeks corrected gestation; death; and bronchopulmonary dysplasia at 36 weeks corrected gestation. CPAP=continuous positive airway pressure
We identified significant heterogeneity for administered surfactant and any mechanical ventilation and therefore these results must be interpreted with caution. All trials assessed surfactant, with a significant reduction in administered surfactant in the nasal CPAP group (relative risk 0.40, 0.23 to 0.70, risk difference −0.51, −0.79 to −0.23, with 98% heterogeneity). All trials assessed the need for any mechanical ventilation, with a significant reduction in the nasal CPAP group (relative risk 0.56, 0.32 to 0.97, risk difference −0.34, −0.68 to −0.01, with 99% heterogeneity).
Other outcomes (including postnatal corticosteroid and patent ductus arteriosus) were not significantly different between those treated with nasal CPAP or with intubation (table 3). Subgroup analyses based on gestational age were not possible because the results of individual studies used different gestational ages for stratification. In addition, one trial was stopped after recruitment reached 74% of the projected sample size, owing to difficulties with enrolment.9
Long term outcomes have been reported recently for the SUPPORT trial (Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial),8 and Dunn et al are planning to report their long term outcomes at two years corrected age.9 The SUPPORT trial did not show any difference in death or neurodevelopmental impairment at 18 to 22 months between groups.19
Discussion
We identified four randomised controlled trials that compared nasal CPAP with intubation,7 8 9 10 as they are somewhat difficult to undertake during the emergent timeframe of neonatal resuscitation. Our meta-analysis shows that one additional infant could survive to 36 weeks without bronchopulmonary dysplasia for every 25 babies treated with nasal continuous positive airway pressure (CPAP) in the delivery room rather than being intubated and mechanically ventilated. In addition, the reduction in bronchopulmonary dysplasia was of borderline significance.
However, caution should be exercised in generalising the results from our review. All the studies required antenatal consent, preselecting more stable pregnancies and lowering the observed incidence of acute or serious antenatal and neonatal complications. In addition, 95% of the infants included in our analysis received antenatal steroids. Furthermore, none of the studies were blinded, raising the possibility of bias influencing the outcomes if caregivers did not follow the trial guidelines or changed subsequent management based on resuscitation interventions. We did not conduct any formal tests for publication bias; however, funnel plots (data not shown) revealed no obvious asymmetry. Only one trial reported long term outcomes and reported no difference between the nasal CPAP group and the intubation group.19
None of the trials enrolled infants of 23 weeks gestational age and only one trial included infants of 24 weeks gestational age.8 These extremely premature neonates represent a high risk group, with a high mortality and a high need for early intubation in the delivery room. A further confounder is the variation in how surfactant was administered between the identified studies. The thresholds for intubation and surfactant treatment varied from 40% to 60% oxygen, potentially influencing lung injury acutely (that is, pneumothorax) or chronically as bronchopulmonary dysplasia.
These studies were conducted when 21% oxygen was not recommended as the initial oxygen concentration used for neonatal resuscitation. In addition, the use of 100% oxygen in neonatal resuscitation has been associated with increased oxidative stress and related pulmonary injury.20 It will be interesting to examine if similar effects on mortality and morbidity are observed when nasal CPAP is compared with intubation and mechanical ventilation for extremely low birth weight infants using 21-40% fractional inspired oxygen for initiating resuscitation at birth. None the less, the rush to intubate and mechanically ventilate at birth could be avoided with the increasing knowledge of normal oxygen saturation levels in the first minutes after birth.21 22 23
Despite these limitations, clinicians remain obliged to resuscitate newborn infants on the basis of best available evidence, and researchers should be encouraged to build on existing trials to deal with this important problem. The studies included in this review prove that large trials could be undertaken in the delivery room, with random allocation of all eligible babies.24 Future trials should investigate different levels of nasal CPAP and different strategies and thresholds for administering surfactant and should ensure long term follow-up of neurodevelopment. Waiver or deferral of consent might be considered as a means of avoiding selection bias and improving generalisability.
Conclusions
Nasal CPAP initiated in the delivery room compared with intubation reduces death or bronchopulmonary dysplasia in very preterm babies. One additional infant could survive to 36 weeks without bronchopulmonary dysplasia for every 25 babies treated with nasal CPAP in the delivery room rather than being intubated and mechanically ventilated.
What is already known on this topic
Most infants who develop bronchopulmonary dysplasia are born prematurely, and most of these babies weigh less than 1000 g at birth and are mechanically ventilated
Providing nasal continuous positive airway pressure (CPAP) at birth has been advocated to avoid lung injury and potentially lessen the risk of bronchopulmonary dysplasia
What this study adds
One additional infant could survive to 36 weeks without bronchopulmonary dysplasia for every 25 babies treated with nasal CPAP in the delivery room rather than being intubated
Contributors: GMS, MK, and PYC conceived and designed the study. All authors collected, assembled, analysed, and interpreted the data, drafted the article, critically revised the article for important intellectual content, and approved the final version of the article. GMS and PYC are the guarantors. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors were included in every step of the review and had full access to all data. The corresponding author had final responsibility to submit for publication.
Funding: This study received no specific funding.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: GMS had support from Banting postdoctoral fellowship, Canadian Institutes of Health Research and an Alberta Innovates—Health Solutions clinical fellowship for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Cite this as: BMJ 2013;347:f5980
Web Extra. Extra material supplied by the author
Search strategy
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723-9. [DOI] [PubMed] [Google Scholar]
- 2.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946-55. [DOI] [PubMed] [Google Scholar]
- 3.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet 2006;367:1421-31. [DOI] [PubMed] [Google Scholar]
- 4.Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics 2006;118:108-13. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Anderson PJ. Long-term outcomes of bronchopulmonary dysplasia. Semin Fetal Neonatal Med 2009;14:391-5. [DOI] [PubMed] [Google Scholar]
- 6.Schmölzer GM, Te Pas AB, Davis PG, Morley CJ. Reducing lung injury during neonatal resuscitation of preterm infants. J Pediatr 2008;153:741-5. [DOI] [PubMed] [Google Scholar]
- 7.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 2008;358:700-8. [DOI] [PubMed] [Google Scholar]
- 8.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010;362:1970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128:e1069-76. [DOI] [PubMed] [Google Scholar]
- 10.Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 2010;125:e1402-9. [DOI] [PubMed] [Google Scholar]
- 11.Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987;79:26-30. [PubMed] [Google Scholar]
- 12.Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? Pediatrics 2000;105:1194-201. [DOI] [PubMed] [Google Scholar]
- 13.Ammari A, Suri M, Milisavljevic V, Sahni R, Bateman D, Sanocka U, et al. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J Pediatr 2005;147:341-7. [DOI] [PubMed] [Google Scholar]
- 14.Gagliardi L, Bellù R, Lista G, Zanini R, and Network Neonatale Lombardo Study Group. Do differences in delivery room intubation explain different rates of bronchopulmonary dysplasia between hospitals? Arch Dis Child—Fetal Neonatal Ed 2011;96:F30-5. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 17.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820-6. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med 2007;4:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaucher YE, Peralta-Carcelen M, Finer NN, Carlo WA, Gantz MG, Walsh MC, et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med 2012;367:2495-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vento M, Cheung P-Y, Aguar M. The first golden minutes of the extremely-low-gestational-age neonate: a gentle approach. Neonatology 2009;95:286-98. [DOI] [PubMed] [Google Scholar]
- 21.Dawson JA, Kamlin COF, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010;125:e1340-7. [DOI] [PubMed] [Google Scholar]
- 22.Dawson JA, Vento M, Finer NN, Rich W, Saugstad OD, Morley CJ, et al. Managing oxygen therapy during delivery room stabilization of preterm infants. J Pediatr 2012;160:158-61. [DOI] [PubMed] [Google Scholar]
- 23.Rabi Y, Yee W, Chen SY, Singhal N. Oxygen saturation trends immediately after birth. J Pediatr 2006;148:590-4. [DOI] [PubMed] [Google Scholar]
- 24.Davis PG, Tan A, O’Donnell CPF, Schulze A. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet 2004;364:1329-33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy