Abstract
Opiates such as morphine and fentanyl, a major class of analgesics used in the clinical management of pain, exert their effects through the activation of opioid receptors. Opioids are among the most commonly prescribed and frequently abused drugs in the USA; however, the prolonged use of opiates often leads to the development of tolerance and addiction. Although blockade of opioid receptors with antagonists such as naltrexone and naloxone can lessen addictive impulses and facilitate recovery from overdose, systemic disruption of endogenous opioid receptor signalling through the use of these antagonistic drugs can have severe side effects. In the light of these challenges, current efforts have focused on identifying new therapeutic targets that selectively and specifically modulate opioid receptor signalling and function so as to achieve analgesia without the adverse effects associated with chronic opiate use. We have previously reported that opioid receptors interact with each other to form heteromeric complexes and that these interactions affect morphine signalling. Since chronic morphine administration leads to an enhanced level of these heteromers, these opioid receptor heteromeric complexes represent novel therapeutic targets for the treatment of pain and opiate addiction. In this review, we discuss the role of heteromeric opioid receptor complexes with a focus on mu opioid receptor (MOR) and delta opioid receptor (DOR) heteromers. We also highlight the evidence for altered pharmacological properties of opioid ligands and changes in ligand function resulting from the heteromer formation.
Opioid receptors are members of the seven types of opioid receptors: mu opioid receptor transmembrane G-protein-coupled receptor (MOR), kappa opioid receptor (KOR) and delta (GPCR) superfamily (Ref. 1). There are three opioid receptor (DOR). At the cellular level, opioid receptors are coupled to Gi/Go proteins and their activation leads to inhibition of adenylyl cyclase activity and voltage-gated Ca2+ channels, increases in mitogen-activated protein kinase (MAPK) phosphorylation and in the activity of inwardly rectifying K+ channels and phospholipase C beta (Ref. 2). The signalling cascades initiated by the activation of opioid receptors induce the transcription of genes that regulate cellular differentiation, proliferation and survival (Ref. 3). At the systems level, opioid receptor activation leads to a number of physiological outcomes including analgesia, feelings of euphoria and anxiety, respiratory depression, constipation, immunosuppression and changes in feeding and locomotor activity (Ref. 2). Despite the large number of physiological outcomes associated with opioid signalling, these receptors are largely valued for their role in inducing analgesia.
In general, studies show that MOR- or DOR-selective agonists can induce both analgesia and reward (Ref. 2). The use of transgenic mice has provided greater insight into the potential roles of individual opioid receptor subtypes. These studies indicate that the analgesic effects of clinical opioids such as morphine and the rewarding properties associated with nonopiate drugs of abuse such as marijuana (Ref. 4) are primarily mediated by the activation of MOR (Ref. 5). However, DOR has been shown to have a role in the regulation of emotional responses associated with opioid use (Refs 2, 6). Despite this knowledge, the major clinical limitation of opioids – tolerance and development of addiction following chronic use – has not yet been overcome (Refs 1, 2, 5, 7). To better understand how tolerance and addiction can be dissociated from the desirable outcome of analgesia, the field has begun to take a closer look at the interactions between MOR and DOR, and more recently on the role of the MOR–DOR heteromer.
Over the last decade, several studies have shown that GPCRs can form homomers (associations between same receptor subtype) or heteromers (associations between different receptor subtypes or between different GPCRs) (Ref. 7). Such receptor interactions have been shown to modulate ligand binding, to affect interactions with intracellular scaffolding proteins, to alter signalling cascades induced following receptor activation and to alter receptor trafficking (reviewed in Refs 8, 9, 10). In the case of opioid receptors, several studies have reported receptor heteromers that show receptor binding, signalling and trafficking properties that are distinct from those of receptor monomers or homomers (Refs 7, 11, 12, 13, 14, 15, 16). In addition, studies have shown that regulation of heteromer formation at the cellular level has the potential to modulate opioid signalling both in vitro and in vivo (Refs 12, 13). Finally, a recent study suggested that opioid receptor signalling could be modulated not only by the levels and types of heteromers present in a cell, but also by the activation state of both the opioid receptor and its partner GPCR (Ref. 11). This suggests the intriguing possibility of tissue- or disease-specific expression of heteromeric complexes that could be targeted to develop drugs that specifically target the heteromer in a disease state. In this review, we discuss MOR–DOR heteromers and the potential for targeting these complexes to achieve antinociception or analgesia without the adverse effects of tolerance, dependence and addiction associated with chronic opioid use.
Opioid receptor complexes
The existence of mu–delta ‘opioid receptor complexes’ was postulated as early as 1981 (Ref. 17), and the possibility that allosteric binding could contribute to the heterogeneity of opioid receptor interactions was considered even earlier (Ref. 18). Such hypotheses were drawn from the results of studies probing the activity of opioid peptides in radiolabelled binding assays and in guinea pig ileum and mouse vas deferens contraction assays (Ref. 19). These studies revealed a surprising diversity in opioid receptor activity that, at the time, was attributed to ligand binding at several sites of a single receptor or binding to altered forms of the receptor (Ref. 19). These opioid ligands also demonstrated heterogeneity in binding characteristics, under a range of ligand concentrations and in the presence of endogenous opioids (Ref. 19).
Support for MOR–DOR complex formation was provided by competitive binding assays that measured the ability of a test drug to displace the binding of a known radiolabelled ligand in cellular membrane preparations (Ref. 20). In this type of assay, inhibition of radiolabelled ligand binding is usually attributed to competitive displacement of the ligand by the test drug. However, studies examining competitive displacement of radiolabelled Leu-enkephalin by unlabelled Leu-enkephalin (a DOR receptor agonist) in the absence or presence of different concentrations of morphine (recently shown to exert its effects through MOR using transgenic animals) found that the latter shifted the Leu-enkephalin binding kinetics from being competitive to noncompetitive (Refs 17, 20). This suggested that morphine, bound to a receptor not labelled by radiolabelled Leu-enkephalin, allosterically induced an apparent loss of Leu-enkephalin-labelled receptors (Refs 17, 20).
Studies using the tail-flick assay to measure the analgesic properties of different opioid ligands in vivo showed that their potency was consistently greater than what would have been predicted from data obtained from in vitro binding assays (Ref. 21). For example, subanalgesic doses of the DOR-selective agonist, Leu-enkephalin, could potentiate morphine-mediated analgesia (Ref. 22). This suggested the possibility of interactions between opioid receptor subtypes and provided a potential mechanism by which the site-specific activity of paired receptor complexes could dictate the outcome of opioid drug treatment (Ref. 22). This model assumed that the availability of opioid receptors and their endogenous ligands is regionally localised and regulated. The observed effect of any given opioid treatment would therefore depend on the interaction, or interference, of region-specific factors in the microenvironment of the receptors. Thus, it becomes apparent that in vitro binding assay measurements cannot necessarily predict the in vivo analgesic utility of opioid ligands (Ref. 22). Furthermore, both in vitro and in vivo assays could be affected by differences in the ratio of MOR to DOR as well as the presence or absence of endogenous ligands (Ref. 11).
Studies examining receptor expression and interaction at the cellular level have implicated MOR in the regulation of DOR expression at the plasma membrane (Refs 23, 24, 25). For example, chronic exposure to morphine leads to an increase in the surface expression of DOR in cultured cortical neurons and in neurons in the dorsal horn of the spinal cord in vivo (Refs 24, 25); this does not occur in MOR-deficient animals (Ref. 24). Similarly, increases in the surface expression of DOR were also described in cultured dorsal root ganglion (DRG) neurons following chronic exposure to morphine in vitro (Ref. 23). These studies indicate a role for MOR in mediating DOR trafficking and cell surface availability, thereby providing further support for interactions between MOR and DOR. Such results suggest that DOR regulates morphine dependence – possibly by modulating MOR expression – and reciprocally, that MOR regulates DOR expression and availability in response to chronic morphine. Although MOR–DOR signalling and the definitive contributions of each receptor to heteromer-mediated signalling are still under scrutiny, these studies make clear the functional relevance of MOR–DOR interactions in pain and tolerance development. Substantiating these findings through investigation of the physical interaction between MOR and DOR has become essential to delineate the contribution of each receptor towards MOR–DOR interactions. Studies examining the coexpression of these receptors, as well as their direct physical interaction, are discussed below.
MOR and DOR transgenic mice
Studies using transgenic mice with genetic deletions of either MOR or DOR provided tremendous insight into the functional roles of these receptors in antinociception and other physiological and pharmacological outcomes such as tolerance (Refs 5, 26, 27, 28). These transgenic mice served as appropriate controls to determine the contribution of each opioid receptor subtype to a given physiological response. In addition, these transgenic mice have enabled studies to assess the contribution of receptor–receptor interactions to opioid-receptor-mediated signalling and behavioural responses (Ref. 27).
Early investigations by Matthes and colleagues found that MOR-deficient mice did not show morphine-mediated analgesia, physical dependence or conditioned place preference (Ref. 5). These results indicated the unique requirement for MOR in morphine-mediated antinociceptive responses and suggested that MOR is the target of morphine in vivo and indeed the receptor responsible for morphine-induced antinociception and behaviour. However, this finding was unexpected, given previous studies showing that, at high doses, morphine can also bind to DOR and KOR and that all three opioid receptor subtypes are involved in the blockade of pain at the level of the spinal cord (Ref. 29). In addition, administration of DOR-selective agonists to MOR-deficient mice led to reduced antinociception in the tail-flick test (Refs 5, 30). This suggested that either the predicted involvement of DOR in spinal analgesia was incorrect, or that the availability of MOR was required for DOR-mediated spinal analgesia. One possibility for reduced DOR-mediated antinociception in MOR-deficient mice could be due to impairment of DOR-mediated activity. However, examination of signalling responses using G-protein binding and adenylyl cyclase assays found DOR-mediated signalling to be intact in these animals (Ref. 30). In addition, DOR agonists continued to mediate a peripheral effect, such as inhibition of muscle contraction (using the vas deferens twitch assay) in MOR-deficient mice (Ref. 30). Taken together, these results indicated a normal DOR function in MOR-deficient mice and suggested that optimal DOR-mediated antinociception requires the presence of MOR.
In the case of DOR-deficient animals, studies with Dor1 mutant mice generated by deletion of exon 2 demonstrated a strong reduction in antinociceptive responses to intrathecally administered DOR-selective agonists compared with wild-type animals (Ref. 26). However, these agonists showed antinociceptive effects following intracerebroventricular administration, suggesting that they exerted their supraspinal analgesic effects at a receptor other than DOR (Ref. 26). Interestingly, these DOR-deficient mice showed normal morphine-mediated antinociceptive responses though the development of tolerance to morphine was abolished (Ref. 26). Thus, these studies suggest interactions between MOR and DOR and that the latter has a role in the development of tolerance to morphine.
Further support for functional interactions between MOR and DOR comes from a study examining the coupling of MOR to voltage-gated Ca2+ channels in DRG neurons from wild type and from animals lacking DOR (Ref. 31). This work found that the MOR-selective agonists, morphine and DAMGO, were less effective at inhibiting the activity of voltage-gated Ca2+ channels in neurons lacking DOR compared with wild-type neurons (Ref. 31). These effects were neither because of decrease in the density and function of voltage-gated Ca2+ channels nor because of changes in MOR mRNA levels; this suggests functional interactions between MOR and DOR at the level of inhibition of voltage-gated Ca2+ channel activity.
MOR–DOR interacting complexes –anatomical and molecular evidence
Demonstration of MOR–DOR heteromer formation requires that MOR and DOR be present not only in the same cell, but also in the same subcellular compartment. Studies investigating the distribution of MOR and DOR in the dorsal horn of the rat spinal cord using dual immunocytochemical analysis and electron microscopy revealed the presence of both MOR and DOR in the same somatodendritic compartments, both in discrete areas of the plasma membrane and in organelles (Ref. 32). Expression of MOR and DOR in the same cells was also revealed using in situ hybridisation (Ref. 33). These studies detected coexpression of mRNA encoding MOR and DOR in spinally projecting neurons of the rostral ventromedial medulla (RVM) (Ref. 33). These data provided one of the first demonstrations that MOR and DOR colocalised in neurons of central nervous system regions associated with nociception.
The existence of MOR–DOR interacting complexes has been demonstrated more directly through the use of heterologous expression systems (Refs 34, 35, 36, 37). Early work from our laboratory revealed that interacting complexes could be isolated both from heterologous cells expressing recombinant receptors as well as from endogenous tissue expressing native opioid receptors (Refs 34, 37). Furthermore, close proximity to form interacting complexes between MOR and DOR was demonstrated by coimmunoprecipitation experiments (Refs 34, 37). In these studies, cells were transfected with either FLAG-tagged MOR, Myc-tagged DOR or both epitope-tagged receptors. The lysates from these cells were then immunoprecipitated with antibodies directed against the Myc epitope. The resulting immunoprecipitates were probed with anti-FLAG antibodies by Western blot, revealing a distinct band at ~150 kDa only in cells coexpressing both receptors (Ref. 37). Bioluminescence resonance energy transfer assays using MOR–luciferase- and DOR–YFP (yellow fluorescent protein)-tagged receptors in heterologous live cells supported the existence of receptors that are in close enough proximity (<5–10 nm apart), to form interacting complexes (Ref. 34).
However, it is important to note that there is a debate on whether MOR–DOR heteromerisation occurs, given a recently published study challenging the presence of MOR and DOR in the same cells (Ref. 38). This study used a knock-in mouse model with DOR tagged with enhanced green fluorescent protein (eGFP) and used antibodies directed against GFP or MOR to examine colocalisation between the eGFP-tagged DOR and endogenous MOR. The authors reported colocalisation in <5% of DRG neurons in vivo (Ref. 38); these data contradicted results from other studies reporting substantial MOR–DOR colocalisation (Refs 33, 39). This might be attributable to a number of important factors. Of note is the relatively higher level of expression of DOR in these knock-in mice (Ref. 40) and the possibility that there is an increase in surface expression of DOR in these mice as a direct consequence of the incorporated C-terminal GFP tag (Ref. 41). In addition, the antibody directed against GFP used in this study for the visualisation of eGFP-tagged DOR could exhibit higher avidity for GFP than the MOR-directed antibody exhibits for MOR (Ref. 40). Finally, a recent study reported that increased surface expression of DOR attenuated the maturation of MOR (Ref. 42). These factors could contribute to an underestimation of MOR–DOR colocalisation in vivo.
The dynamics underlying the formation of MOR–DOR heteromers, as well as the molecular mechanisms underlying their trafficking and surface expression, remains an issue of significant interest to the field. It has been hypothesised that GPCR heteromers are formed intracellularly before trafficking and insertion at the plasma membrane (Refs 39, 42). Early reports using cells that constitutively express MOR and where DOR expression was induced by ponasterone A treatment suggested that the formation of MOR–DOR complexes occurred at the cell surface and required interactions with Gi/o proteins (Ref. 43). This study, however, examined trafficking of biotinylated cell surface receptors and so could not examine whether the induced DOR was associated with pre-existing MOR (which is constitutively expressed) or with newly synthesised MOR nor did it examine colocalisation of MOR and DOR in various cellular compartments. Work from our own laboratory has showed that putative MOR–DOR heteromers are retained in the Golgi complex and require the presence of a specific receptor transport protein such as RTP4 (Ref. 42). This transport protein prevents ubiquitylation and proteasomal degradation of MOR–DOR complexes, leading to enhanced heteromer trafficking to the cell surface (Ref. 42). We further used antibodies that selectively recognise MOR–DOR heteromers to show that long-term morphine treatment leads to an increase in heteromer levels in cells endogenously expressing both receptors (Ref. 16). Taken together with a report showing that morphine could help rescue cell surface expression of mutant MOR (Ref. 44), our findings indicate that morphine can function as a pharmaco-chaperone that promotes MOR–DOR heteromer trafficking from the Golgi to the cell surface. This possibility is interesting in the light of studies showing that prolonged morphine treatment increases cell surface availability of DOR (Refs 23, 24, 25). Additional studies will be needed to further elucidate molecular mechanisms underlying maturation and trafficking of the MOR–DOR heteromers.
Although not much is known about the mechanism of MOR–DOR heteromer formation, there is strong molecular and immunohistochemical evidence showing that DOR is coexpressed with MOR in the same cellular environment. Cells expressing both MOR and DOR have been identified in the GABAergic neurons of the hippocampus (Ref. 45). Additionally, small DRG neurons were found to coexpress MOR and DOR at the single-cell level (Ref. 39) and functional studies indicated a role for MOR–DOR interaction in synaptic transmission and opioid analgesia (Ref. 39). Although the debate over the existence of MOR–DOR heteromers continues, the recent generation of tools such as monoclonal antibodies that selectively recognise MOR–DOR heteromers (Ref. 16) or of agents that disrupt the heteromer in vivo (Ref. 46) represents an important step towards the resolution of these findings. These important experimental results will be discussed in greater detail below.
Biochemical characterisation of the MOR–DOR heteromer
Numerous investigations have contributed to our current understanding of the biochemical characteristics of MOR–DOR complexes through the use of recombinant systems (reviewed in Ref. 7). These studies indicate that MOR–DOR heteromers show a pharmacological profile that is distinct from that of either MOR or DOR alone (Fig. 1). Such work has provided direct evidence for the formation of MOR–DOR heteromers, heteromer-induced alteration of ligand-binding properties, alteration of cyclic adenosine monophosphate (cAMP) regulation and signalling, and for changes in the induction of MAPK phosphorylation (Refs 34, 35, 36, 37). Furthermore, MOR–DOR heteromers have been found to couple to distinct signalling pathways (Refs 36, 47). One study reported that the MOR–DOR heteromer couples to pertussistoxin-insensitive inhibitory G protein (Gαz) (Ref. 36), whereas another study found that the heteromers are constitutively coupled to β-arrestin-2 (Ref. 48). Although these findings appear divergent, they are not necessarily in opposition, given the potential for the MOR–DOR heteromer to couple to and signal through different pathways in different brain regions and under a variety of different conditions. Indeed, such distinct coupling might be one of several ways in which MOR–DOR heteromerisation can contribute to alterations in the induction of tolerance in response to morphine and other opiate compounds.
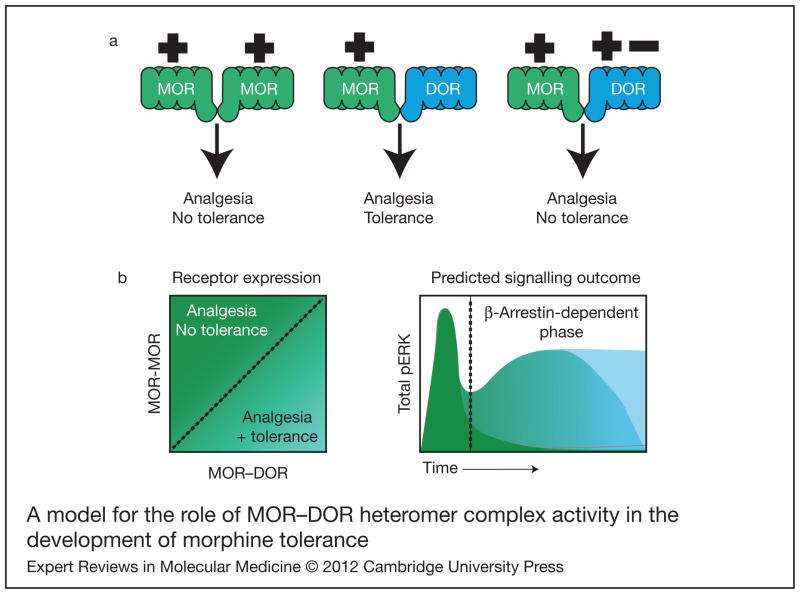
Figure 1. A model for the role of MOR–DOR heteromer complex activity in the development of morphine tolerance.
(a) Signalling of MOR in the absence of DOR (left pair), as found in Dor1 mutant mice, is not sufficient to the development of tolerance. The presence of DOR (centre pair) leads to the development of tolerance, but the occupancy of DOR (right pair) by agonist (+) or antagonist (−) in the context of MOR–DOR is sufficient to abrogate this effect. (b) Increased expression of MOR–DOR heteromer complexes produces a sustained increase in pERK that is associated with β-arrestin-dependent signalling and tolerance induction (dark green). Reduction of MOR–DOR pairs (blue/green) reduces the duration of pERK signalling and subsequent tolerance formation. When there is an abundance of MOR homomers (light blue), there is no secondary, sustained pERK generation over time and tolerance is not developed. Abbreviations: DOR, delta opioid receptor; MOR, mu opioid receptor; pERK, phosphorylated extracellular signal-regulated kinases.
The MOR–DOR heteromerisation-mediated switch from a G-protein to a β-arrestin-2-mediated signalling pathway (Ref. 47) is intriguing because β-arrestin-2 is found to colocalise at the plasma membrane in cells coexpressing MOR–DOR, but not in cells where either MOR or DOR were expressed alone (Ref. 47). Furthermore, β-arrestin-2 was found to coimmunoprecipitate with MOR–DOR only in cells coexpressing MOR and DOR (Refs 47, 48). Accompanying the shift from G-protein- to β-arrestin-2-dependent signalling were marked alterations in the temporal dynamics of extracellular signal-regulated kinases (ERK) phosphorylation in cells coexpressing MOR and DOR, relative to the kinetics observed for cells expressing only MOR. In particular, stimulation with the MOR-selective agonist, DAMGO, produced a slow, but sustained, increase in phosphorylated ERK (pERK) levels in cells coexpressing MOR and DOR; this is in contrast to a rapid, yet transient, increase in pERK levels in cells that expressed only MOR (Ref. 47). Of critical importance was the observation that the expression level of the MOR–DOR heteromer correlated directly to the duration of the sustained increase in pERK: as the ratio of MOR–DOR heteromers to individual receptor homomers increased, so did the duration of ERK phosphorylation. Additionally, it was shown that this sustained phase of pERK induction was a direct consequence of MOR–DOR heteromer signalling in a β-arrestin-2-dependent manner, because siRNA against β-arrestin-2 shifted the temporal dynamics and kinetics of DAMGO-mediated pERK formation to that observed in cells expressing only MOR (Ref. 47). These findings are relevant, considering that β-arrestin-2 has been implicated in modulation of analgesia and in the development of tolerance. In particular, studies have shown that β-arrestin-2-knockout mice show enhanced morphine-induced analgesia (Refs 49, 50) and do not develop tolerance to morphine (Refs 49, 50). Taken together, these studies suggest a role for the MOR–DOR heteromer in analgesia and tolerance induction through modulation of β-arrestin-2-dependent signalling pathways. Thus, the unique binding and signalling properties of the MOR–DOR heteromer (Refs 34, 35, 36, 37) suggest that it represents a novel functional unit distinct from either MOR or DOR alone and reveals the dynamic nature of signalling through these receptors, which might offer insights into the development of opiate-induced tolerance.
MOR–DOR heteromers and chronic opiate administration
Evidence suggests that there is an increased abundance of MOR–DOR heteromers in pathophysiological conditions such as chronic pain or subsequent to chronic exposure to opiate drugs such as morphine, relative to the normal or naive state (Ref. 16). In the case of morphine, several studies have previously demonstrated that chronic exposure increases the total abundance of opioid receptors (Refs 16, 23, 25). An increase in the colocalisation of MOR and DOR at the plasma membrane and within intracellular compartments was observed in cultured DRG neurons, following chronic exposure to morphine (Ref. 16). In addition, using an antibody that selectively recognises MOR–DOR heteromers, we showed that exposure of mice to escalating dose of morphine administration known to reliably induce both tolerance and physical dependence (Ref. 51) resulted in increased heteromer expression in both the medial nucleus of the trapezoid body (MNTB) and the RVM (Ref. 16), brain regions implicated in the processing of painful stimuli. The mRNA encoding MOR and DOR, as shown in the Allen Brain Atlas (http://www.brain-map.org/), exist in these brain regions, consistent with the presence of both proteins in these regions. Furthermore, an increase in the abundance of MOR–DOR heteromers was detected by ELISA in membranes prepared from cortex, MNTB, RVM, nucleus accumbens and the ventral tegmental area of animals subjected to chronic morphine treatment, compared with saline-injected controls (Ref. 16). Because the increases in MOR–DOR heteromer levels following chronic morphine administration are not observed in saline-injected controls or in animals acutely administered with morphine, this would suggest that upregulation of MOR–DOR heteromers represents a compensatory homeostatic response to chronic morphine-mediated signalling (Ref. 16).
New tools for the study of opioid heteromers in vivo
Many in vivo studies have focused on the induction of tolerance to morphine at the cellular level, because the action of morphine is unique from that of other opioid receptor agonists. Classical models predict that following receptor activation, opioid receptors are usually desensitised, endocytosed and then either recycled to the cell surface or targeted for degradation (Ref. 52). It is generally thought that cellular tolerance develops when the number of functional receptors at the cell surface is reduced, thereby limiting the cell’s ability to signal in response to a drug. However, morphine has been shown to elicit significantly less desensitisation and endocytosis of MOR (Ref. 53) even in tolerant animals (Ref. 54). Instead, tolerance to morphine has been attributed to superactivation of the cAMP signalling pathway (reviewed in Refs 55, 56); this is supported by studies showing that MOR mutations that increase morphine-mediated receptor endocytosis reduce cAMP superactivation and development of tolerance (Ref. 56). Studies have also reported that MOR–DOR heteromerisation induces alterations in protomer trafficking (Refs 42, 53), which might lead to changes in receptor availability, and as a result, contribute to the alteration of signalling pathways and regulation of the development of tolerance.
Findings to date strongly suggest that DOR receptor availability is required for tolerance induction. In vivo, the development of tolerance has been found to correlate with enhanced surface expression of DOR in neurons expressing MOR (Refs 24, 26, 57, 58). Indeed, an increase in MOR–DOR complex formation and subsequent increase in heteromer-specific signalling might accompany tolerance induction (Ref. 8). The development of MOR–DOR heteromer-specific agonists and antibodies that specifically recognise this heteromer has greatly contributed to the understanding of the role of MOR–DOR heteromers in tolerance.
Bivalent ligands of the MOR agonist–DOR antagonist series can be used to directly test the effect of MOR–DOR heteromer-selective activation on the development of tolerance (Refs 59, 60, 61). These bivalent ligands, containing an MOR agonist and a DOR antagonist pharmacophore bound by a linker bridge, were shown to suppress tolerance while preserving antinociceptive response in the tail-flick test (Refs 59, 60, 61). These findings support the concept of the MOR–DOR as a functional unit. Interestingly, varying the spacer length of these bivalent compounds was found to alter the profiles of tolerance and conditioned-place preference as well as the severity of side effects such as gastrointestinal transit (Refs 59, 60, 61), indicating that different mechanisms of heteromer complex activity might contribute to the development of morphine-induced behaviours.
Recently, our group generated a novel heteromer-specific antibody to directly assess the formation and function of MOR–DOR heteromers (Ref. 16). This antibody recognises an epitope found in membranes of wild type but not MOR- or DOR-knockout animals. Interestingly, this heteromer-specific antibody also selectively inhibits heteromer signalling, as indicated by its ability to block signalling potentiation of MOR agonist DAMGO by a low dose of the DOR-antagonist TIPPψ (Ref. 16). We used this MOR–DOR heteromer-specific antibody to assess the level of MOR–DOR in the brain following tolerance induction. The antibody detected enhanced levels of MOR–DOR in neurons after chronic morphine treatment (associated with tolerance induction), but not acute morphine treatment (not associated with tolerance induction). MOR–DOR heteromer abundance was increased in the RVM (Ref. 16), a brain region that has been implicated in the development of neuropathic pain, a condition characterised by supersensitivity to painful stimuli (hyperalgesia) and painful sensation by normally nonpainful stimuli (tactile allodynia) (Refs 62, 63). Interestingly, a recent study using in situ hybridisation, single-cell PCR and immunostaining demonstrated expression of both MOR and DOR in the small neurons of the DRG, which convey nociceptive signals to the spinal cord (Ref. 39). These findings indicate an abundance of MOR–DOR heteromers in neural regions associated with descending inhibitory pain pathways, which is of interest in the light of the unclear role of DOR in mediating pain sensitivity in the MOR-deficient mouse (Refs 30, 64).
Another recently developed tool for probing the functioning of the MOR–DOR heteromer is a peptide able to selectively target the heteromer (Ref. 46). In this study, the authors demonstrated that the heteromeric interaction between MOR and DOR could be disrupted in cultured DRG neurons by the expression of an interfering fusion protein, consisting of the first transmembrane region of MOR and the TAT peptide (Ref. 46). This novel peptide tool was used to show that the cotrafficking of MOR and DOR to postendocytotic degradation pathways that typically occur subsequent to DOR activation could be disrupted by expression of the fusion protein in DRG cells, in turn leading to the desensitisation of MOR (Ref. 46). More importantly, the small interfering peptide had the ability to disrupt MOR–DOR heteromer-mediated activity in the spinal cord and in small DRG neurons in vivo (Ref. 46). As a result of this disruption, animals treated with the small interfering peptide showed an enhanced analgesic response to morphine administration, as well as a concomitant reduction in tolerance to the drug (Ref. 46). Although these results are fascinating, it is clear that further study of MOR–DOR heteromers in preclinical models will be necessary to understand more fully the role of these receptor complexes in analgesia and tolerance induction.
MOR and DOR interactions with other analgesic receptors
Several heteromer-like interactions between MOR, DOR and other GPCRs have been observed and experimentally verified (Refs 8, 12, 13). Of specific interests are those heteromers associated with antinociception, because these receptor complexes might share signalling pathways in common with those of the MOR–DOR heteromer. Included among these complexes are heteromers consisting of the cannabinoid receptor type 1(CB1R) and either MOR or DOR (Ref. 65). Because cannabinoids are known to mediate analgesic effects, it is of clinical relevance to determine the capacity for both cannabinoid and opioid receptor activity to modulate pain relief without side effects.
Several studies suggested the possibility of interactions between CB1R and MOR (extensively reviewed in Ref. 66). In this section, we will describe a few of the studies supporting the possibility of CB1R–MOR interactions. Tetrahydrocannabinol (THC)-mediated activation of dopamine outflow from the nucleus accumbens can be blocked by the opioid antagonist, naloxone (Refs 67, 68). In studies examining the effects of morphine and THC on antinociception it was observed that nonanalgesic doses of THC enhanced the potency of morphine-mediated antinociception (Refs 69, 70) and that nonanalgesic doses of morphine could enhance THC-mediated antinociception (Ref. 71). In addition, administration of the CB1R antagonist, SR 141716A, precipitates withdrawal in morphine-dependent animals (Ref. 72). Interestingly, administration of either THC or the endogenous cannabinoid, anandamide, decreased the somatic signs of naloxone-precipitated withdrawal symptoms in mice chronically treated with morphine (Refs 73, 74, 75). Several studies demonstrated that the somatic signs of morphine withdrawal are markedly decreased in CB1R-knockout mice (Refs 74, 76), but conflicting results were reported in studies using MOR-knockout animals. One study reported no change in the severity of cannabinoid withdrawal effects (Ref. 77), whereas another reported a decrease (Ref. 74). Interestingly, the somatic signs of cannabinoid withdrawal were reduced in mice lacking both MOR and DOR (Ref. 78), which would suggest that these two receptors have a role in the development of tolerance to and dependence on cannabinoids. Conflicting results were also reported with studies examining CB1R levels following chronic morphine administration. One study reported that chronic morphine administration decreased CB1R density and function as measured using a G-protein activity assay (Ref. 79), whereas other studies reported either an increase (Refs 80, 81, 82) or no change in CB1R levels (Refs 83, 84). Nevertheless, the expression of CB1R was increased at the spinal level following chronic intrathecal administration of morphine (Ref. 85). Studies examining the localisation of mRNA encoding CB1R and MOR provide additional support for the possibility that these two receptors could interact as heteromeric complexes in several areas of the central nervous system, including the limbic system, mesencephalon, brain stem and spinal cord (Refs 86, 87). Furthermore, studies have reported colocalisation of CB1R and MOR in striatal GABAergic neurons (Refs 87, 88, 89), in areas of the dorsal horn of the spinal cord (Refs 86, 88, 90), as well as areas of the brain controlling nociceptive responses such as the periaqueductal grey, raphe nuclei and central-medial thalamic nuclei (Refs 91, 92, 93). Taken together, these studies are consistent with the hypothesis that CB1R and MOR interact and in some cases they could directly associate to form heteromers.
We used bioluminescence energy transfer assays to demonstrate that opioid and cannabinoid receptors are in close enough proximity (<10 nm apart) to form heteromeric complexes. We found that the CB1R agonist attenuated MOR-mediated signalling and this effect was reciprocal, in that MOR agonists could attenuate CB1R-mediated signalling (Ref. 94). Further support for the formation of functional CB1R–MOR heteromers comes from a study using coimmunoprecipitation, fluorescence resonance energy transfer assay and electrophysiology (Ref. 95).
In the case of CB1R–DOR heteromerisation, there are only a small number of studies showing interactions between these two receptors. In vitro studies using heterologous cells have shown that there is a crosstalk between CB1R and DOR at the level of signal desensitisation (Refs 96, 97, 98, 99, 100). In vivo studies showed that a DOR antagonist could block the anxiolytic activity of THC. Research on DOR-knockout mice shows increased levels and functional activity of CB1R in the substantia nigra (Ref. 101) and CB1R-knockout mice also show increased functional activity of DOR in the caudate putamen (Ref. 102). We have recently reported that CB1R and DOR directly interact to form heteromers, and this leads to the targeting of CB1R to the cell surface. We found that CB1R–DOR recruits distinct signalling complexes leading to the activation of a novel antiapoptotic signalling pathway that has a role in neuronal survival (Ref. 103). These results suggest that CB1R–DOR heteromerisation is involved in increasing the diversity of endocannabinoid signalling and indicate that heteromerisation has an important role in enhancing the repertoire of GPCR signalling.
Interactions between cannabinoid and opioid receptors have clinical relevance. The Institute of Medicine has reported that cannabis would be useful to treat pain, should there be synergistic interactions with opioid analgesics (Ref. 104). In fact, the cannabinoid receptor agonist, THC (Marinol/Dronabinol), is currently in phase III clinical trial as an add-on therapy for patients using opioids in the treatment of chronic pain (Clinical Trial Identifier: NCT00153192). This trial represents a novel use of Marinol, which is generally not indicated for chronic somatic nociceptive pain syndrome. Results from a phase I double-blinded, randomised, placebo-controlled study indicate that patients who received Marinol experienced decreased pain intensity compared with those who received placebo (Ref. 104). These results suggest that the use of cannabinoids might benefit patients who continue to experience pain despite treatment with opioid drugs. However, these studies were not designed to test for synergistic effects between the cannabinoid and opioid pathways, or to gauge the influence of cannabinoid receptor agonists on the development of tolerance to opioids. Thus, careful preclinical studies are needed to elucidate the existence and function of CB1R-opioid receptor complexes.
Summary and perspectives
MOR–DOR heteromers represent a novel signalling complex whose distinct pharmacological profile reveals the potential to induce analgesia without tolerance. Modulation of this receptor complex might significantly alter the way clinicians treat pain. Thus, discovery of nontoxic, bioavailable drugs that can mediate this effect in the clinic would be a major advance in the field of pain management. Furthermore, better understanding of the local effect of MOR–DOR heteromers will probably contribute to our knowledge of mechanisms underlying both tolerance development and regulation of peripheral pain.
Indeed, studies have shown that in addition to MOR–DOR, several types of opioid receptor heteromers as well as opioid-cannabinoid receptor heteromers exist. Although we have not discussed KOR–DOR heteromer formation and signalling (Ref. 105), or that of MOR–nociceptin (Ref. 106) and MOR–KOR (Refs 105, 107), many groups are working to provide evidence of the physiological and pathophysiological roles of these receptor complexes. Current understanding of these heteromers is yet to establish their active presence in a tissue- or disease-specific manner, although the development of new tools such as heteromer-selective agonists, antagonists and antibodies will greatly aid in the discovery of microenvironment-specific activity and function of these and other receptor complexes.
Acknowledgments
Acknowledgements and funding
L.A.D. is supported by NIH grants DA008863 and DA019521. C.M.C. is supported by NIH CTSA grant UL1RR029887 awarded to the Mount Sinai School of Medicine.
References
- 1.Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cellular and Molecular Neurobiology. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annual Review of Biochemistry. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 3.Chen YL, Law PY, Loh HH. The other side of the opioid story: modulation of cell growth and survival signaling. Current Medicinal Chemistry. 2008;15:772–778. doi: 10.2174/092986708783955518. [DOI] [PubMed] [Google Scholar]
- 4.Filbey FM, et al. Marijuana craving in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 6.Kieffer BL, Evans CJ. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology. 2009;56(Suppl 1):205–212. doi: 10.1016/j.neuropharm.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacological Reviews. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes I, et al. G protein coupled receptor dimerization: implications in modulating receptor function. Journal of Molecular Medicine (Berlin) 2001;79:226–242. doi: 10.1007/s001090100219. [DOI] [PubMed] [Google Scholar]
- 9.Satake H, Sakai T. Recent advances and perceptions in studies of heterodimerization between G protein-coupled receptors. Protein and Peptide Letters. 2008;15:300–308. doi: 10.2174/092986608783744207. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Maeso J. GPCR oligomers in pharmacology and signaling. Molecular Brain. 2011;4:20. doi: 10.1186/1756-6606-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes I, et al. G protein-coupled receptor heteromerization: a role in allosteric modulation of ligand binding. Molecular Pharmacology. 2011;79:1044–1052. doi: 10.1124/mol.110.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochemical Journal. 2011;433:11–18. doi: 10.1042/BJ20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends in Pharmacological Sciences. 2010;31:124–130. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams D, Devi LA. Escorts take the lead molecular chaperones as therapeutic targets. Progress in Molecular Biology and Translational Sciences. 2010;91:121–149. doi: 10.1016/S1877-1173(10)91005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakrabarti S, Liu NJ, Gintzler AR. Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20115–20119. doi: 10.1073/pnas.1009923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Science Signalling. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman RB, Westfall TC. Allosteric modulation by leucine-enkephalin of [3H]naloxone binding in rat brain. European Journal of Pharmacology. 1981;72:365–368. doi: 10.1016/0014-2999(81)90577-x. [DOI] [PubMed] [Google Scholar]
- 18.Lee NM, Smith AP. A protein-lipid model of the opiate receptor. Life Sciences. 1980;26:1459–1464. doi: 10.1016/0024-3205(80)90266-0. [DOI] [PubMed] [Google Scholar]
- 19.Lord JA, et al. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 20.Rothman RB, Westfall TC. Morphine allosterically modulates the binding of [3H]leucine enkephalin to a particulate fraction of rat brain. Molecular Pharmacology. 1982;21:538–547. [PubMed] [Google Scholar]
- 21.Barrett RW, Vaught JL. The effects of receptor selective opioid peptides on morphine-induced analgesia. European Journal of Pharmacology. 1982;80:427–430. doi: 10.1016/0014-2999(82)90090-5. [DOI] [PubMed] [Google Scholar]
- 22.Vaught JL, Rothman RB, Westfall TC. Mu and delta receptors: their role in analgesia in the differential effects of opioid peptides on analgesia. Life Sciences. 1982;30:1443–1455. doi: 10.1016/0024-3205(82)90558-6. [DOI] [PubMed] [Google Scholar]
- 23.Gendron L, et al. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. Journal of Neuroscience. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morinville A, et al. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. Journal of Neuroscience. 2003;23:4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill CM, et al. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. Journal of Neuroscience. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 27.Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Progress in Neurobiology. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 28.Billa SK, Xia Y, Moron JA. Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: study of mu-opioid and delta-opioid receptor expression at the synapse. European Journal of Neuroscience. 2010;32:625–631. doi: 10.1111/j.1460-9568.2010.07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickenson AH. Mechanisms of the analgesic actions of opiates and opioids. British Medical Bulletin. 1991;47:690–702. doi: 10.1093/oxfordjournals.bmb.a072501. [DOI] [PubMed] [Google Scholar]
- 30.Matthes HW, et al. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. Journal of Neuroscience. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walwyn W, et al. Delta receptors are required for full inhibitory coupling of mu-receptors to voltage-dependent Ca(2+) channels in dorsal root ganglion neurons. Molecular Pharmacology. 2009;76:134–143. doi: 10.1124/mol.109.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chengm PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Research. 1997;778:367–380. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in spinally projecting serotonergic and nonserotonergic neurons of the rostral ventromedial medulla. Journal of Comparative Neurology. 1999;404:183–196. doi: 10.1002/(sici)1096-9861(19990208)404:2<183::aid-cne4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 34.Gomes I, et al. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomes I, Filipovska J, Devi LA. Opioid receptor oligomerization. Detection and functional characterization of interacting receptors. Methods in Molecular Medicine. 2003;84:157–183. doi: 10.1385/1-59259-379-8:157. [DOI] [PubMed] [Google Scholar]
- 36.George SR, et al. Oligomerization of mu-and delta-opioid receptors. Generation of novel functional properties. Journal of Biological Chemistry. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 37.Gomes I, et al. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. Journal of Neuroscience. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherrer G, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang HB, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scherrer G, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang HB, et al. Distinct subcellular distribution of delta-opioid receptor fused with various tags in PC12 cells. Neurochemical Research. 2008;33:2028–2034. doi: 10.1007/s11064-008-9678-9. [DOI] [PubMed] [Google Scholar]
- 42.Decaillot FM, et al. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Law PY, et al. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. Journal of Biological Chemistry. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- 44.Chaipatikul V, et al. Rescuing the traffic-deficient mutants of rat mu-opioid receptors with hydrophobic ligands. Molecular Pharmacology. 2003;64:32–41. doi: 10.1124/mol.64.1.32. [DOI] [PubMed] [Google Scholar]
- 45.Stumm RK, et al. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. Journal of Comparative Neurology. 2004;469:107–118. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- 46.He SQ, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB Journal. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozenfeld R, et al. An emerging role for the delta opioid receptor in the regulation of mu opioid receptor function. Scientific World Journal. 2007;7:64–73. doi: 10.1100/tsw.2007.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bohn LM, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 50.Bohn LM, et al. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 51.Trang T, et al. Spinal administration of lipoxygenase inhibitors suppresses behavioural and neurochemical manifestations of naloxone-precipitated opioid withdrawal. British Journal of Pharmacology. 2003;140:295–304. doi: 10.1038/sj.bjp.0705440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lefkowitz RJ, et al. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Advances in Pharmacology. 1998;42:416–420. doi: 10.1016/s1054-3589(08)60777-2. [DOI] [PubMed] [Google Scholar]
- 53.He L, et al. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 54.Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiological Reviews. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 55.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 56.Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 57.Guan JS, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biological Psychiatry. 2009;65:169–174. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daniels DJ, et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenard NR, et al. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. European Journal of Pharmacology. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 61.Lenard NR, Roerig SC. Development of antinociceptive tolerance and physical dependence following morphine i.c.v. infusion in mice. European Journal of Pharmacology. 2005;527:71–76. doi: 10.1016/j.ejphar.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 62.Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 63.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in Neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 64.Sora I, et al. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bushlin I, Rozenfeld R, Devi LA. Cannabinoid–opioid interactions during neuropathic pain and analgesia. Current Opinion in Pharmacology. 2010;10:80–86. doi: 10.1016/j.coph.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robledo P, et al. Advances in the field of cannabinoid–opioid cross-talk. Addiction Biology. 2008;13:213–224. doi: 10.1111/j.1369-1600.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- 67.Chen JP, et al. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berlin) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 68.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 69.Cichewicz DL, McCarthy EA. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. Journal of Pharmacology and Experimental Therapeutics. 2003;304:1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- 70.Cichewicz DL, et al. Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. Journal of Pharmacology and Experimental Therapeutics. 1999;289:859–867. [PubMed] [Google Scholar]
- 71.Reche I, Fuentes JA, Ruiz-Gayo M. Potentiation of delta 9-tetrahydrocannabinol-induced analgesia by morphine in mice: involvement of mu- and kappa-opioid receptors. European Journal of Pharmacology. 1996;318:11–16. doi: 10.1016/s0014-2999(96)00752-2. [DOI] [PubMed] [Google Scholar]
- 72.Navarro M, et al. CB1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. Neuroreport. 1998;9:3397–3402. doi: 10.1097/00001756-199810260-00012. [DOI] [PubMed] [Google Scholar]
- 73.Valverde O, et al. Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. European Journal of Neuroscience. 2001;13:1816–1824. doi: 10.1046/j.0953-816x.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- 74.Lichtman AH, et al. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. Journal of Pharmacology and Experimental Therapeutics. 2001;298:1007–1014. [PubMed] [Google Scholar]
- 75.Vela G, Ruiz-Gayo M, Fuentes JA. Anandamide decreases naloxone-precipitated withdrawal signs in mice chronically treated with morphine. Neuropharmacology. 1995;34:665–668. doi: 10.1016/0028-3908(95)00032-2. [DOI] [PubMed] [Google Scholar]
- 76.Ledent C, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 77.Ghozland S, et al. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. Journal of Neuroscience. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castane A, et al. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knockout mice. European Journal of Neuroscience. 2003;17:155–159. doi: 10.1046/j.1460-9568.2003.02409.x. [DOI] [PubMed] [Google Scholar]
- 79.Vigano D, et al. Molecular mechanisms involved in the asymmetric interaction between cannabinoid and opioid systems. Psychopharmacology (Berlin) 2005;182:527–536. doi: 10.1007/s00213-005-0114-4. [DOI] [PubMed] [Google Scholar]
- 80.Rubino T, et al. Chronic treatment with a synthetic cannabinoid CP-55,940 alters G-protein expression in the rat central nervous system. Brain Research Molecular Brain Research. 1997;44:191–197. [PubMed] [Google Scholar]
- 81.Gonzalez S, et al. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug and Alcohol Dependence. 2002;66:77–84. doi: 10.1016/s0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- 82.Gonzalez S, et al. Region-dependent changes in endocannabinoid transmission in the brain of morphine-dependent rats. Addiction Biology. 2003;8:159–166. doi: 10.1080/1355621031000117383. [DOI] [PubMed] [Google Scholar]
- 83.Thorat SN, Bhargava HN. Evidence for a bidirectional cross-tolerance between morphine and delta 9-tetrahydrocannabinol in mice. European Journal of Pharmacology. 1994;260:5–13. doi: 10.1016/0014-2999(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 84.Romero J, et al. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse. 1998;30:298–308. doi: 10.1002/(SICI)1098-2396(199811)30:3<298::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 85.Lim G, Wang S, Mao J. Central glucocorticoid receptors modulate the expression of spinal cannabinoid receptors induced by chronic morphine exposure. Brain Research. 2005;1059:20–27. doi: 10.1016/j.brainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Salio C, et al. CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12:3689–3692. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- 87.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. Journal of Neuroscience. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hohmann AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 89.Pickel VM, et al. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Welch SP, Stevens DL. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. Journal of Pharmacology and Experimental Therapeutics. 1992;262:10–18. [PubMed] [Google Scholar]
- 91.Herkenham M, et al. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Research. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- 92.Mansour A, et al. Anatomy of CNS opioid receptors. Trends in Neurosciences. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 93.Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. Journal of Pharmacology and Experimental Therapeutics. 1996;276:585–593. [PubMed] [Google Scholar]
- 94.Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. British Journal of Pharmacology. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hojo M, et al. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. Journal of Pharmacological Sciences. 2008;108:308–319. doi: 10.1254/jphs.08244fp. [DOI] [PubMed] [Google Scholar]
- 96.Korzh A, et al. Modulation of extracellular signal-regulated kinase (ERK) by opioid and cannabinoid receptors that are expressed in the same cell. Brain Research. 2008;1189:23–32. doi: 10.1016/j.brainres.2007.10.070. [DOI] [PubMed] [Google Scholar]
- 97.Rubovitch V, Gafni M, Sarne Y. The involvement of VEGF receptors and MAPK in the cannabinoid potentiation of Ca2+ flux into N18TG2 neuroblastoma cells. Brain Research Molecular Brain Research. 2004;120:138–144. doi: 10.1016/j.molbrainres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 98.Shapira M, Gafni M, Sarne Y. Independence of, and interacEtions between, cannabinoid and opioid signal transduction pathways in N18TG2 cells. Brain Research. 1998;806:26–35. doi: 10.1016/s0006-8993(98)00697-0. [DOI] [PubMed] [Google Scholar]
- 99.Law PY, et al. Potentiation of opiate action in neuroblastoma N18TG2 cells by lipid incorporation. Molecular Pharmacology. 1982;21:492–502. [PubMed] [Google Scholar]
- 100.Dill JA, Howlett AC. Regulation of adenylate cyclase by chronic exposure to cannabimimetic drugs. Journal of Pharmacology and Experimental Therapeutics. 1988;244:1157–1163. [PubMed] [Google Scholar]
- 101.Berrendero F, et al. Cannabinoid receptor and WIN 55 212-2-stimulated [35S]-GTPgammaS binding in the brain of mu-, delta- and kappa-opioid receptor knockout mice. European Journal of Neuroscience. 2003;18:2197–2202. doi: 10.1046/j.1460-9568.2003.02951.x. [DOI] [PubMed] [Google Scholar]
- 102.Uriguen L, et al. Kappa- and delta-opioid receptor functional activities are increased in the caudate putamen of cannabinoid CB1 receptor knockout mice. European Journal of Neuroscience. 2005;22:2106–2110. doi: 10.1111/j.1460-9568.2005.04372.x. [DOI] [PubMed] [Google Scholar]
- 103.Rozenfeld R, et al. Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons. PLoS One. 2012;7:e29239. doi: 10.1371/journal.pone.0029239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Narang S, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. Journal of Pain. 2008;9:254–264. doi: 10.1016/j.jpain.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 105.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pan YX, Bolan E, Pasternak GW. Dimerization of morphine and orphanin FQ/nociceptin receptors: generation of a novel opioid receptor subtype. Biochemical and Biophysical Research Communications. 2002;297:659–663. doi: 10.1016/s0006-291x(02)02258-1. [DOI] [PubMed] [Google Scholar]
- 107.Wang D, et al. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Molecular Pharmacology. 2005;67:2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]