Abstract
Pancreatic cancer is a highly lethal disease that is difficult to diagnose and treat. The advances of proteomics technology, especially quantitative proteomics, have stimulated a great interest to apply this technology for pancreatic cancer study. A variety of tissue proteomics approaches have been applied to investigate pancreatic cancer and the associated diseases. These studies were carried out with various goals, aiming to better understand the molecular mechanisms underlying pancreatic tumorigenesis, to improve therapeutic treatment and to identify cancer associated protein signatures, signaling events as well as interactions between cancer cells and tumor microenvironment. Here, we provide an overview on the tissue proteomics studies of pancreatic cancer reported in the past few years in light of discovery and technology development.
Keywords: Proteomics, pancreatic cancer, mass spectrometry, biomarker, chronic pancreatitis, tissues, pancreas, formalin-fixed paraffin embedded (FFPE) tissue
Introduction
Pancreatic cancer is an almost uniformly lethal disease, and has the highest mortality rate of all major solid cancers [1,2]. Pancreatic ductal adenocarinoma represents the majority of pancreatic cancer, accounting for 80–90% of pancreatic cancer cases. While progress has been made in computed tomography, magnet resonance imaging, and endoscopic retrograde cholangiopancreatography, diagnosis of pancreatic cancer at an early, asymptomatic stage when the disease is surgically curable is still a challenging task. Furthermore, chemotherapy treatments have thus far failed to significantly improve the prognosis and survival rate of this deadly disease. The molecular mechanisms underlying the pancreatic tumorigenesis remain to be better understood for improved diagnosis and therapy.
Over the past decade, proteomics, as an emerging technology, has increasingly been applied in pancreatic cancer studies, offering a wide range of opportunities to investigate malignancy associated molecular alterations at protein level. Proteins are the essential functional biomolecules that participate in all sorts of biological processes and regulate many physiological activities. While cancer associated mutations at genomic level can affect protein characteristics and functions at various levels, the quantitative changes at the mRNA level do not simply correlate with protein expression in a linear fashion [3–5]. The emerging technology of proteomics has provided a unique and powerful approach allowing global protein identification and quantification in complex biological systems and to systematically reveal proteome changes implicated in tumorigenesis, including protein abundance, protein post-translational modifications (PTMs), protein complex and pathway interactions.
The advance in proteomics technology, especially quantitative proteomics, has stimulated a great interest in applying the technology to study pancreatic cancer. A large number of proteomics studies in this field have been reported in the literature, as summarized in a number of recent reviews [6–11], with various goals ranging from investigating the molecular mechanism of tumorigenesis to discovering protein biomarkers for diagnosis or therapeutic targets. These efforts have provided a wealth of information and have made use of a variety of sample types, including tissue, plasma/serum, pancreatic juice, cyst fluid and cell culture products. Among the different clinical specimens, tumor tissue is the most direct source to investigate protein alterations associated with pancreatic cancer. Pancreatic ductal adenocarcinoma is generally thought to arise originally from ductal epithelium. Recent evidences also support the important roles of other pancreas cell types (including pancreas stellate cells, acinar cells and endocrine cells) in the progression of pancreatic ductal adenocarcinoma. With this report, we attempt to assess the current status and challenge of tissue proteomics in pancreatic cancer study by surveying recent discoveries and technical developments in this area.
Quantitative proteomics techniques
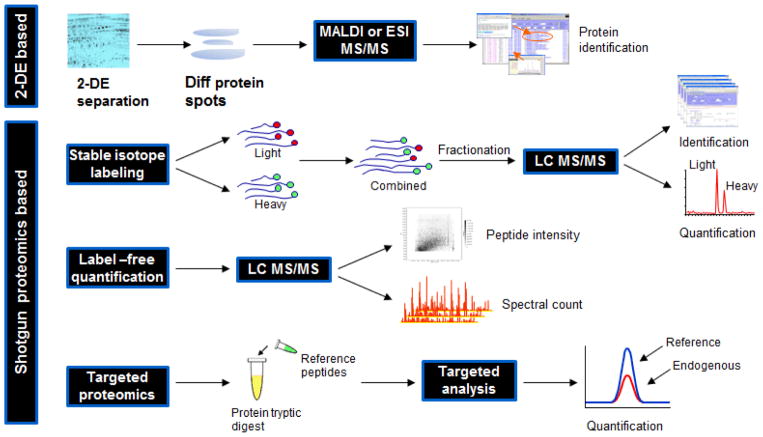
The most versatile and widely used proteomics approach is the “shotgun” proteomics or “bottom-up” approach [12–14], in which proteins are digested into peptides and analyzed by tandem mass spectrometry for peptide and protein identification. A typical proteomics pipeline usually consists of the following technical modules: sample preparation, protein/peptide separation, tandem mass spectrometric analysis and bioinformatic data analysis [13]. In cancer-associated studies, much of the interest is centered on identifying significant proteins, protein PTMs, protein interactions or pathways that are implicated in tumorigenesis. In such context, quantitative proteomics, which allows systematic identification and comparison of a static state or the perturbation-induced changes in proteomes between two or more biological systems, is frequently applied. Such an approach typically involves the comparison of cancerous tissue with control tissue, and is built on systematic registry of peptides and proteins in the tissue samples and at least semi-quantitative comparison of protein abundance between the disease and control tissues. With the enormous complexity of protein species and the substantial dynamic difference in protein abundance in tissue, it requires a concerted approach drawing from different technologies to accomplish a quantitative proteomics analysis. To reduce the sample complexity and enhance the analytical sensitivity, proteins extracted from tissue specimens are typically subjected to orthogonal fractionation and separation at either protein or peptide level, prior to mass spectrometric analysis. After tandem mass spectrometric analysis, the obtained MS/MS spectra are processed and searched against established protein database using database search algorithm, such as SEQUEST [15], MASCOT [16] or X!tandem [17] for peptide/protein identification, followed by peptide/protein quantification using a suite of bioinformatics software [18]. Different quantitative proteomics strategies are schematically illustrated in Figure 1 and discussed below.
Figure 1.
Schematic illustration of different strategies for quantitative proteomics analysis.
For shotgun proteomics, the most common approach for quantitative analysis utilizes stable isotope labeling to introduce differential mass tags, which present a distinct mass difference between the peptides with identical sequence but from different origins, for mass spectrometry identification and quantification. In such settings, proteins or peptides from different samples are labeled with different stable isotopic tags using a variety of methods, including chemical derivatization, metabolic incorporation and enzymatic reaction [13,19]. Among these labeling methods, chemical reaction is the most common approach for incorporation of stable isotopic labeling of proteins or peptides, providing great flexibility for quantitative proteomics analysis. From a mass spectrometry analysis standpoint, stable isotope label-based quantitative analysis can be categorized as “isotopic” or “isobaric”. The main difference between the two is that, the “isotopic” methods, such as ICAT [20] and ICPL [21], quantify peptides at the MS level using the ion current intensity of light and heavy forms of a peptide; and the “isobaric” methods, such as iTRAQ [22] and TMT [23], quantify peptides at MS/MS level using a comparison of the reporting peaks with different isotopic labeling.
Label-free approaches, generally considered less robust than label-based methods, have been increasingly used in the past few years, largely due to the advances in mass spectrometry technology providing higher mass accuracy and better reproducibility, and the development of computation solutions for label free analysis. Label-free quantification can be based on either peptide spectral count [18,24,25] or peptide ion current intensity [18,26,27]. The major caveat for the spectral count method is that it is less sensitive for detecting the quantitative difference between the low abundance proteins. Quantification based on peptide intensity requires more computational effort in data processing and sophisticated methods to normalize peptide intensity for each individual run, to infer peptide quantification for protein quantification and to establish an accurate mass and time (AMT) database to enhance peptide identification and quantification [28–31].
Unlike global scale quantitative protein profiling, which is an unbiased proteome wide analysis, targeted quantitative proteomics is a candidate-based technique that allows specific detection of selected analytes in a complex system. The technology is based on the concept of stable isotope dilution and uses isotopically labeled synthetic peptides mimicking endogenous targets as internal standards to achieve absolute quantification. In such an approach, a mass spectrometer is operated in a data-dependent acquisition mode, which is precisely directed on searching, identifying and quantifying the candidate peptides that are specifically targeted for interrogation in a complex background. Recent studies have demonstrated the potential of this technique for a range of applications for cancer study, including biomarker detection, monitoring specific protein pattern and targeted analysis of protein interaction [32–38]. While targeted proteomics analysis is commonly carried out on triple quadrupole instrument using Selected/Multiple Reaction Monitoring (SRM or MRM) technique, other mass spectrometers, such as MALDI TOF/TOF, ion trap, QT/OF [37] and triple TOF (SWATH) [39], have also been used.
Discovery of differentially expressed proteins in pancreatic cancer tissues
Many surgically-obtained tumor tissues include cancerous lesions and the adjacent stromal components. In pancreatic ductal adenocarcinoma, the solid tumor mass consists of cancer cells interspersed with activated stroma, which could make up to 90% of the tumor volume, including activated fibroblasts, myofibroblast, immune, neo-endothelial cells and extra cellular matrix [40]. Whether to use a whole tumor tissue or purified cancer cells for proteomics profiling depends on the purpose and scope of a study, and may critically impact the outcome of the proteomics results and biological conclusions. Tumor-host microenvironment, which participates in the induction, selection and expansion of the neoplastic cells, plays a pivotal role in malignancy [41,42]. The cross-talk between tumor cells and the surrounding microenvironment may induce production and secretion of stimulatory growth factors and cytokines by tumor cells and the surrounding stromal cells to recruit vasculature or suppress immune response for tumor development [41,43]. A tissue proteomics study of pancreatic ductal adenocarcinoma has evidenced that the stromal-epithelial interaction plays an important role in pancreatic tumorigenesis [44]. Differentially expressed proteins in pancreatic cancer were involved in protein-driven interactions between the ductal epithelium and the extracellular matrix that orchestrate tumor growth, migration, angiogenesis, invasion, metastasis, and immunologic escape, underscoring the importance of the tumor microenvironment in promoting pancreatic cancer progression.
Analysis of whole tumor tissue
Most of the earlier tissue proteomics studies of pancreatic cancer focused on global comparison of the proteome of pancreatic cancer tissue with healthy controls, and have been well discussed in several recent reviews [6,7,9,10]. These studies include 2-DE based [45,46] and ICAT-based [44] quantitative proteomics investigations of pancreatic adenocarcinoma. Despite the limitations of the low-resolution mass spectrometer used, these earlier studies have identified several important proteins that are associated with pancreatic tumorigenesis, including galectin-1 [44,46], gelsolin [44], lumican [44], 14-3-3 protein sigma [44], cathepsin D [44–46], cofilin [44], moesin [44], and plectin-1 [44]. Gelsolin and lumican were later tested in plasma and showed 80% sensitivity at 95% specificity as a composite biomarker in separating the early stage pancreatic cancer patients (stage 1 and 2) from healthy controls and patients with chronic pancreatitis using SRM based targeted proteomics assays [47]. Plectin-1 has been developed into a molecular imaging agent for identifying primary and metastatic pancreatic cancer [48,49]. Cofilin and its isoforms were recently further characterized for their roles in association with pancreatic cancer [50]. Moesin was found with elevated expression in pancreatic cancer with lymph node metastasis compared to pancreatic cancer without lymph node metastasis in addition to c14orf166 and radixin [51]. 14-3-3 sigma was also found up-regulated in the malignant epithelia of lymph node metastases relative to primary pancreatic ductal adenocarcinoma [52], and may play a role in modulating pancreatic cancer cell survival and invasiveness [53]. Galectin-1 was characterized as a functional receptor of tissue-type plasminogen activator (tPA), which was involved in pancreatic cancer progression [54]. More recently, galectin-1 was found with a new role associated with pancreatic cancer survival, with a significantly decreased expression observed in very long term pancreatic cancer survivors, as well as those at the higher end of the survival spectrum following pancreatic cancer resection [55]. In addition to galectin-1, the same study also indicated the association of prolargin (PRELP) and osteoglycin (OGN) with pancreatic cancer survival, both proteins play a role in inhibition of metastasis [55].
Pancreatic Intraepithelial neoplasia (PanIN) is the precursor of pancreatic adenocarcinoma, and PanIN 3 is believed to be the most clinically relevant stage for early detection of curable pancreatic neoplasia. Using both iTRAQ and ICAT techniques, a recent study carried out quantitative proteomics investigation of the tissue proteome of PanIN 3 lesion in comparison to the healthy control, and patients with chronic pancreatitis and pancreatic cancer [56]. The study found that many dysregulated proteins in PanIN 3 lesion were also dysregulated in pancreatic cancer tissue concurrently, suggesting that the dysregulation at protein level may start early before cancer invasion. Among the >200 dysregulated proteins in PanIN 3, the top enrichment protein category is cell motility, which involves many cytoskeleton proteins. This early mobility and invasive behavior of PanIN 3 cells occurring prior to cancer formation was recently documented in an engineered mouse model of pancreatic cancer [57]. It is gratifying to see that the regulatory and signaling pathways invoked in proteomic discovery are clinically relevant in the biologic models of the disease.
Several overexpressed proteins in PanIN 3 were discussed in the report, including laminin beta 1, 14-3-3 theta, decorin, galectin-1, vimentin, and actinin-4 [56]. The follow-up validation studies using immunohistochemistry (IHC) revealed the overexpression of galectin-1 and laminin in the stroma adjacent to PanIN 3; and the overexpression of actinin-4 in both the malignant epithelium and surrounding stroma of pancreatic adenocarcinomas [56]. The overexpression of these proteins may represent a response of the host microenvironment due to the neoplasia. It is notable that in a different study, vimentin, along with fructose-bisphosphate aldolase A and alpha-smooth muscle actin, were also found overexpressed in pancreatic cystadenomas [58].
Chronic pancreatitis is a chronic inflammatory disorder of pancreas, which shares many clinical features with pancreatic cancer [59–61]. The initial attempt to compare the differential proteins expressed in the tissue specimens from patients of pancreatic cancer and chronic pancreatitis was made using immunoblotting analysis [62]. More recently, mass spectrometry-based large scale quantitative proteomics investigations have been carried out to compare the tissue proteome of these two diseases [56,63,64]. The ICAT based quantitative proteomics investigation indicated that about 40% of the 116 differentially expressed proteins identified in chronic pancreatitis were also involved in pancreatic cancer [63]. Among the proteins validated using either IHC or western blotting (WB), annexin A2 and insulin-like growth factor-binding protein 2 were overexpressed in cancer but not in chronic pancreatitis. On the other hand, cathepsin D, integrin 1, and plasminogen were overexpressed in both pancreatic cancer and chronic pancreatitis [63]. In a different study focusing on analyzing the tissue proteome of pancreatic intraepithelial neoplasia, more than 25% of the overexpressed proteins identified in PanIN tissue were also overexpressed in chronic pancreatitis [56]. More recently, a study to reveal the common molecular events associated with pancreatic cancer and chronic pancreatitis was reported using formalin-fixed paraffin embedded (FFPE) tissue and a label-free quantitative proteomics approach [64]. The study involved the quantitative comparison of the tissue proteome of histologically-graded chronic pancreatitis lesions at different stages of chronic inflammation to normal pancreas and pancreatic cancer, evidencing that more than 50% of the differential proteins in chronic pancreatitis were concurrently differentially expressed in pancreatic cancer. The results further evidenced the common molecular events implicated in both diseases and will be discussed in more details in the following section. These studies clearly demonstrated that, at proteome level, chronic pancreatitis and pancreatic cancer share many molecular signatures and highlight the long-standing adage that cancer is a wound that doesn’t heal [65].
Analysis of Formalin-Fixed Paraffin-Embedded (FFPE) tissue
While most tissue proteomics studies used surgically obtained snap-frozen tissue specimens, which are less accessible and require stringent criteria for handling and storage, FFPE tissues have been increasingly used for proteomics study. Proteins and peptides can be extracted from FFPE tissues for shotgun proteomics analysis, as illustrated in Figure 2. A number of studies [66–68] have suggested that for a given analytical sensitivity, the use of FFPE tissue provided comparable identification of proteins to snap-frozen tissue using “shotgun” proteomics. With the range of current proteomics technology, the use of FFPE tissue for proteomics studies has proven to be effective in identifying some disease associated proteins [69–73]. In a recent study, FFPE tissues were used to investigate differential proteins associated with mild and severe chronic pancreatitis in comparison with normal pancreas and pancreatic ductal adenocarcinoma [64]. In the study, the FFPE tissues were de-paraffinized and rehydrated, and the disease lesions were carefully dissected for proteomics analysis. Using a label-free quantitative proteomics approach, the study identified 87, 217 and 298 differential proteins in mild chronic pancreatitis, severe chronic pancreatitis and pancreatic cancer, respectively, compared to normal pancreas tissue. Several groups of proteins were found that behave similarly in both severe chronic pancreatitis and pancreatic cancer, including: 1) the abundance of a group of digestive enzymes were decreased in pancreatitis and cancer, 2) proteins related to the extracelluar matrix and stellate cells were elevated, 3) glycoproteins were significantly enriched among the over-expressed proteins, 4) inflammatory proteins were up-regulated. Three up-regulated proteins, lumican, versican and Col14A1 were confirmed using IHC. The findings also suggested that several molecular events, including acute phase response, prothrombin activation and pancreatic fibrosis/pancreatic stellate cell activation, were commonly shared between chronic pancreatitis and pancreatic cancer, while metabolic changes were significantly associated with pancreatic cancer only. In a different study, non-quantitative proteomics was applied to identify proteins in FFPE tissue specimens from patients with intraductal papillary mucinous neoplasm (IPMN). In total, 523 proteins were identified and the IHC analysis showed over-expression of two proteins: deleted in malignant brain tumors 1 (DMBT1) and tissue transglutaminase 2 (TGM2), in IPMNs [74]. The availability of FFPE tissue specimens for proteomics study has not only provided a rich resource of clinically and pathologically well-defined tissue specimens, but also allows a direct correlation of histological observation with proteomics analysis – a great advantage for clinical proteomics studies.
Figure 2.
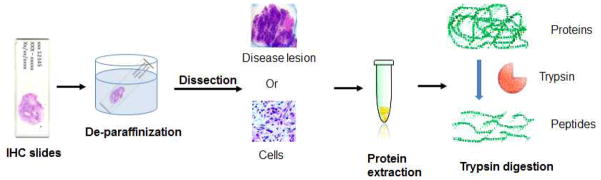
Extraction of proteins/peptides from FFPE tissues for shotgun proteomics analysis.
Functional proteomics analysis
In addition to the non-biased global quantitative tissue proteomics studies, functional proteomics has been used to investigate enzyme activities associated with pancreatic neoplasia [75]. Using an activity-based proteomics approach, the study investigated active serine hydrolases in the soluble pancreas tumor proteome with a fluorophosphonate probe and identified retinoblastoma-binding protein9 (RBBP9) as a tumor-associated serine hydrolase. The study indicated that while the expression of this protein was equivalent between normal and malignant specimens, its activity was elevated in pancreatic carcinomas.
Analysis of isolated neoplastic cells
Analysis of purified cancer cells can provide information that is directly relevant to biologic processes within the cancer cells. There are a variety of approaches for cancer cell enrichment. Laser capture microdissection (LCM) technique works well with both snap-frozen and FFPE samples to isolate neoplastic cells from tumor tissue and has been used in a number of tissue proteomics studies [76–78]. One study employing LCM and 2-DE to compare the proteomes of normal and malignant pancreatic ductal epithelial cells was able to isolate about 50,000 epithelial cells for analysis [79]. The study found nine protein spots which were differentially expressed in malignant epithelial cells, including S1006 [79]. More recently, a study applied LCM coupled with LC MS/MS to investigate the proteome of primary tumours of pancreatic ductal adenocarcinoma in comparison of matched lymph node metastases using FFPE tissues [52]. In the study, the analysis of the malignant epithelia isolated by LCM identified 115 differentially expressed proteins using label-free approach. The IHC analysis further confirmed the up-regulation of S100P and 14-3-3 sigma in lymph node metastases relative to primary pancreatic ductal adenocarcinoma [52]. When sample quantity is an issue, primary culture and xenograft transplantation of tumor cells may be options for expanding the number of cancer cells, however rigorous analysis is required to ensure that the cells for proteomics analysis are derived from the specific tumor and species of origin. Additionally, it is known that cell culture may result in some changes in protein expression and posttranslational modifications. Another approach to obtain isolated epithelial cells is the immuno-affinity based approach, which uses epithelial specific surface antibody to capture epithelial cells using fluorescence activated cell-sorting (FACS) or solid phase immobilized monoclonal antibodies to separate targeted cancer cells from cellular slurry of whole tumor tissue [80]. A study using magnetic beads coupled to epithelial cell surface antigen Ber-Ep4 obtained a large quantity of epithelial cells (>95% purity) from fresh human tissues for subsequent 2-DE analysis [81]. The study also suggested that adding RNase inhibitors to the protocol enabled the enriched samples for both proteomics (Western Blot, 2-DE) and transcriptomic studies (RT-PCR, cDNA microarray).
Analysis of isolated stromal cells
Pancreatic tumor cells are notorious for their exuberant desmoplastic stroma, which can make up the majority of the tumor mass. In addition to being a barrier to intra-tumor perfusion, the dense stroma plays important mechanistic roles in the progression of pancreatic cancer. Accumulated evidence indicates that cancer cells can activate their surrounding stroma by multiple signaling pathways. The crosstalk of neoplastic cells and stroma components can stimulate activation of surrounding fibroblasts to cancer-associated myofibroblasts, and recruitment of immunologic and neovascular endothelial cells which jointly promote cancer progression, invasion and metastasis. Quantitative proteomics analysis of whole pancreatic cancer tissues had previously underscored the importance of the host microenvironment [44]. To further elucidate the role of stromal cells in pancreatic tumorigenesis, one study investigated protein expression in stromal cells surrounding malignant pancreatic ductal cells using 2-DE [82]. Stromal cells were isolated from malignant pancreatic tissue by LCM and compared to the counterparts isolated from benign pancreatic tissue. The study found high expression of S100A8 and S100A9 in tumor-associated stroma but not in benign or malignant epithelia. The cells that were strongly positive for S100A8 or S100A9 were indeed immunologic cells in the cancer stroma.
Analysis of stem cells
Cancer stem cells represent a small fraction of heterogeneous tumor cells that are exclusively tumorigenic and essential drivers for tumor progression and metastasis. In pancreatic cancer, a subpopulation of pancreatic tumor cells with cell surface markers CD44+CD24+ESA+ has been identified as pancreatic cancer stem cells [83]. Functional studies verified that this subpopulation possessed the ability of self-renewal and producing differentiated progeny. The challenge of performing global proteomics analysis of pancreatic stem cells is the extremely low quantity of stem cells that could be obtained for analysis. Aiming to overcome this problem, a recent study employed offline capillary isoelectric focusing and LC-MS/MS to analyze protein lysate derived from ~10,000 cancer stem cells (about 1 ug of total protein) isolated from xenograft tumors in mice [84]. The study identified 169 differentially expressed proteins in pancreatic cancer stem cells in comparison with a nontumorigenic tumor cell sample using label-free approach. Among these differential expression proteins, signaling pathways related to apoptosis, cell proliferation, inflammation, and metastasis were significantly involved in the cancer stem cells. Emerging evidence has suggested that cancer stem cells are highly resistant to chemo- and radiotherapy resulting in their relative enrichment during treatment and rapid relapse of disease [85]. Many research interests have now been directed on in-depth characterization of these cancer stem cells in order to provide novel treatment modalities for fighting this deadly disease [85].
Mass spectrometry imaging of tissue
Mass spectrometry based tissue imaging allows the investigation of spatial distribution of a targeted molecule (such as protein, peptide, lipids, drugs, and metabolites) in intact tissue sections. It provides important insights into biological processes because the native distribution of various proteins are minimally disturbed, and histological features remain intact throughout the analysis [86]. Several studies have recently been reported demonstrating the MALDI MS imaging analysis of pancreas tissue. One study performed imaging analysis of pancreas to investigate peptide expression patterns in a mouse model of diabetes and obesity, and observed that the distribution of C-peptide of insulin was significantly increased in the obese mice, reflecting more and larger islets, a typical characteristic in that mouse model [87]. A different study used on-tissue reduction followed by MALDI-MS imaging to identify insulin B chain peptides and localize pancreatic islets of langerhans in human pancreas tissue specimens [88]. MALDI MS imaging was also used to analyze FFPE human pancreatic cancer tissue sections for identification and localization of glucose-regulated protein 78 kDa (Grp78) in-situ using ion mobility mass spectrometry [89]. When comparing the IHC staining images and the MALDI-IMS-MS images obtained from the tumor tissue sections, Grp78 was found mainly located in the tumor region. The MALDI images were found to be in good concordance with images obtained by the companion IHC study [89]. With the continuous advancement of the technology, it is foreseeable that mass spectrometry imaging could be applied in clinical settings in conjunction with histopathological evaluation in the future.
Cell lines as in-vitro model systems
Cell lines as in-vitro model systems offer great advantages in hypothesis-driven investigations and facilitate experimental manipulation of the systems that can not be achieved with human specimens. Recent proteomic investigations using pancreatic cancer cell lines include identification and characterization of cancer-associated receptors, microRNA targets, signaling pathways, as well as drug resistance targets. While cancer cell lines offer convenient ways to systematically study cancer in-vitro, interpretation of the results derived from long-established cell lines should be cautious as cancer cell lines which are under continuous tissue culture may not accurately reflect the cancer cells that were present in the originating organ of the patient.
Analysis of stellate cells
One of the cellular components of the pancreas parenchyma is the stellate cells. In the normal pancreas, pancreatic stellate cells are located in close proximity to the basal aspect of pancreatic acinar cells and remain in a quiescent state. In response to pancreatic injury, quiescent pancreatic stellate cells become activated with myofibroblast-like phenotype and secrete excessive amounts of the extracellular matrix (ECM) proteins that comprise fibrous tissue [90]. It is now known that stellate cells are important mediators in chronic pancreatitis and pancreatic cancer pathogenesis. Recent evidence from animal experiments suggests that pancreatic stellate cells can promote local tumor growth and metastatic spread and can also increase resistance to chemo and radiation treatment [90]. Investigating the proteome difference between activated and quiescent stellate cells may provide valuable insights into the development of chronic pancreatitis and pancreatic cancer [91]. In such a study, the immortalized mouse pancreatic stellate cell line at activated stage was compared to a pseudo-quiescent stage using LC-MS/MS coupled with label-free analysis. The study revealed several hundred proteins that were differential in abundance between the two different cell states [91]. Proteins that were more abundant in the activated stage included cytoskeletal proteins and ribosomal proteins, while those that were more abundant in pseudo-quiescent stage included proteins involved in protein degradation-related pathways (lysosome, ubiquitin-mediated proteolysis, and the proteasome). Despite the limitations of using immortalized cell line and a serum–starved pseudo-quiescent stage, the study provided the first analysis of the proteome differences in activated versus non-activated stellate cells.
Identification of receptors
Proteins typically exert their function in protein complexes within or outside the cell. Mass spectrometry combined with affinity purification has become a powerful tool for studying protein complexes, identifying protein binding partners or receptors. Tissue-type plasminogen activator (tPA) is involved in the degradation of extracellular matrix and activation of growth factors in the processes of tissue remodeling, cell migration and tumor invasiveness [92]. To identify putative receptors for tPA, a proteomic approach employing affinity capture (antibody pull-down) followed by 2-DE was used to identify proteins pulled down from either total lysates or raft membrane fractions from pancreatic cancer cell lines, in comparison with those from a total lysate of an endothelial cell line used as a non-pancreatic cancer control [93]. The study identified 31 proteins, including annexin A2, which was known as a tPA receptor in both pancreas and endothelial cells [94]. In a follow-up study from the same group, one of the putative tPA receptors, galectin-1, was characterized and validated as functional receptor that participated in pancreatic cancer progression [54]. The interaction of tPA and Gal-1 was direct, specific, and of high affinity. Down-regulation of galectin-1 abolished the effects of tPA on extracellular signal-regulated kinase 1/2 activation, cell proliferation, and invasion.
Study of chemo- and drug- resistance
Pancreatic cancer is a fatal disease and commonly acquires resistance to chemotherapy. Proteomics identification of significant proteins that are associated with chemo- or drug- resistance may open an avenue to develop therapeutic targets to improve current pancreatic cancer treatment. Several studies have applied proteomics to investigate gemcitabine resistance in pancreatic cancer using cellular models. The comparison of the proteome of gemcitabine-sensitive and -resistance cell lines using 2-DE and mass spectrometry has identified a subset of proteins associated with gemcitabine-resistance, highlighted by heat shock protein 27 (HSP27), whose over-expression was related to gemcitabine induced drug resistance [95–98]. Using 2D-DIGE and MALDI-TOF mass spectrometry, another study suggested that gemcitabine-resistance of PANC-1 pancreatic cancer cells might involve tumor suppressor protein p53 [99]. In addition to gemcitabine, a study investigated the anticancer compound 2-methoxyestradiol in drug resistant pancreatic cancer cell line using 2-DE-based proteomics [100]. The study indicated that the up-regulation of mitochondrial protein manganese superoxide dismutase (SOD2) might play an important role in acquiring resistance to anticancer drugs that are based on reactive oxygen species (ROS)-induced mitochondrion-dependent apoptosis. Other study investigated the development of thermoresistance of pancreatic cell lines that are with or without multi-drug resistance using 2-DE and MADLI-TOF, and has identified sets of differential proteins associated with thermoresistance of pancreatic cells [101]. Many of these differential proteins belong to the group of molecular chaperones including heat shock proteins and calcium-binding proteins, and the proteins involved drug detoxification activities.
Identification of microRNA targets
MicroRNAs (miRNAs) are about 22-nucleotide-long RNAs that are important regulators of gene expression. They act by leading the target mRNA for degradation or inhibiting the translation process. The typical experimental approach for identifying microRNA targets involves identifying dysregulated mRNA or proteins when a specific miRNA is over-expressed and/or knock-downed. Stable isotope labeling by amino acids in cell culture (SILAC)-based quantitative proteomics has been used in the identification of microRNA targets in cell lines. miR-143 is frequently down-regulated in several cancers including pancreatic cancer. To identify target proteins of miR-143, one study analyzed the dysregulated proteins after over-expressing miR-143 in pancreatic cancer cell line MiaPaCa2, which has a low endogenous expression of miR-143 [102]. Using SILAC-based quantitative proteomics, the study identified 94 putative targets of miR-143, including 93 down-regulated and one up-regulated protein following transfection of a miR-143 mimic. Using luciferase assays, a subset of these putative targets was validated to be the direct targets of miR-143 [102].
Invadopodia component
Invadopodia are dynamics protrusions of tumor cells and transformed cells which mediate proteolysis of extra cellular matrix constituents, including fibronectin, laminins and collagens [103]. Proteolytic degradation of the extra cellular matrix is an essential property of cancer cells, enabling them to invade through the surrounding tissue. Proteomics techniques are well-suited to investigate the molecular components of these invadopodia in order to dissect signaling pathways and activity in invadopodia. In a study to investigate fibroblast activation by palladin in the setting of pancreatic cancer, it was found that overexpression of palladin could induce activation of stromal fibroblasts into a myofibroblast phenotype accompanied by increased invasion capacity [104]. A study showed that palladin was overexpressed in the cancer-associated fibroblasts in 96% of pancreatic cancer patients [105]. To unravel the mechanism underlying the invasive capability of the palladin-expressing fibroblasts, the invadopodia of the palladin-activated fibroblasts, which had become more prominent with the transition into myofibroblasts, were analyzed by proteomics analysis [104]. The invadopodia that invaded through the matrix-covered pores from palladin over-expressing fibroblasts were isolated and analyzed using SILAC-based quantitative proteomics. Over 200 proteins were differentially expressed in the invadopodia of palladin-expressing fibroblasts relative to those of control cells. These differentially expressed proteins included known invadopodia proteins, ras-related and GTP binding proteins, and proteolytic enzymes. The enhanced capacity of extracellular matrix degradation in palladin-overexpressing cells was likely due to the increased proteolytic enzymes in those invadopodia.
Signaling pathway
Cancer could be considered as a disease of abnormal signal transduction. Targeting the aberrant signaling pathways driving tumorigenesis represents a promising strategy for cancer treatment. To identify aberrantly activated tyrosine kinase signaling pathways in pancreatic cancers, a systematic study was carried out using SILAC-based quantitative proteomics to compare tyrosine phosphorylated proteins from a pancreatic cancer cell line with a control pancreatic epithelial cell line [106]. Anti-phosphotyrosine immuno-precipitation was used to enrich the proteins for phosphotyrosine profiling. The enriched tyrosine phosphoproteins were resolved using SDS-PAGE and the resulting protein bands were tryptic digested and analyzed by LC MS/MS. The study found hyper-phosphorylation of epidermal growth factor (EGF) receptor and many of its pathway substrates in a low passage pancreatic cancer cell line. At least 14 downstream substrates of EGF receptor were found to be hyper-phosphorylated. Pancreatic cancer xenografts of pancreatic cancer cells with activated EGF receptor showed a dramatic response to erlotinib (small molecule targeting EGF receptor) as evidenced by near complete reduction of tumor [106]. In light of the results, activated EGF receptor (pEGFR1068) could potentially be a companion therapeutic biomarker to select patients who are most likely to respond to EGF receptor inhibitors.
Oxythiamine, a transketolase inhibitor, is known to inhibit cancer cell growth by suppression of the cell cycle. To understand the molecular mechanisms that mediates the inhibitory effect of oxythiamine treatment, a study used 2-DE to investigate the cellular phosphoproteome in a pancreatic cancer cell line after oxythiamine treatment [107]. The overall cellular phosphorylated proteins were reduced and 12 phosphoproteins were down-regulated by the drug treatment. Among them, phosphorylated heat shock protein 27 (HSP27) was substantially inhibited by oxythiamine. Phosphorylation at serine 78 of HSP27 was further shown to be the only phosphorylation site affected by drug treatment. The finding of deactivation of phosphor HSP27 by oxythiamine from this study supports and expands the concept that phosphorylated Hsp27 could be used as a biomarker for drug resistance in pancreatic cancer cells [108].
Conclusion
Pancreatic tumor tissues and cancer cell lines represent the most direct sources to investigate pancreatic tumorigenesis and discover biomarkers for clinical applications. Tissue proteomics investigations of pancreatic cancer reported in the past few years have provided important information to shed light on the functional molecular events underlying this deadly disease. Some of the novel differentially-expressed proteins discovered in the profiling studies are now under further investigation to better define their biological significance in pancreatic tumorigenesis and their utility in clinical applications. The review of these studies also clearly reveals the important role of the technology in interrogating the complexity of the tissue proteome. As an emerging technology, important issues, including scope of proteome coverage, the reproducibility between different methodologies and definition of sample sources remain to be improved. A variety of sources can affect the outcome of a tissue proteomics experiment, especially those for quantitative analysis, including specimens used, sample handling and storage, sample preparation procedure, quantitative methods, protein/peptide separation, mass spectrometric analysis as well as database search. These factors need to be clearly defined and stated while a proteomics study is described. In tissue proteomics, investigating whole disease lesion may represent different emphasis than single cell type, thus the obtained data may contain different biological messages. Even in a well-designed and executed tissue proteomics experiment, the obtained differential proteins represent a complex and convoluted outcome of multi-factors. In addition to the proteome alterations mechanically associated with pancreatic malignancy, other contributors may include the nature of the samples, biological heterogeneity, perturbations induced or stimulated by the associated diseases. Moreover, many proteins and pathways may be multi-functional among pancreatic cancer and the associated diseases, such as chronic pancreatitis and type-2 diabetes. A comprehensive interpretation of a tissue proteomics dataset, even if it is well-defined, may require more information than the dataset itself. While tissue proteomics profiling provides a high throughput survey to reveal the steady or induced perturbations in the proteome of a pancreatic cancer specimen, the work to follow up the meaningful leads may be enormous, and research interests have increasingly been directed on these studies. Functional proteomics, targeted proteomics, system biology approaches and other molecular techniques are quickly emerging and have been applied to facilitate proteomics study of pancreatic cancer. To date, despite the current technical challenges, tissue proteomics studies of pancreatic cancer have laid important groundwork in this field and may offer guidance to future experimentation. Recognition of the challenges and achievements presented by these studies could help us better define the role of proteomics technology and facilitate our future effort in battling pancreatic cancer.
Acknowledgments
We are grateful to the supports from the National Institutes of Health under grants K25CA137222, R01CA107209, R21CA161575, K07CA116296, R01DK081368 and R21CA149772, and gifts from the Canary Foundation and Gene and Mary Ann Walters Pancreatic Cancer Foundation.
References
- 1.Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012 doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.de Godoy LM, Olsen JV, Cox J, Nielsen ML, Hubner NC, Frohlich F, Walther TC, Mann M. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature. 2008;455:1251–1254. doi: 10.1038/nature07341. [DOI] [PubMed] [Google Scholar]
- 4.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK, Bumgarner R, Goodlett DR, Aebersold R, Hood L. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science. 2001;292:929–934. doi: 10.1126/science.292.5518.929. [DOI] [PubMed] [Google Scholar]
- 6.Cecconi D, Palmieri M, Donadelli M. Proteomics in pancreatic cancer research. Proteomics. 2011;11:816–828. doi: 10.1002/pmic.201000401. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Pan S, Aebersold R, Brentnall T. Proteomics studies of pancreatic cancer. Proteomics Clin Appl. 2007;1:1582–1591. doi: 10.1002/prca.200700414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Pan S, Brentnall TA, Aebersold R. Proteomic profiling of pancreatic cancer for biomarker discovery. Mol Cell Proteomics. 2005;4:523–533. doi: 10.1074/mcp.R500004-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Grantzdorffer I, Carl-McGrath S, Ebert MP, Rocken C. Proteomics of pancreatic cancer. Pancreas. 2008;36:329–336. doi: 10.1097/MPA.0b013e31815cc452. [DOI] [PubMed] [Google Scholar]
- 10.Sun C, Rosendahl AH, Ansari D, Andersson R. Proteome-based biomarkers in pancreatic cancer. World J Gastroenterol. 2011;17:4845–4852. doi: 10.3748/wjg.v17.i44.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonack S, Aspinall-O’Dea M, Neoptolemos JP, Costello E. Pancreatic cancer: proteomic approaches to a challenging disease. Pancreatology. 2009;9:567–576. doi: 10.1159/000212083. [DOI] [PubMed] [Google Scholar]
- 12.Aebersold R, Goodlett DR. Mass spectrometry in proteomics. Chem Rev. 2001;101:269–295. doi: 10.1021/cr990076h. [DOI] [PubMed] [Google Scholar]
- 13.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 14.McDonald WH, Yates JR., III Shotgun proteomics: integrating technologies to answer biological questions. Curr Opin Mol Ther. 2003;5:302–309. [PubMed] [Google Scholar]
- 15.Eng J, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 16.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Craig R, Beavis RC. TANDEM: matching proteins with tandem mass spectra. Bioinformatics. 2004;20:1466–1467. doi: 10.1093/bioinformatics/bth092. [DOI] [PubMed] [Google Scholar]
- 18.Mueller LN, Brusniak MY, Mani DR, Aebersold R. An assessment of software solutions for the analysis of mass spectrometry based quantitative proteomics data. J Proteome Res. 2008;7:51–61. doi: 10.1021/pr700758r. [DOI] [PubMed] [Google Scholar]
- 19.Pan S, Aebersold R. Quantitative proteomics by stable isotope labeling and mass spectrometry. Methods Mol Biol. 2007;367:209–218. doi: 10.1385/1-59745-275-0:209. [DOI] [PubMed] [Google Scholar]
- 20.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt A, Kellermann J, Lottspeich F. A novel strategy for quantitative proteomics using isotope-coded protein labels. Proteomics. 2005;5:4–15. doi: 10.1002/pmic.200400873. [DOI] [PubMed] [Google Scholar]
- 22.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Thompson A, Schafer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK, Hamon C. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem. 2003;75:1895–1904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- 24.America AH, Cordewener JH. Comparative LC-MS: a landscape of peaks and valleys. Proteomics. 2008;8:731–749. doi: 10.1002/pmic.200700694. [DOI] [PubMed] [Google Scholar]
- 25.Choi H, Fermin D, Nesvizhskii AI. Significance analysis of spectral count data in label-free shotgun proteomics. Mol Cell Proteomics. 2008;7:2373–2385. doi: 10.1074/mcp.M800203-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Ryu S, Gallis B, Goo YA, Shaffer SA, Radulovic D, Goodlett DR. Comparison of a label-free quantitative proteomic method based on peptide ion current area to the isotope coded affinity tag method. Cancer Inform. 2008;6:243–255. doi: 10.4137/cin.s385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellew M, Coram M, Fitzgibbon M, Igra M, Randolph T, Wang P, May D, Eng J, Fang R, Lin C, Chen J, Goodlett D, Whiteaker J, Paulovich A, McIntosh M. A suite of algorithms for the comprehensive analysis of complex protein mixtures using high-resolution LC-MS. Bioinformatics. 2006;22:1902–1909. doi: 10.1093/bioinformatics/btl276. [DOI] [PubMed] [Google Scholar]
- 29.May D, Liu Y, Law W, Fitzgibbon M, Wang H, Hanash S, McIntosh M. Peptide sequence confidence in accurate mass and time analysis and its use in complex proteomics experiments. J Proteome Res. 2008;7:5148–5156. doi: 10.1021/pr8004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May D, Pan S, Crispin DA, Lai K, Bronner MP, Hogan J, Hockenbery DM, McIntosh M, Brentnall TA, Chen R. Investigating neoplastic progression of ulcerative colitis with label-free comparative proteomics. J Proteome Res. 2011;10:200–209. doi: 10.1021/pr100574p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P, Tang H, Zhang H, Whiteaker J, Paulovich AG, McIntosh M. Normalization regarding non-random missing values in high-throughput mass spectrometry data. Pac Symp Biocomput. 2006:315–326. [PubMed] [Google Scholar]
- 32.Boja ES, Rodriguez H. Mass spectrometry-based targeted quantitative proteomics: Achieving sensitive and reproducible detection of proteins. Proteomics. 2012;12:1093–1110. doi: 10.1002/pmic.201100387. [DOI] [PubMed] [Google Scholar]
- 33.Elschenbroich S, Kislinger T. Targeted proteomics by selected reaction monitoring mass spectrometry: applications to systems biology and biomarker discovery. Mol Biosyst. 2011;7:292–303. doi: 10.1039/c0mb00159g. [DOI] [PubMed] [Google Scholar]
- 34.Gallien S, Duriez E, Domon B. Selected reaction monitoring applied to proteomics. J Mass Spectrom. 2011;46:298–312. doi: 10.1002/jms.1895. [DOI] [PubMed] [Google Scholar]
- 35.Huttenhain R, Malmstrom J, Picotti P, Aebersold R. Perspectives of targeted mass spectrometry for protein biomarker verification. Curr Opin Chem Biol. 2009;13:518–525. doi: 10.1016/j.cbpa.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng Z, Veenstra TD. Targeted mass spectrometry approaches for protein biomarker verification. J Proteomics. 2011;74:2650–2659. doi: 10.1016/j.jprot.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Pan S, Aebersold R, Chen R, Rush J, Goodlett DR, McIntosh MW, Zhang J, Brentnall TA. Mass spectrometry based targeted protein quantification: methods and applications. J Proteome Res. 2009;8:787–797. doi: 10.1021/pr800538n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiess R, Wollscheid B, Aebersold R. Targeted proteomic strategy for clinical biomarker discovery. Mol Oncol. 2009;3:33–44. doi: 10.1016/j.molonc.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillet LC, Navarro P, Tate S, Rost H, Selevsek N, Reiter L, Bonner R, Aebersold R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics. 2012;11:O111. doi: 10.1074/mcp.O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, Tuveson DA. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 41.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 42.Wernert N. The multiple roles of tumour stroma. Virchows Arch. 1997;430:433–443. doi: 10.1007/s004280050053. [DOI] [PubMed] [Google Scholar]
- 43.Brown LF, Guidi AJ, Schnitt SJ, Van De WL, Iruela-Arispe ML, Yeo TK, Tognazzi K, Dvorak HF. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–1056. [PubMed] [Google Scholar]
- 44.Chen R, Yi EC, Donohoe S, Pan S, Eng J, Cooke K, Crispin DA, Lane Z, Goodlett DR, Bronner MP, Aebersold R, Brentnall TA. Pancreatic cancer proteome: the proteins that underlie invasion, metastasis, and immunologic escape. Gastroenterology. 2005;129:1187–1197. doi: 10.1053/j.gastro.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Lu Z, Hu L, Evers S, Chen J, Shen Y. Differential expression profiling of human pancreatic adenocarcinoma and healthy pancreatic tissue. Proteomics. 2004;4:3975–3988. doi: 10.1002/pmic.200300863. [DOI] [PubMed] [Google Scholar]
- 46.Shen J, Person MD, Zhu J, Abbruzzese JL, Li D. Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res. 2004;64:9018–9026. doi: 10.1158/0008-5472.CAN-04-3262. [DOI] [PubMed] [Google Scholar]
- 47.Pan S, Chen R, Brand RE, Hawley S, Tamura Y, Gafken PR, Milless BP, Goodlett DR, Rush J, Brentnall TA. Multiplex targeted proteomic assay for biomarker detection in plasma: a pancreatic cancer biomarker case study. J Proteome Res. 2012;11:1937–1948. doi: 10.1021/pr201117w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bausch D, Thomas S, Mino-Kenudson M, Fernandez D, Bauer TW, Williams M, Warshaw AL, Thayer SP, Kelly KA. Plectin-1 as a novel biomarker for pancreatic cancer. Clin Cancer Res. 2011;17:302–309. doi: 10.1158/1078-0432.CCR-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, DePinho RA, Mahmood U, Weissleder R. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008;5:e85. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Kuramitsu Y, Ueno T, Suzuki N, Yoshino S, Iizuka N, Zhang X, Oka M, Nakamura K. Differential expression of up-regulated cofilin-1 and down-regulated cofilin-2 characteristic of pancreatic cancer tissues. Oncol Rep. 2011;26:1595–1599. doi: 10.3892/or.2011.1447. [DOI] [PubMed] [Google Scholar]
- 51.Cui Y, Wu J, Zong M, Song G, Jia Q, Jiang J, Han J. Proteomic profiling in pancreatic cancer with and without lymph node metastasis. Int J Cancer. 2009;124:1614–1621. doi: 10.1002/ijc.24163. [DOI] [PubMed] [Google Scholar]
- 52.Naidoo K, Jones R, Dmitrovic B, Wijesuriya N, Kocher H, Hart IR, Crnogorac-Jurcevic T. Proteome of formalin-fixed paraffin-embedded pancreatic ductal adenocarcinoma and lymph node metastases. J Pathol. 2012;226:756–763. doi: 10.1002/path.3959. [DOI] [PubMed] [Google Scholar]
- 53.Neupane D, Korc M. 14-3-3sigma Modulates pancreatic cancer cell survival and invasiveness. Clin Cancer Res. 2008;14:7614–7623. doi: 10.1158/1078-0432.CCR-08-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roda O, Ortiz-Zapater E, Martinez-Bosch N, Gutierrez-Gallego R, Vila-Perello M, Ampurdanes C, Gabius HJ, Andre S, Andreu D, Real FX, Navarro P. Galectin-1 is a novel functional receptor for tissue plasminogen activator in pancreatic cancer. Gastroenterology. 2009;136:1379–5. doi: 10.1053/j.gastro.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 55.Chen R, Pan S, Ottenhof N, de Wilde R, Wolfgang C, Lane Z, Pos TJ, Bronner M, Willmann J, Maitra A, Brentnall T. Stromal galectin-1 expression is associated with long-term survival in resectable pancreatic ductal adenocarcinoma. Cancer Biology & Therapy. 2012;13 doi: 10.4161/cbt.20842. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan S, Chen R, Reimel BA, Crispin DA, Mirzaei H, Cooke K, Coleman JF, Lane Z, Bronner MP, Goodlett DR, McIntosh MW, Traverso W, Aebersold R, Brentnall TA. Quantitative proteomics investigation of pancreatic intraepithelial neoplasia. Electrophoresis. 2009;30:1132–1144. doi: 10.1002/elps.200800752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui Y, Tian M, Zong M, Teng M, Chen Y, Lu J, Jiang J, Liu X, Han J. Proteomic analysis of pancreatic ductal adenocarcinoma compared with normal adjacent pancreatic tissue and pancreatic benign cystadenoma. Pancreatology. 2009;9:89–98. doi: 10.1159/000178879. [DOI] [PubMed] [Google Scholar]
- 59.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andren-Sandberg A, Domellof L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 60.Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Levy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosty C, Geradts J, Sato N, Wilentz RE, Roberts H, Sohn T, Cameron JL, Yeo CJ, Hruban RH, Goggins M. p16 Inactivation in pancreatic intraepithelial neoplasias (PanINs) arising in patients with chronic pancreatitis. Am J Surg Pathol. 2003;27:1495–1501. doi: 10.1097/00000478-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Crnogorac-Jurcevic T, Gangeswaran R, Bhakta V, Capurso G, Lattimore S, Akada M, Sunamura M, Prime W, Campbell F, Brentnall TA, Costello E, Neoptolemos J, Lemoine NR. Proteomic analysis of chronic pancreatitis and pancreatic adenocarcinoma. Gastroenterology. 2005;129:1454–1463. doi: 10.1053/j.gastro.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 63.Chen R, Brentnall TA, Pan S, Cooke K, Moyes KW, Lane Z, Crispin DA, Goodlett DR, Aebersold R, Bronner MP. Quantitative proteomics analysis reveals that proteins differentially expressed in chronic pancreatitis are also frequently involved in pancreatic cancer. Mol Cell Proteomics. 2007;6:1331–1342. doi: 10.1074/mcp.M700072-MCP200. [DOI] [PubMed] [Google Scholar]
- 64.Pan S, Chen R, Stevens T, Bronner MP, May D, Tamura Y, McIntosh MW, Brentnall TA. Proteomics portrait of archival lesions of chronic pancreatitis. PLoS One. 2011;6:e27574. doi: 10.1371/journal.pone.0027574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 66.Blonder J, Veenstra TD. Clinical proteomic applications of formalin-fixed paraffin-embedded tissues. Clin Lab Med. 2009;29:101–113. doi: 10.1016/j.cll.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Hood BL, Conrads TP, Veenstra TD. Mass spectrometric analysis of formalin-fixed paraffin-embedded tissue: unlocking the proteome within. Proteomics. 2006;6:4106–4114. doi: 10.1002/pmic.200600016. [DOI] [PubMed] [Google Scholar]
- 68.Ralton LD, Murray GI. The use of formalin fixed wax embedded tissue for proteomic analysis. J Clin Pathol. 2011;64:297–302. doi: 10.1136/jcp.2010.086835. [DOI] [PubMed] [Google Scholar]
- 69.Bell LN, Saxena R, Mattar SG, You J, Wang M, Chalasani N. Utility of formalin-fixed, paraffin-embedded liver biopsy specimens for global proteomic analysis in nonalcoholic steatohepatitis. Proteomics Clin Appl. 2011;5:397–404. doi: 10.1002/prca.201000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hood BL, Darfler MM, Guiel TG, Furusato B, Lucas DA, Ringeisen BR, Sesterhenn IA, Conrads TP, Veenstra TD, Krizman DB. Proteomic analysis of formalin-fixed prostate cancer tissue. Mol Cell Proteomics. 2005;4:1741–1753. doi: 10.1074/mcp.M500102-MCP200. [DOI] [PubMed] [Google Scholar]
- 71.Hwang SI, Thumar J, Lundgren DH, Rezaul K, Mayya V, Wu L, Eng J, Wright ME, Han DK. Direct cancer tissue proteomics: a method to identify candidate cancer biomarkers from formalin-fixed paraffin-embedded archival tissues. Oncogene. 2007;26:65–76. doi: 10.1038/sj.onc.1209755. [DOI] [PubMed] [Google Scholar]
- 72.Negishi A, Masuda M, Ono M, Honda K, Shitashige M, Satow R, Sakuma T, Kuwabara H, Nakanishi Y, Kanai Y, Omura K, Hirohashi S, Yamada T. Quantitative proteomics using formalin-fixed paraffin-embedded tissues of oral squamous cell carcinoma. Cancer Sci. 2009;100:1605–1611. doi: 10.1111/j.1349-7006.2009.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanca A, Addis MF, Pagnozzi D, Cossu-Rocca P, Tonelli R, Falchi G, Eccher A, Roggio T, Fanciulli G, Uzzau S. Proteomic analysis of formalin-fixed, paraffin-embedded lung neuroendocrine tumor samples from hospital archives. J Proteomics. 2011;74:359–370. doi: 10.1016/j.jprot.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Cheung W, Darfler MM, Alvarez H, Hood BL, Conrads TP, Habbe N, Krizman DB, Mollenhauer J, Feldmann G, Maitra A. Application of a global proteomic approach to archival precursor lesions: deleted in malignant brain tumors 1 and tissue transglutaminase 2 are upregulated in pancreatic cancer precursors. Pancreatology. 2008;8:608–616. doi: 10.1159/000161012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shields DJ, Niessen S, Murphy EA, Mielgo A, Desgrosellier JS, Lau SK, Barnes LA, Lesperance J, Bouvet M, Tarin D, Cravatt BF, Cheresh DA. RBBP9: a tumor-associated serine hydrolase activity required for pancreatic neoplasia. Proc Natl Acad Sci USA. 2010;107:2189–2194. doi: 10.1073/pnas.0911646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Funel N, Giovannetti E, Pollina LE, del Chiaro M, Mosca F, Boggi U, Campani D. Critical role of laser microdissection for genetic, epigenetic and proteomic analyses in pancreatic cancer. Expert Rev Mol Diagn. 2011;11:695–701. doi: 10.1586/erm.11.62. [DOI] [PubMed] [Google Scholar]
- 77.Gutstein HB, Morris JS. Laser capture sampling and analytical issues in proteomics. Expert Rev Proteomics. 2007;4:627–637. doi: 10.1586/14789450.4.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mustafa D, Kros JM, Luider T. Combining laser capture microdissection and proteomics techniques. Methods Mol Biol. 2008;428:159–178. doi: 10.1007/978-1-59745-117-8_9. [DOI] [PubMed] [Google Scholar]
- 79.Shekouh AR, Thompson CC, Prime W, Campbell F, Hamlett J, Herrington CS, Lemoine NR, Crnogorac-Jurcevic T, Buechler MW, Friess H, Neoptolemos JP, Pennington SR, Costello E. Application of laser capture microdissection combined with two-dimensional electrophoresis for the discovery of differentially regulated proteins in pancreatic ductal adenocarcinoma. Proteomics. 2003;3:1988–2001. doi: 10.1002/pmic.200300466. [DOI] [PubMed] [Google Scholar]
- 80.Fu AY, Spence C, Scherer A, Arnold FH, Quake SR. A microfabricated fluorescence-activated cell sorter. Nat Biotechnol. 1999;17:1109–1111. doi: 10.1038/15095. [DOI] [PubMed] [Google Scholar]
- 81.Kellner U, Steinert R, Seibert V, Heim S, Kellner A, Schulz HU, Roessner A, Kruger S, Reymond M. Epithelial cell preparation for proteomic and transcriptomic analysis in human pancreatic tissue. Pathol Res Pract. 2004;200:155–163. doi: 10.1016/j.prp.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Sheikh AA, Vimalachandran D, Thompson CC, Jenkins RE, Nedjadi T, Shekouh A, Campbell F, Dodson A, Prime W, Crnogorac-Jurcevic T, Lemoine NR, Costello E. The expression of S100A8 in pancreatic cancer-associated monocytes is associated with the Smad4 status of pancreatic cancer cells. Proteomics. 2007;7:1929–1940. doi: 10.1002/pmic.200700072. [DOI] [PubMed] [Google Scholar]
- 83.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 84.Dai L, Li C, Shedden KA, Lee CJ, Li C, Quoc H, Simeone DM, Lubman DM. Quantitative proteomic profiling studies of pancreatic cancer stem cells. J Proteome Res. 2010;9:3394–3402. doi: 10.1021/pr100231m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balic A, Dorado J, Alonso-Gomez M, Heeschen C. Stem cells as the root of pancreatic ductal adenocarcinoma. Exp Cell Res. 2012;318:691–704. doi: 10.1016/j.yexcr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Seeley EH, Schwamborn K, Caprioli RM. Imaging of intact tissue sections: moving beyond the microscope. J Biol Chem. 2011;286:25459–25466. doi: 10.1074/jbc.R111.225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minerva L, Clerens S, Baggerman G, Arckens L. Direct profiling and identification of peptide expression differences in the pancreas of control and ob/ob mice by imaging mass spectrometry. Proteomics. 2008;8:3763–3774. doi: 10.1002/pmic.200800237. [DOI] [PubMed] [Google Scholar]
- 88.Green-Mitchell SM, Cazares LH, Semmes OJ, Nadler JL, Nyalwidhe JO. On-tissue identification of insulin: in situ reduction coupled with mass spectrometry imaging. Proteomics Clin Appl. 2011;5:448–453. doi: 10.1002/prca.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Djidja MC, Claude E, Snel MF, Scriven P, Francese S, Carolan V, Clench MR. MALDI-ion mobility separation-mass spectrometry imaging of glucose-regulated protein 78 kDa (Grp78) in human formalin-fixed, paraffin-embedded pancreatic adenocarcinoma tissue sections. J Proteome Res. 2009;8:4876–4884. doi: 10.1021/pr900522m. [DOI] [PubMed] [Google Scholar]
- 90.Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ, Hwang RF, Jaster R, Kleeff J, Kloppel G, Kordes C, Logsdon CD, Masamune A, Michalski CW, Oh J, Phillips PA, Pinzani M, Reiser-Erkan C, Tsukamoto H, Wilson J. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172–178. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paulo JA, Urrutia R, Banks PA, Conwell DL, Steen H. Proteomic analysis of an immortalized mouse pancreatic stellate cell line identifies differentially-expressed proteins in activated vs nonproliferating cell states. J Proteome Res. 2011;10:4835–4844. doi: 10.1021/pr2006318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roda O, Chiva C, Espuna G, Gabius HJ, Real FX, Navarro P, Andreu D. A proteomic approach to the identification of new tPA receptors in pancreatic cancer cells. Proteomics. 2006;6(Suppl 1):S36–S41. doi: 10.1002/pmic.200500376. [DOI] [PubMed] [Google Scholar]
- 94.Hajjar KA, Krishnan S. Annexin II: a mediator of the plasmin/plasminogen activator system. Trends Cardiovasc Med. 1999;9:128–138. doi: 10.1016/s1050-1738(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 95.Kuramitsu Y, Taba K, Ryozawa S, Yoshida K, Zhang X, Tanaka T, Maehara S, Maehara Y, Sakaida I, Nakamura K. Identification of up- and down-regulated proteins in gemcitabine-resistant pancreatic cancer cells using two-dimensional gel electrophoresis and mass spectrometry. Anticancer Res. 2010;30:3367–3372. [PubMed] [Google Scholar]
- 96.Kuramitsu Y, Wang Y, Taba K, Suenaga S, Ryozawa S, Kaino S, Sakaida I, Nakamura K. Heat-shock Protein 27 Plays the Key Role in Gemcitabine-resistance of Pancreatic Cancer Cells. Anticancer Res. 2012;32:2295–2299. [PubMed] [Google Scholar]
- 97.Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, Mikuria K, Fujimoto M, Maehara S, Maehara Y, Okita K, Nakamura K, Sakaida I. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int J Oncol. 2007;31:1345–1350. [PubMed] [Google Scholar]
- 98.Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, Taba K, Fujimoto M, Okita K, Nakamura K, Sakaida I. A proteomic profiling of gemcitabine resistance in pancreatic cancer cell lines. Mol Med Report. 2008;1:429–434. [PubMed] [Google Scholar]
- 99.Chen YW, Liu JY, Lin ST, Li JM, Huang SH, Chen JY, Wu JY, Kuo CC, Wu CL, Lu YC, Chen YH, Fan CY, Huang PC, Law CH, Lyu PC, Chou HC, Chan HL. Proteomic analysis of gemcitabine-induced drug resistance in pancreatic cancer cells. Mol Biosyst. 2011;7:3065–3074. doi: 10.1039/c1mb05125c. [DOI] [PubMed] [Google Scholar]
- 100.Zhou J, Du Y. Acquisition of resistance of pancreatic cancer cells to 2-methoxyestradiol is associated with the upregulation of manganese superoxide dismutase. Mol Cancer Res. 2012;10:768–777. doi: 10.1158/1541-7786.MCR-11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poland J, Urbani A, Lage H, Schnolzer M, Sinha P. Study of the development of thermoresistance in human pancreatic carcinoma cell lines using proteome analysis. Electrophoresis. 2004;25:173–183. doi: 10.1002/elps.200305698. [DOI] [PubMed] [Google Scholar]
- 102.Yang Y, Chaerkady R, Kandasamy K, Huang TC, Selvan LD, Dwivedi SB, Kent OA, Mendell JT, Pandey A. Identifying targets of miR-143 using a SILAC-based proteomic approach. Mol Biosyst. 2010;6:1873–1882. doi: 10.1039/c004401f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stylli SS, Kaye AH, Lock P. Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–737. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 104.Brentnall TA, Lai LA, Coleman J, Bronner MP, Pan S, Chen R. Arousal of cancer-associated stroma: overexpression of palladin activates fibroblasts to promote tumor invasion. PLoS One. 2012;7:e30219. doi: 10.1371/journal.pone.0030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salaria SN, Illei P, Sharma R, Walter KM, Klein AP, Eshleman JR, Maitra A, Schulick R, Winter J, Ouellette MM, Goggins M, Hruban R. Palladin is overexpressed in the non-neoplastic stroma of infiltrating ductal adenocarcinomas of the pancreas, but is only rarely overexpressed in neoplastic cells. Cancer Biol Ther. 2007;6:324–328. doi: 10.4161/cbt.6.3.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harsha HC, Jimeno A, Molina H, Mihalas AB, Goggins MG, Hruban RH, Schulick RD, Kamath U, Maitra A, Hidalgo M, Pandey A. Activated epidermal growth factor receptor as a novel target in pancreatic cancer therapy. J Proteome Res. 2008;7:4651–4658. doi: 10.1021/pr800139r. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H, Cao R, Lee WN, Deng C, Zhao Y, Lappe J, Recker R, Yen Y, Wang Q, Tsai MY, Go VL, Xiao GG. Inhibition of protein phosphorylation in MIA pancreatic cancer cells: confluence of metabolic and signaling pathways. J Proteome Res. 2010;9:980–989. doi: 10.1021/pr9008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taba K, Kuramitsu Y, Ryozawa S, Yoshida K, Tanaka T, Maehara S, Maehara Y, Sakaida I, Nakamura K. Heat-shock protein 27 is phosphorylated in gemcitabine-resistant pancreatic cancer cells. Anticancer Res. 2010;30:2539–2543. [PubMed] [Google Scholar]