Abstract
OBJECTIVE
To determine the practice patterns of Canadian hematologists and neonatologists/paediatricians who care for newborns with hemophilia, with regard to vitamin K administration, use of empirical clotting factor replacement therapy, neuroimaging and timing of hematology consultation.
METHODS:
Hematologists and neonatologists/paediatricians, identified from membership lists of Canadian professional organizations, were provided electronic and/or paper versions of the survey instrument. Questions were posed in the context of specific clinical scenarios. Differences in response proportions between groups were compared for selected questions.
RESULTS:
There were 171 respondents among 616 eligible persons who were sent the survey; 58 respondents had recent experience managing a newborn with hemophilia. There was a consensus not to provide empirical treatment to well newborns after uncomplicated deliveries, to provide empirical treatment to symptomatic newborns and to obtain neuroimaging for symptomatic newborns. Systematic differences between hematologists and neonatologists/paediatricians existed with regard to the timing of hematology consultation when the diagnosis of hemophilia had not been confirmed antenatally, the route of vitamin K administration for newborns with hemophilia and the choice of product to use for empirical treatment of a symptomatic newborn.
CONCLUSIONS:
The observed lack of consensus regarding important management decisions indicates a need for ongoing research in the care of newborns with hemophilia. Systematic differences between hematologists and neonatologists/paediatricians suggest a role for improved communication and collaboration between these two groups of practitioners.
Keywords: Hemophilia A, Hemophilia B, Intracranial hemorrhages, Neuroimaging, Newborn
Abstract
OBJECTIF:
Déterminer les profils de pratique des hématologues et des néonatologistes/pédiatres canadiens qui soignent des nouveau-nés hémophiles à l’égard de l’administration de vitamine K, de l’utilisation empirique du traitement par le facteur de remplacement de coagulation, de la neuro-imagerie et du moment de la consultation en hématologie.
MÉTHODOLOGIE:
Les hématologues et les néonatologistes/pédiatres, repérés grâce aux listes de membres d’organismes professionnels canadiens, ont reçu une version virtuelle, une version papier ou les deux versions du sondage. Les questions étaient posées dans le contexte de scénarios cliniques précis. Les différences dans les proportions de réponses entre les groupes étaient comparées à l’égard de questions sélectionnées.
RÉSULTATS:
Sur les 616 personnes admissibles, 171 ont répondu au sondage. De ce nombre, 58 avaient eu une expérience récente de prise en charge d’un nouveau-né hémophile. On observait un consensus de ne pas administrer de traitement empirique aux nouveau-nés en santé après un accouchement sans complication, d’administrer un traitement empirique aux nouveau-nés symptomatiques et d’obtenir une neuroimagerie chez ces nouveau-nés symptomatiques. Il y avait des différences systématiques entre les hématologues et les néonatologistes/pédiatres pour ce qui est du moment de la consultation en hématologie lorsque le diagnostic n’avait pas été confirmé pendant la période anténatale, de la voie d’administration de la vitamine K aux nouveau-nés hémophiles et du choix de produit à utiliser pour administrer un traitement empirique à un nouveau-né symptomatique.
CONCLUSIONS:
L’absence de consensus observé au sujet d’importantes décisions de prise en charge démontre la nécessité de poursuivre les recherches sur les soins aux nouveau-nés hémophiles. En raison des différences systématiques entre les hématologues et les néonatologistes/pédiatres, il y aurait lieu d’améliorer les communications et la collaboration entre ces deux groupes de praticiens.
Hemophilia A (a deficiency of coagulation factor VIII [FVIII] due to mutation in the F8 gene) and hemophilia B (a deficiency of coagulation factor IX [FIX] due to mutation in the F9 gene) are bleeding disorders with X-linked inheritance. Newborns with hemophilia are at risk for intracranial hemorrhage (ICH); in one report, 5.9% of patients with hemophilia A and 3.2% with hemophilia B experienced ICH in the first three months of life, the majority occurring within five days of birth (1). In addition to causing acute morbidity, ICH can have serious chronic sequelae (1–3). There are no proven strategies for the prevention of ICH in newborns with hemophilia. Although there is ongoing debate regarding the optimal mode of delivery for fetuses with hemophilia (4,5), less attention has been given to aspects of postnatal care that may contribute to the prevention, diagnosis and treatment of neonatal ICH.
A statement from the United Kingdom Haemophilia Centres Doctors Organization (UKHCDO) in 2011 (6) is the only published guideline that addresses these issues, and it is unknown how well this guideline reflects current practice. Surveys published in the United States in 1999 (7) and in the United Kingdom in 2005 (8) demonstrated that there was no consensus with respect to the use of factor concentrates and imaging. However, these surveys have important limitations: specific circumstances that may prompt particular interventions were not identified, and only hematologists were surveyed. Because other practitioners may be involved in the care of newborns with hemophilia, we conducted a survey of hematologists and neonatologists/paediatricians who care for newborns, to describe current practice in specific clinical situations and to identify systematic differences in practice between these groups.
METHODS
The survey instrument was drafted in English by the first author, and reviewed by all the authors. The instrument was translated into French by the second author and the fourth author (a native Francophone). A convenience sample of hematologists and neonatologists pilot tested the instrument for readability and clarity, and to establish the time necessary for its completion (5 min to 10 min).
The survey consisted of 31 multiple-choice questions. The first question identified respondents who had treated a newborn with hemophilia in the past five years. Eighteen questions examining the management of hemophilia were posed in the context of three clinical scenarios that are detailed in Box 1. These questions explored the administration of vitamin K, the use of factor concentrates and other hemostatic products, the use of tests to confirm the diagnosis of hemophilia, the use of neuroimaging and the timing of hematology consultation. The final 12 questions included demographic information about the respondents and their institutions (the survey instrument is available on request).
Box 1: Clinical scenarios used in the survey instrument.
Scenario 1: Maternal carrier, uncomplicated delivery, well newborn
A 26-year-old G2P1 woman gives birth to a boy at 39 weeks’ gestation by uncomplicated vaginal delivery. The mother is a known carrier of a mutation for severe hemophilia A. The baby boy is clinically well.
Scenario 2: Maternal carrier, difficult delivery/Caesarian section, well newborn
A 29-year-old G1P0 woman gives birth to a baby boy at 39 weeks’ gestation. The mother is a known carrier of a mutation for severe hemophilia A. Vaginal delivery is attempted but is converted to an emergency Caesarian section because of fetal bradycardia and difficulty extracting the baby’s head. The baby boy is clinically well after birth, but he has facial bruising and a cephalohematoma.
Scenario 3: Prenatal diagnosis, uncomplicated delivery, symptomatic newborn
A 33-year-old G3P2 woman gives birth to a baby boy at 38 weeks’ gestation. Prenatal testing has confirmed that the baby has a factor VIII mutation associated with severe hemophilia A. The birth is by uncomplicated vaginal delivery. Within 6 h of birth, the baby is observed to feed poorly, and subsequently the baby has a seizure.
To be included in the study, hematologists and neonatologists/paediatricians had to have been practicing in Canada and to have treated a newborn with hemophilia in the past five years. Only respondents who met these criteria were invited to complete the entire survey. The sample was identified from available membership lists for the Association of Hemophilia Clinic Directors of Canada, the C17 Research Network (Canadian academic paediatric hematology/oncology centres), the American Society of Pediatric Hematology/Oncology (members at Canadian institutions only), the Canadian Hematology Society, the Canadian Paediatric Society Neonatal-Perinatal Medicine Section and the Canadian Neonatal Network.
Some physicians identified from these lists were found to not be currently practicing and were excluded. Two of the present study’s authors were identified in these lists, but were excluded. In August 2010, persons identified as possible participants received an invitation e-mail explaining the purpose of the study and requesting their participation. This was followed by an electronic mailing of the instrument through Survey Monkey; a reminder e-mail was sent one week later to individuals who had not yet responded. In September 2010, the electronic survey was closed and a paper version of the instrument was mailed, along with a nominal incentive (a $2 coffee card), to physicians who had not responded to the electronic instrument and those for whom a valid e-mail address was not available.
Response proportions were examined for all survey items. All responses were weighted equally. For selected survey items, intergroup differences in response proportions were compared using Pearson’s χ2 test or Fisher’s exact test, as appropriate. Two-sided P<0.05 were considered to be statistically significant, and no adjustments for multiple comparisons were made.
The present study was approved by the Research Ethics Board of the Children’s Hospital of Eastern Ontario (Ottawa, Ontario).
RESULTS
Of the 616 individuals who were identified as potentially eligible to complete the survey, 529 were sent the electronic instrument and 547 were sent the paper instrument. The conduct of the survey is detailed in Figure 1.
Figure 1).
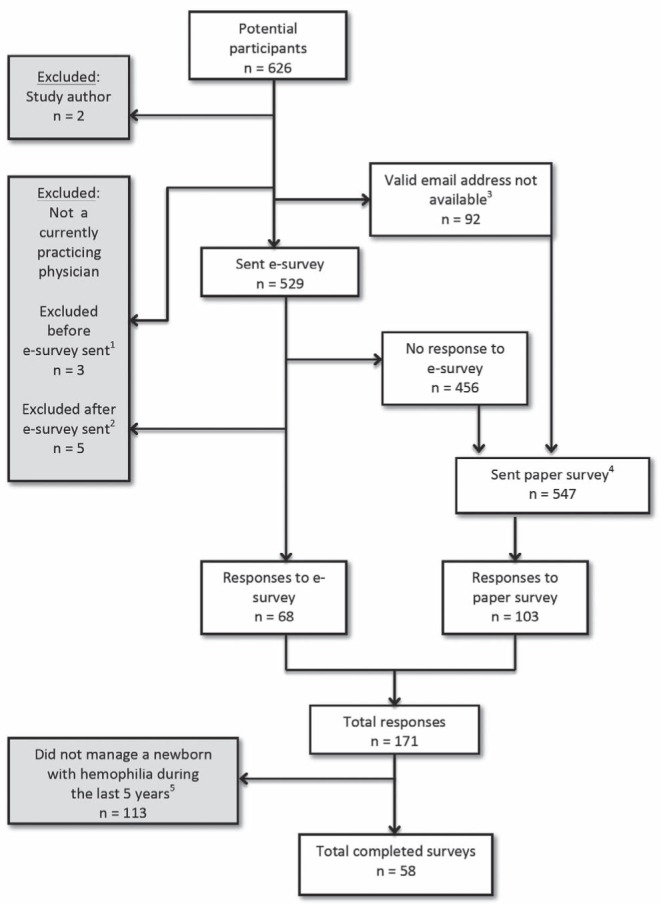
Conduct of the survey. 1Physicians, not currently practicing: retired (n=1) and maternity leave (n = 2); 2Nonphysicians identified by Internet research: registered nurse (n=1), graduate student (n=1) and researcher (n=3); 3Valid e-mail address not available: Canadian Hematology Society members (n=49), invalid e-mail (n=32), no e-mail address available (n=6), previously opted out of Survey Monkey (n=5); 4A mailing address was not available for one person who had been sent the e-survey; 5Six respondents indicated by e-mail that they do not treat paediatric patients with hemophilia, which was considered equivalent to having responded to the survey and indicating that they had no recent experience managing newborns with hemophilia
The response rates were 12.9% for the electronic instrument (68 replies from 524 potential respondents) and 18.8% for the paper instrument (103 replies from 547 potential respondents). The pooled response rate for both forms of the instrument was 27.8% (171 replies from 616 potential respondents). Fifty-eight respondents (29 hematologists and 29 neonatologists/paediatricians; 34% of all respondents) had managed a newborn with hemophilia in the past five years and were eligible to complete the entire survey instrument.
Respondents who completed the entire survey had been practicing for a mean of 14 years, and 33% were members of the Association of Hemophilia Clinic Directors of Canada. The estimated average number of newborns with hemophilia treated at respondents’ institutions was <1 per year for 30% of respondents, one to two per year for 47% and >2 per year for 23%. Fourteen per cent of respondents reported that their institution had a written protocol for the management of newborns with hemophilia, and 5% reported that protocols for the monitoring of infants at high risk of ICH existed at their institution. Sixteen per cent of respondents reported treating a newborn with hemophilia who had experienced a neonatal ICH in the past five years.
Significantly more hematologists than neonatologists/paediatricians preferred hematology consultation regarding the care of the newborn occur before delivery in the two scenarios in which the mother was a known carrier but the diagnosis of hemophilia A had not been confirmed antenatally (26 of 29 [90%] versus 18 of 29 [62%] in scenario 1; P=0.01; 24 of 28 [86%] versus 17 of 29 [59%] in scenario 2; P=0.003). There was a consensus in favour of prenatal paediatric hematology consultation when a prenatal diagnosis of hemophilia had been made (28 of 29 [97%] versus 25 of 28 [89%]; P=0.48).
Respondents were asked about testing to confirm a diagnosis of hemophilia in the two scenarios in which the diagnosis had not been established prenatally. In the scenario involving a newborn with bruising following a difficult delivery, hematologists were more likely than neonatologists/paediatricians to measure the FVIII level in the cord blood (21 of 29 [72%] versus 12 of 29 [41%]; P=0.017) and less likely to measure the FVIII level in peripheral blood (nine of 29 [31%] versus 17 of 29 [59%]; P=0.035) compared with neonatologists/paediatricians. A similar pattern was observed in the scenario involving a well newborn after an easy delivery, but the difference did not reach statistical significance.
Questions pertaining to vitamin K administration were asked in two of the three scenarios. Figure 2 demonstrates the considerable heterogeneity of responses. There was a significant difference between groups with regard to the scenario involving a newborn with prenatally diagnosed hemophilia, due to the preference of neonatologists/paediatricians for oral vitamin K in this circumstance.
Figure 2).
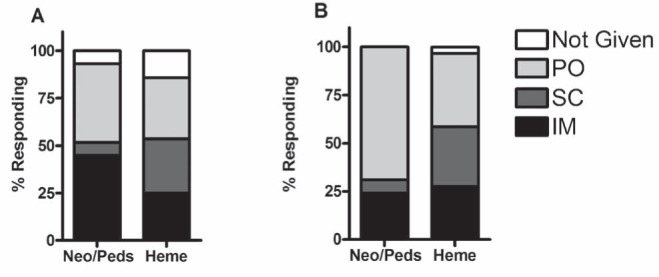
Response proportions for questions involving vitamin K administration. A Scenario 1: P>0.05.B Scenario 3: P=0.043. Questions regarding vitamin K administration were asked only in scenarios 1 and 3. Heme Hematologists; IM Intramuscular; Neo/Peds Neonatologists/paediatricians; PO Per os; SC Subcutaneous
Respondents were asked about empirical treatment in the three scenarios, with results presented in Figure 3. Overwhelmingly, respondents preferred not to treat a well newborn after an easy delivery, while comparable minorities in both groups preferred to treat a bruised but otherwise asymptomatic newborn. In the case of a symptomatic newborn, treatment was preferred by large majorities, but there was a significant difference between groups: hematologists almost exclusively preferred to treat with FVIII concentrate rather than other products.
Figure 3).
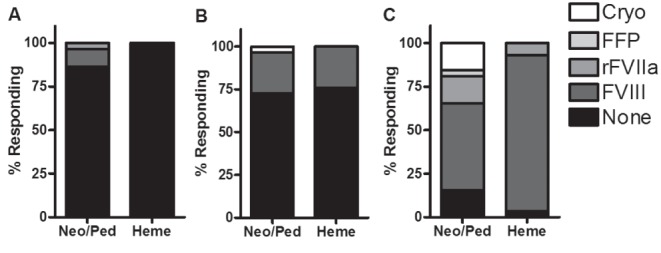
Response proportions for questions involving empirical treatment. A Scenario 1: P>0.05. B Scenario 2: P>0.05. C Scenario 3: P=0.02. Cryo Cryoprecipitate; FFP Fresh frozen plasma; Heme Hematologists; Neo/Peds Neonatologists/paediatricians; rFVIIa Recombinant activated factor VIIa; FVIII Factor VIII (either plasma-derived or recombinant)
Figure 4 illustrates respondents’ preferences for neuroimaging studies in the different scenarios. Majorities in both groups suggested cranial ultrasound for a bruised newborn after a difficult delivery, and minorities in both groups suggested use of this modality after an easy delivery. Although nearly all respondents recommended imaging of a symptomatic newborn, there was no consensus as to the preferred modality.
Figure 4).
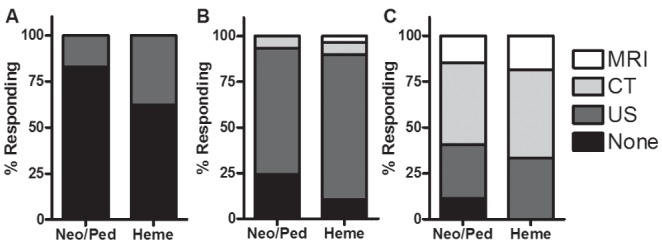
Response proportions for questions involving cranial imaging. A Scenario 1: P>0.05. B Scenario 2: P>0.05. C Scenario 3: P>0.05. CT Computed tomography; Heme Hematologists; MRI Magnetic resonance imaging; Neo/Peds Neonatologists/paediatricians; US Ultrasound
DISCUSSION
The present study was the first systematic examination of clinicians’ preferred management of newborns with hemophilia in specific clinical scenarios and was the first to compare the opinions of hematologists with those of neonatologists/paediatricians. There were systematic differences in responses between the two groups with regard to testing to confirm a diagnosis of hemophilia, vitamin K administration and choice of treatment product for a symptomatic newborn. These discrepancies in hemophilia-specific areas may indicate a need for improved communication and for education of neonatologists/paediatricians regarding the care of newborns with hemophilia.
This may be achieved through earlier paediatric hematology consultation, another area in which we observed a difference between the groups. In the two scenarios in which a prenatal diagnosis of hemophilia had not been made, 86% and 90% of hematologists preferred prenatal paediatric hematology consultation, compared with only 59% and 62% of neonatologists and paediatricians (in comparison, the rate of prenatal involvement of Hemophilia Treatment Centres from the Universal Data Collection project in the United States was approximately 60% [9]). Prenatal paediatric hematology consultation would enable advance planning for diagnostic testing, vitamin K administration, choice of product, if treatment is required, and other issues. We therefore recommend that paediatric hematology services be involved prenatally with all pregnant women who are known to be carriers of F8 or F9 mutations, in collaboration with the adult hematology services that provide care to the mothers.
The results demonstrated an expected variation within groups in some controversial areas, such as the use of factor concentrates after delivery as prophylaxis against ICH. In a previous survey (8), only 19% of UKHCDO members would consider prophylactic treatment in all cases and 50% would consider treatment of a newborn born prematurely or by traumatic delivery. In comparison, nearly all of our respondents preferred not to treat an asymptomatic newborn after an easy delivery, while approximately one-quarter of respondents would treat a newborn with bruising after a difficult delivery. Systematic data that help clinicians decide which newborns require prophylactic treatment do not exist, and research in this area is required.
Although there were no significant differences between hematologists and neonatologists/paediatricians with regard to ordering neuroimaging tests, there was significant variability within both groups. Cranial ultrasound was preferred when imaging asymptomatic newborns. The recent UKHCDO guideline (6) refers to ultrasound as an option for a ‘screening’ test in this setting, but also states that ultrasound may detect fewer hemorrhagic lesions than computed tomography or magnetic resonance imaging. A true screening test should have high sensitivity, a characteristic that ultrasound lacks. However, computed tomography involves radiation exposure and magnetic resonance imaging often requires sedation; as a result, these modalities also have important limitations. The need for routine imaging, the timing of this imaging and the appropriate imaging technology to use remain open issues requiring additional study.
The decision to obtain neuroimaging of a symptomatic newborn was nearly unanimous in the survey, consistent with the UKHCDO’s recommendation. However, there was no consensus as to the appropriate modality for its use. The presumed seriousness of a ‘false negative’ result (ie, a missed ICH) should be considered when choosing a neuroimaging test for a symptomatic newborn with hemophilia, but optimal evidence-based diagnostic strategies are not known.
There was no consensus regarding the route of administration of vitamin K. Both the Canadian Paediatric Society and the American Academy of Pediatrics, motivated by concerns regarding late-onset vitamin K deficiency bleeding (a hemorrhagic disease of newborns), recommend that vitamin K be given parenterally to all infants (10,11). However, these recommendations are made without specific reference to hemophilia and, in the present survey, neonatologists/paediatricians preferred oral vitamin K when a newborn’s diagnosis of hemophilia had been confirmed. This is consistent with the existing UKHCDO recommendation (6). This preference for oral vitamin K may be related to concerns regarding the safety of intramuscular injections in newborns with hemophilia, but the validity of these concerns has not been established. In fact, one-half of the hematologists responding to our survey give vitamin K parenterally, either subcutaneously or intramuscularly; these routes of administration would likely not be preferred if serious hemorrhagic complications were apparent. Additionally, the site of first bleeding event was an intramuscular injection in only 4% of children in the Universal Data Collection project, and in only 2.8% of children whose first bleeding event occurred in the first 30 days of life (9). Finally, the World Federation of Hemophilia makes no recommendation regarding vitamin K injections, but recommends that vaccines be given subcutaneously to children with hemophilia rather than be omitted, indicating the accepted safety of subcutaneous injection for these patients (12). Although the UKHCDO recommends oral vitamin K for newborns with hemophilia, parenteral vitamin K is preferred in general and has not been demonstrated to cause significant bleeding complications in newborns with hemophilia. Parenteral vitamin K is therefore an option for newborns with hemophilia.
Study limitations
The overall response rate of 27.8% was low but in the expected range for this type of survey. The low number of respondents completing the entire survey was a consequence, not of methodological problems, but of the limited experience of hematologists and neonatologists/paediatricians in managing newborns with hemophilia: only one-third of those who responded to the survey had managed such a patient within the past five years. Those who did not respond to our survey are even less likely to have had this experience. This scarcity of experience supports the need for Canadian guidelines to aid clinicians.
Another limitation of the study was that, to allow for brevity and clarity in the survey instrument, the clinical scenarios described involved only hemophilia A. There are some significant differences between hemophilia A and hemophilia B: FIX concentrates can cause anaphylaxis (13), which is seen only rarely with FVIII concentrates; chronic administration of FIX can cause membranous glomerulonephritis (14,15); and patients receiving FIX have a much lower incidence of developing inhibitory antibodies to this protein than do those receiving FVIII (16–18). It is unclear whether any of these factors would create systematic differences between the management of newborns with hemophilia A and those with hemophilia B. We also only inquired about cases of severe hemophilia A, and our results may not describe that management of newborns with moderate or mild disease. We did not ask questions about the management of premature newborns with hemophilia, which has been described only in case reports (19–23).
A final limitation was that the study’s findings may not describe patterns of practice in countries other than Canada, which may have different systems of health care organization, especially with regard to the availability of neuroimaging tests and replacement factor concentrates.
CONCLUSION
The present survey demonstrates systematic differences in practice between hematologists and neonatologists/paediatricians, as well as significant heterogeneity among practitioners with regard to empirical treatment with factor concentrate, cranial imaging and vitamin K administration for babies with hemophilia. This heterogeneity reflects the lack of data to support recommendations in these areas, and additional research is required. Improved education and multi-disciplinary Canadian practice guidelines created by hematologists, neonatologists, neurologists and radiologists are required.
Acknowledgments
The present work was supported by grants from the Children’s Hospital of Eastern Ontario Research Institute (Ottawa, Ontario).
Footnotes
CONTRIBUTORS’ STATEMENTS: Paul Moorehead was the principal investigator and was involved in the conception and design of the study, analysis and interpretation of data, drafting the manuscript and approval of the final version. Jamie Ray was the study coordinator and was involved in study implementation, data collection, data analysis, drafting the manuscript and approval of the final version. Nicholas J Barrowman was the study statistician and was involved in study design, data analysis and approval of the final version of the manuscript. Brigitte Lemyre was one of the senior investigators and was involved in survey design, data analysis and approval of the final version of the manuscript. Robert Klaassen is one of the senior investigators and was involved in protocol development, study implementation, revision of multiple drafts of the manuscript and approval of the final version.
REFERENCES
- 1.Yoffe G, Buchanan GR. Intracranial hemorrhage in newborn and young infants with hemophilia. J Peds. 1988;113:333–6. doi: 10.1016/s0022-3476(88)80277-4. [DOI] [PubMed] [Google Scholar]
- 2.Stieltjes N, Calvez T, Demiguel V, et al. Intracranial haemorrhages in French haemophilia patients (1991–2001): Clinical presentation, management and prognosis factors for death. Haemophilia. 2005;11:452–8. doi: 10.1111/j.1365-2516.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- 3.Miles BS, Anderson P, Agostino A, et al. Effect of intracranial bleeds on the neurocognitive, academic, behavioural and adaptive functioning of boys with haemophilia. Haemophilia. 2012;18:229–34. doi: 10.1111/j.1365-2516.2011.02632.x. [DOI] [PubMed] [Google Scholar]
- 4.James AH, Hoots K. The optimal mode of delivery for the haemophilia carrier expecting an affected infant is caesarean delivery. Haemophilia. 2010;16:420–4. doi: 10.1111/j.1365-2516.2009.02142.x. [DOI] [PubMed] [Google Scholar]
- 5.Ljung R. The optimal mode of delivery for the haemophilia carrier expecting an affected infant is vaginal delivery. Haemophilia. 2010;16:415–9. doi: 10.1111/j.1365-2516.2009.02144.x. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers E, Williams M, Brennand J, et al. Guideline on the management of haemophilia in the fetus and neonate. Br J Haematol. 2011;154:208–15. doi: 10.1111/j.1365-2141.2010.08545.x. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni R, Lusher JM, Lansing E. Current practices regarding newborn intracranial haemorrhage and obstetrical care and mode of delivery of pregnant haemophilia carriers: A survey of obstetricians, neonatologists and haematologists in the United States, on behalf of the National Hemophili. Haemophilia. 1999;5:410–5. doi: 10.1046/j.1365-2516.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers EA, Williams MD, Richards M, et al. Management of neonates with inherited bleeding disorders – a survey of current UK practice. Haemophilia. 2005;11:186–7. doi: 10.1111/j.1365-2516.2005.01072.x. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni R, Soucie JM, Lusher J, et al. Sites of initial bleeding episodes, mode of delivery and age of diagnosis in babies with haemophilia diagnosed before the age of 2 years: A report from The Centers for Disease Control and Prevention’s (CDC) Universal Data Collection (UDC) project. Haemophilia. 2009;15:1281–90. doi: 10.1111/j.1365-2516.2009.02074.x. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics, Committee on Fetus and Newborn Controversies Concerning Vitamin K and the Newborn. Pediatrics. 2003;112:191–2. [PubMed] [Google Scholar]
- 11.Macmillan D, Canadian Paediatric Society, Fetus and Newborn Committee Routine administration of vitamin K to newborns. Paediatr Child Health. 1997;2:429–31. doi: 10.1093/pch/2.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava A, Brewer AK, Mauser-Bunschoten, et al. Guidelines for the management of hemophilia. Hemophilia. 2013;19:e1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 13.Recht M, Pollmann H, Tagliaferri A, et al. A retrospective study to describe the incidence of moderate to severe allergic reactions to factor IX in subjects with haemophilia B. Haemophilia. 2011;17:494–9. doi: 10.1111/j.1365-2516.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- 14.Ewenstein BM, Takemoto C, Warrier I, et al. Nephrotic syndrome as a complication of immune tolerance in hemophilia B. Blood. 1997;89:1115–6. [PubMed] [Google Scholar]
- 15.Chitlur M, Warrier I, Rajpurkar M, et al. Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997–2006) Haemophilia. 2009;15:1027–31. doi: 10.1111/j.1365-2516.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 16.Darby SC, Keeling DM, Spooner RJD, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–99. J Thromb Haemost. 2004;2:1047–54. doi: 10.1046/j.1538-7836.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 17.Katz J. Prevalence of factor IX inhibitors among patients with haemophilia B: Results of a large-scale North American survey. Haemophilia. 1996;2:28–31. doi: 10.1111/j.1365-2516.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 18.Sultan Y. Prevalence of inhibitors in a population of 3435 hemophilia patients in France. French Hemophilia Study Group. Thromb Haemost. 1992;67:600–2. [PubMed] [Google Scholar]
- 19.Gale RF, Hird MF, Colvin BT. Management of a premature infant with moderate haemophilia A using recombinant factor VIII. Haemophilia. 1998;4:850–3. doi: 10.1046/j.1365-2516.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- 20.Bidlingmaier C, Bergmann F, Kurnik K. Haemophilia A in two premature infants. Eur J Pediatrics. 2005;164:70–2. doi: 10.1007/s00431-004-1542-6. [DOI] [PubMed] [Google Scholar]
- 21.Kraft KE, Verlaak R, van Heijst AF, Nováková I, Brons PP. Management of haemophilia in three premature infants. Haemophilia. 2008;14:378–80. doi: 10.1111/j.1365-2516.2007.01645.x. [DOI] [PubMed] [Google Scholar]
- 22.Gelbart B, Barnes C. Severe haemophilia and extreme prematurity – a case report. Haemophilia. 2009;15:352–4. doi: 10.1111/j.1365-2516.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 23.Cartledge P, Deakin K, McKecknie L, et al. A case report of a premature infant with haemophilia A and factor VIII inhibitor. Haemophilia. 2011;17:711–2. doi: 10.1111/j.1365-2516.2010.02455.x. [DOI] [PubMed] [Google Scholar]


