Abstract
The transverse upper gracilis free flap is a well-described option for breast reconstruction. The technique is a secondary choice for autologous breast reconstruction because the abdomen remains the primary donor site for breast reconstruction. However, in appropriately selected patients, the authors believe that the transverse upper gracilis flap remains a reliable flap for breast reconstruction. Its consistent anatomy, potentially reasonable donor site scar, limited functional morbidity and simultaneous two-team surgical approach make this flap a viable option for many patients. The technique, however, is not without drawbacks – known numbness of the medial thigh and the potential for chronic lymphedema of the lower leg, contour deformities of the medial thigh, and widening of the medial thigh scar need to be considered.
The current article presents a harvest technique that is reliable, rapid and addresses each of the above-mentioned limitations with specific changes in the traditional technique. The article provides video documentation of the modified harvest technique using only monopolar cautery for the dissection.
Keywords: Autologous breast reconstruction, Breast reconstruction, Dissection technique, Free flap, Gracilis free flap, TUG flap
Abstract
Le lambeau supérieur transverse du muscle gracile est une méthode bien connue de reconstruction mammaire. Cette technique est un choix secondaire en cas de reconstruction mammaire autologue, car l’abdomen demeure le principal foyer de prélèvement en vue de ce type de reconstruction. Cependant, chez certains patients bien choisis, les auteurs sont d’avis que le lambeau supérieur transverse du muscle gracile demeure fiable pour procéder à cette reconstruction. Son anatomie uniforme, la cicatrice raisonnable potentielle au foyer du prélèvement, la morbidité fonctionnelle limitée et l’approche chirurgicale à deux équipes font de ce prélèvement de lambeau une option viable pour de nombreux patients. La technique n’est toutefois pas sans défauts : il faut tenir compte de l’engourdissement connu de la cuisse, du potentiel de lymphœdème chronique de la jambe inférieure, de la déformation du contour de la cuisse et de l’élargissement de la cicatrice de la cuisse de la partie médiale.
Le présent article propose une technique de prélèvement à la fois fiable et rapide et remplace chacune des limites susmentionnées par des changements particuliers à la technique traditionnelle. Il présente une vidéo de cette technique, faisant seulement appel à la cautérisation monopolaire de la dissection.
The majority of autologous free flap breast reconstructions use tissue from the lower anterior abdominal wall. Traditionally, this involved harvesting a ipsilateral or bilateral transverse rectus abdominus (TRAM) flap, muscle-sparing TRAM flap, perforator-based flap (deep inferior epigastric perforator artery) or superficial inferior epigastric artery (SIEA) flap. In patients who have undergone significant previous abdominal surgery or in whom insufficient abdominal tissue exists, alternative donor sites are needed. In these situations, the upper inner thigh skin and fat is typically a valuable secondary donor site for autologous breast reconstruction. The transverse upper gracilis (TUG) flap harvests this skin and fat in a horizontally oriented ellipse along the medial aspect of the thigh, which was initially described by Yousif et al (1) in 1992, with the first clinical reports in 1993 (2). This myocutaneous flap uses the medial circumflex artery and venae comitantes to reliably perfuse a sizable, horizontally oriented skin paddle, with the associated underlying fat and a small portion of the gracilis muscle. The flap has a well-known, dependable and easy-to-harvest vascular pedicle, rarely associated with previous surgical scars (3–5). It can have significant amounts of adipose tissue and excess skin, especially in patients with a higher body mass index. In patients who have undergone previous cosmetic abdominal surgery, such as abdominoplasties or liposuction to the abdominal wall, the proportional excess fat and skin of the medial thigh can be significant, and the cosmetic advantage of tightening the upper inner thigh skin as a thigh lift can be an added benefit. While having many advantages and a proposed low donor site morbidity, distinct limitations have become apparent with the routine use of this flap (6–9). The primary limitations include the potential damage to the lymphatic drainage of the leg, the limited amount of fat and, particularly, available skin, the widening and lowering of the donor site closure scar with time, and the relatively short pedicle length. Modifications to the standard harvest technique reduce these limitations without compromising the advantages of this flap as a secondary autologous tissue-free breast reconstruction donor site (9–13). The standard gracilis myocutaneous flap remains a well-known and well-used flap for multiple reconstructive problems; however, due to the perceived donor site issues, the TUG flap continues to be under-used for autologous breast reconstruction.
The TUG is perfused by a branch of the medial circumflex artery and its venae that reliably perfuse the gracilis muscle and the ipsilateral medial upper thigh skin and fat. The skin paddle and underlying fat are horizontally oriented as a wide ellipse over the proximal upper one-third of the gracilis muscle. The amount of skin and fat taken is dependent on the amount of donor tissue available and the volume requirements for the breast reconstruction. Early in our experience, every attempt was made to harvest a sufficiently large flap from one thigh to reconstruct each mastectomy defect. In patients with large breast volumes, the large flap volume required resulted in a donor site defect with an unsatisfactory contour deformity, widened and lowered donor scars, delayed wound healing and increased incidence of prolonged lower leg lymphedema. Due to these problems, our technique evolved to using bilateral TUG flaps for unilateral breast reconstructions whenever limited amounts of medial thigh skin and fat were present. In so doing, larger breast volumes can be reconstructed and the medial thigh contour deformities are reduced, both thighs are symmetrical, donor scar widening is limited, and the incidence of delayed wound healing and lymphedema decreased. Further refinements of the harvest technique include limiting the anterior boundary of harvest to the axis of the femoral neurovascular bundle and preservation of the saphenous vein. Extending the harvest of tissue anterior and beyond the femoral axis uniformly results in fat necrosis of this end of the flap, and preserving the saphenous vein preserves the lymphatics that lie just below. Despite being a valuable donor site, the TUG flap continues to be used sparingly in breast reconstruction. We believe our modifications of the TUG flap enable us to avoid many of the previously cited drawbacks of this flap.
TUG AUTOLOGOUS TISSUE HARVEST ALGORITHM
The TUG flap is always a secondary choice in free breast reconstruction. When the abdominal wall tissue cannot be used, all secondary donor sites are examined for their respective appropriateness.
The algorithm for deciding whether to use a TUG flap is based on the following considerations. First, the abdominal tissue is assessed. If the patient does not have sufficient abdominal adipose tissue or the patient has a contraindication to having the abdominal skin harvested, the TUG donor site is evaluated. The volume of breast tissue to be reconstructed is estimated and compared with the excess tissue on the medial upper thighs. The maximum volume limit of fat and skin to be harvested from the thigh is that which would be resected if the patient was to undergo a cosmetic medial thigh lift. With this limitation, if the breast reconstruction requires a larger volume than a single leg can offer, both medial thighs are committed to a single breast reconstruction. The donor vessels remain the internal mammary vessels. In the event that two flaps are required for a single breast reconstruction, most commonly one flap is connected retrograde and the second flap connected antegrade to the internal mammary vessels. Rarely can the two flaps can be connected to one another by means of a side branch and then subsequently one connected antegrade to the internal mammary vessels.
TUG HARVEST TECHNIQUE
The TUG harvest is performed using strictly monopolar cautery. The anterior border of the flap is drawn medial to the femoral neurovascular bundle (Figure 1). The posterior border of the flap is the posterior midline of the inferior buttock fold (Figure 2). The superior horizontal skin marking is drawn approximately 1 cm to 2 cm below the inguinal crease and the inferior gluteal fold (Figure 3). The widest part of the skin ellipse is just posterior to the posterior edge of the gracilis muscle and is estimated by pinching the skin to determine the amount of skin that can be closed without undue tension (Figure 3). With this skin mark drawn on the medial thigh, the inferior horizontal skin marking is made so as to connect the anterior and posterior limits of the flap as a curved line. The majority of the adipose tissue is actually obtained from the subcutaneous fat behind the posterior aspect of the gracilis muscle.
Figure 1).
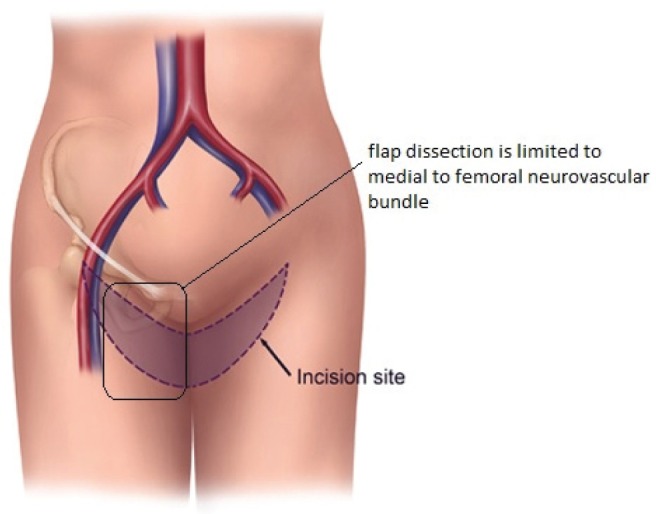
Anterior dissection limit of transverse upper gracilis flap
Figure 2).
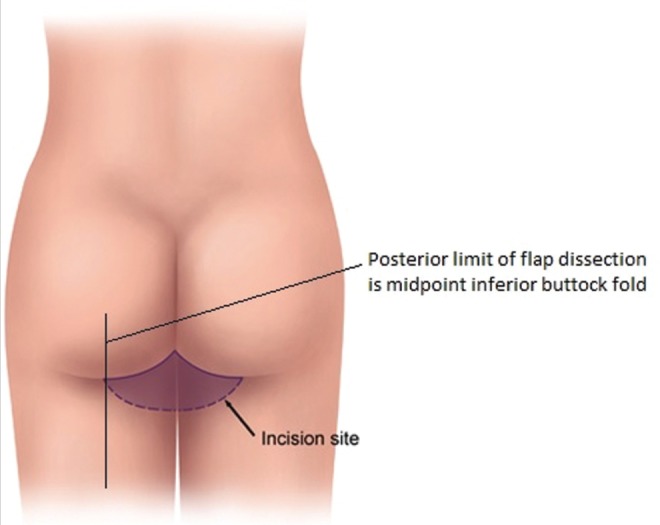
Posterior limit of transverse upper gracilis flap dissection
Figure 3).
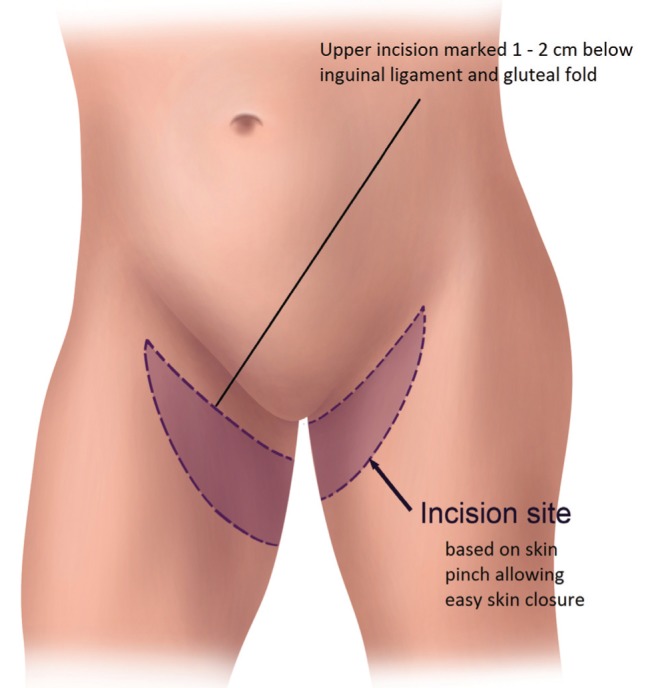
Transverse upper gracilis flap horizontal skin incision markings
The initial skin incisions are made and superficial dissection of the subcutaneous fat proceeds from anterior to posterior until the saphenous vein is located and preserved (Figure 4). From this point forward, a deeper dissection of all the underlying fat can be performed. Inferior undermining is performed down the medial thigh so as to capture additional but limited amounts of adipose tissue. This undermining captures only modest amounts of tissue along the central axis of the medial thigh but enables the harvest of considerably more tissue posterior to the gracilis in the buttock region. The majority of adipose tissue is harvested posterior to the gracilis because the thickness of adipose tissue is significantly greater and leads to a limited contour deformity and no lymphatic drainage disruption. This dissection continues all the way inferiorly and posteriorly over portions of the semimembranosus and semitendinosus muscles. Once the flap has been dissected out superficially on the thigh, dissection of the underlying medial thigh muscles can begin. The posterior aspect of the flap is lifted off the semimembranosus and semitendinosus, moving anteriorly until the adductor magnus is visible. Subsequently, the gracilis is visualized and then cut distal to the inferior edge of the flap and as proximal on the thigh as possible. At this point, the pedicle is visualized entering the gracilis. The adductor longus muscle is then elevated off the medial thigh, just anterior to the gracilis muscle. Two small branches off the pedicle enter each border of the adductor longus muscle. These need to be dissected into the muscle for approximately 1 cm and then clipped. The adductor muscle is now freely mobile, allowing the vascular pedicle to the gracilis to be easily and rapidly dissected (Figure 5). Finally, the dissection continues deep to the anterior border of the adductor longus muscle. The vascular pedicle to the gracilis is dissected to its origin from the profunda vessels. The crescent-shaped flap is then able to be lifted off the medial thigh, typically with an 8 cm pedicle (Figure 6). A short cuff of muscle is typically taken with each flap, but if an increased pedicle length is needed, the vessels can be dissected through the muscle in the style of a perforator flap and the muscle discarded. A video demonstration of this dissection is available for review (go to www.pulsus.com).
Figure 4).
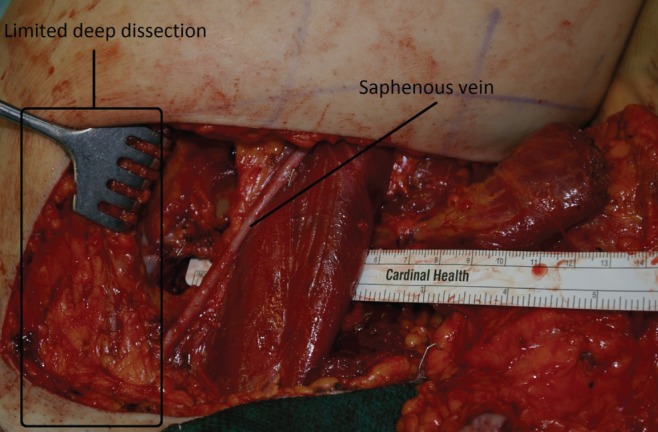
Saphenous vein preserved in anterior transverse upper gracilis dissection
Figure 5).
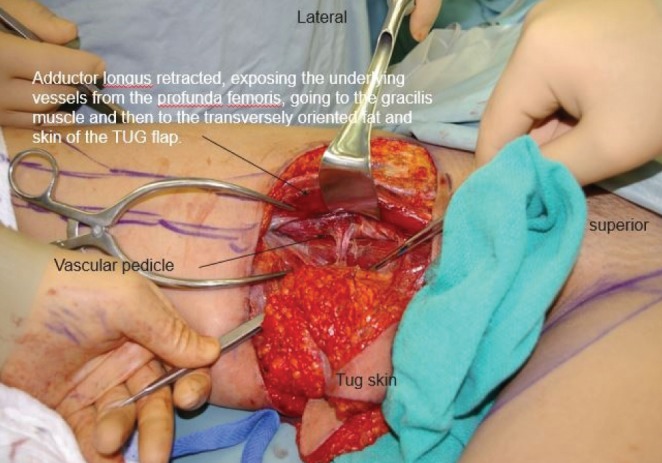
Transverse upper gracilis (TUG) flap dissected with adductor longus muscle retracted exposing vascular pedicle
Figure 6).
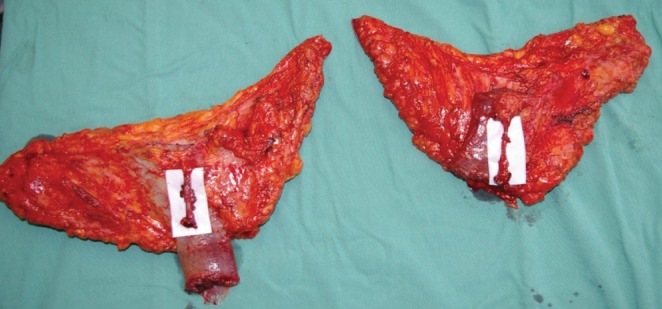
Harvested transverse upper gracilis flaps
CONCLUSION
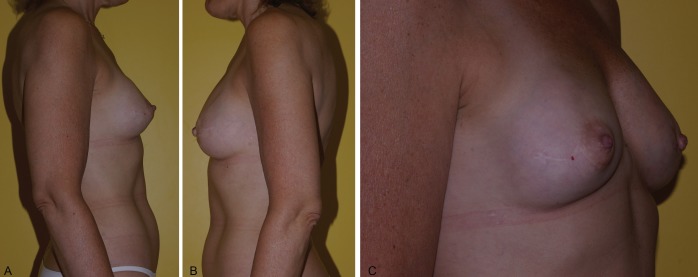
Autologous free breast reconstruction still uses the lower abdominal skin and fat as the most common donor site. If this site is not available, a variety of secondary choices exist. The upper medial thigh skin and fat used as a TUG flap is a valuable choice. The morbidity associated with the donor site can be reduced significantly by limiting the anterior limits of flap dissection to avoid fat necrosis, preserving the saphenous vein to eliminate prolonged leg swelling, and using bilateral flaps for volume to eliminate contour deformities and widened scars. The harvest technique presented here outlines the evolution of this technique, which has resulted in donor site complication rates more comparable with the abdominal donor site (Figures 7 to 16).
Figure 7).
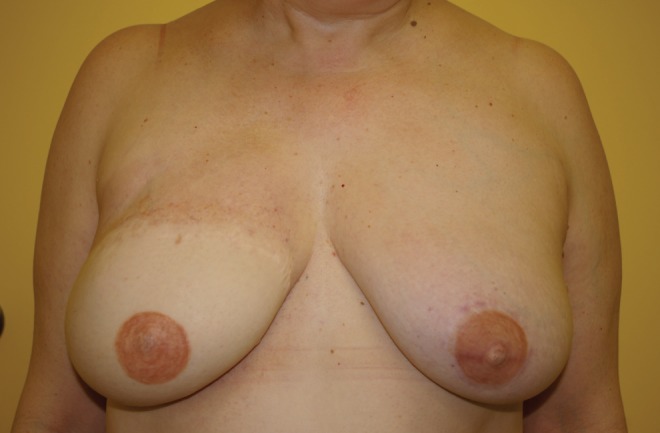
Previous right delayed deep inferior epigastric perforator artery. Immediate left transvers upper gracilis flap
Figure 16).
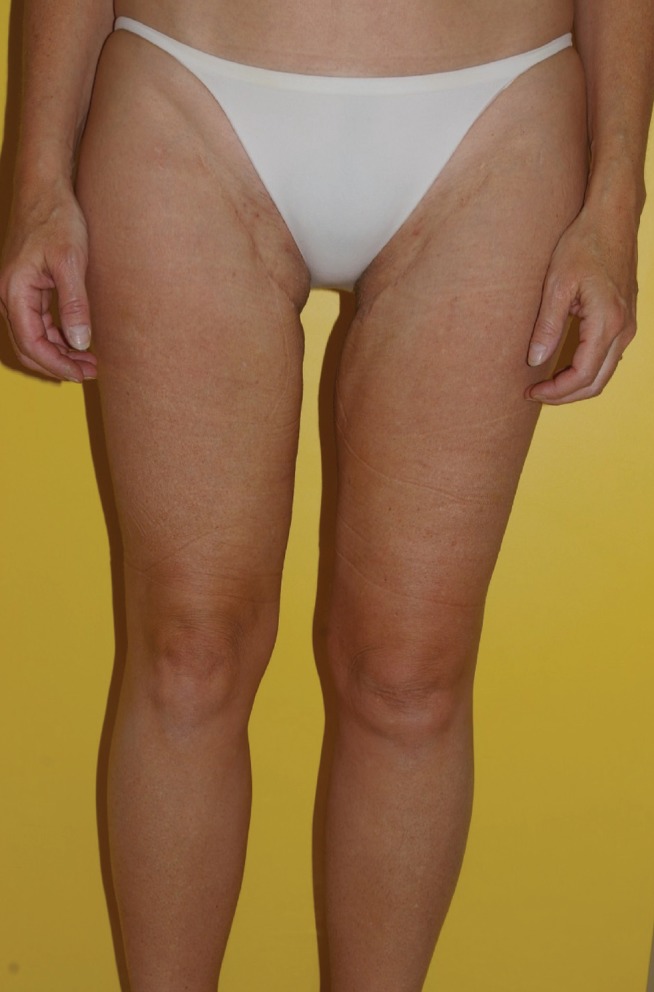
Bilateral transverse upper gracilis flaps to reconstruct bilateral breasts postoperative donor. Right nipple-sparing – prophylactic mastectomy. Left skin-sparing mastectomy for cancer
Figure 8).
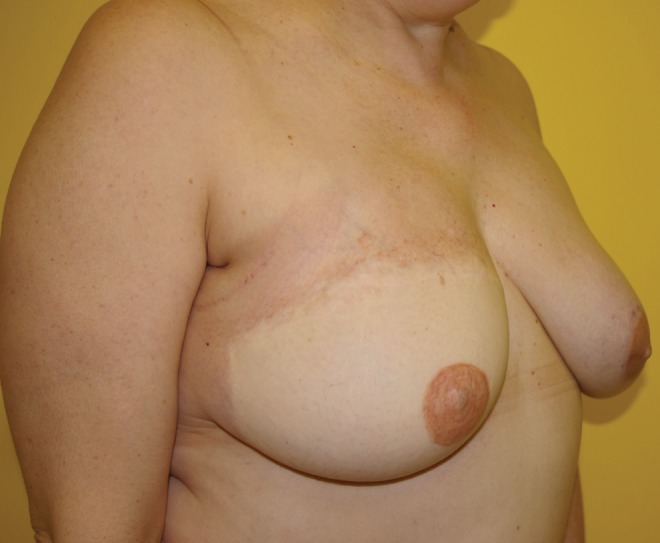
Previous right delayed deep inferior epigastric perforator artery flap. Immediate left transverse upper gracilis flap
Figure 9).
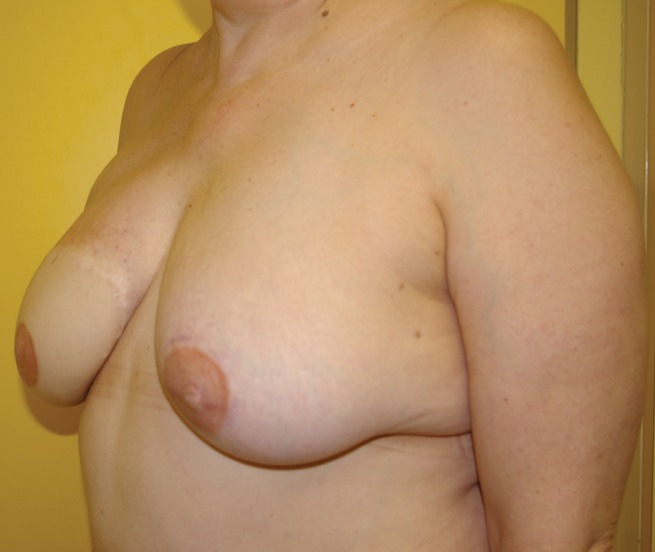
Previous right delayed deep inferior epigastric perforator artery. Immediate left transverse upper gracilis
Figure 10).
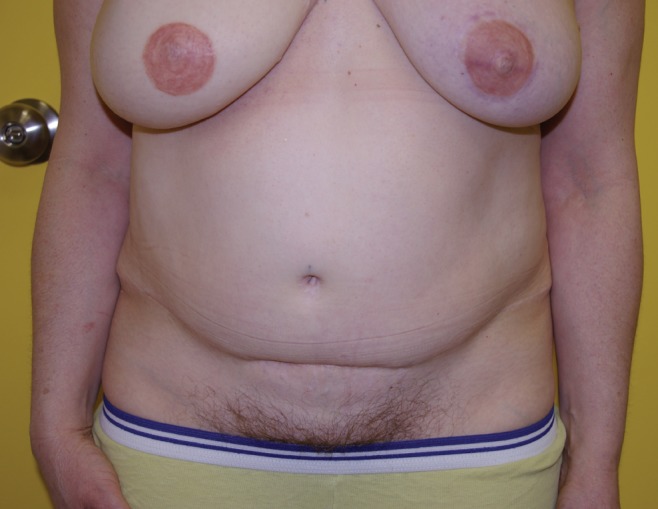
Previous right delayed deep inferior epigastric perforator artery flap. Immediate left transverse upper gracilis flap – abdomen scar
Figure 11).

Previous right delayed deep inferior epigastric perforator artery flap. Immediate left transverse upper gracilis flap – transverse upper gracilis donor site scar
Figure 12).
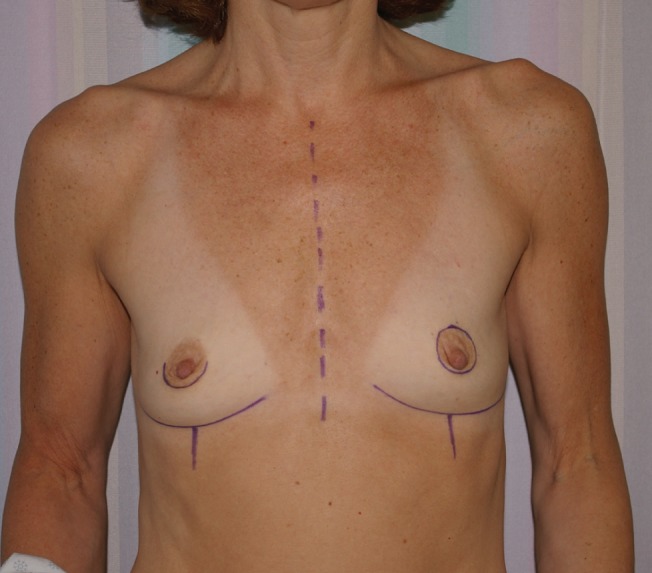
Bilateral transverse upper gracilis flaps to reconstruct bilateral breasts preoperatively. Right nipple sparing – prophylactic mastectomy. Left skin-sparing mastectomy for cancer
Figure 13).
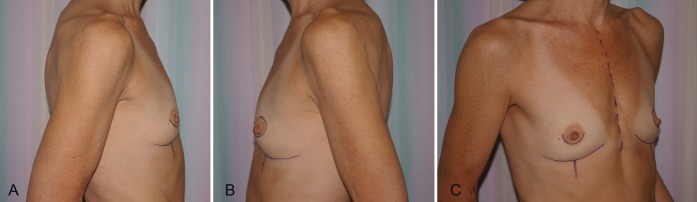
Bilateral transverse upper gracilis flaps to reconstruct bilateral breasts preoperatively. Right nipple-sparing – prophylactic mastectomy. Left skin-sparing mastectomy for cancer
Figure 14).
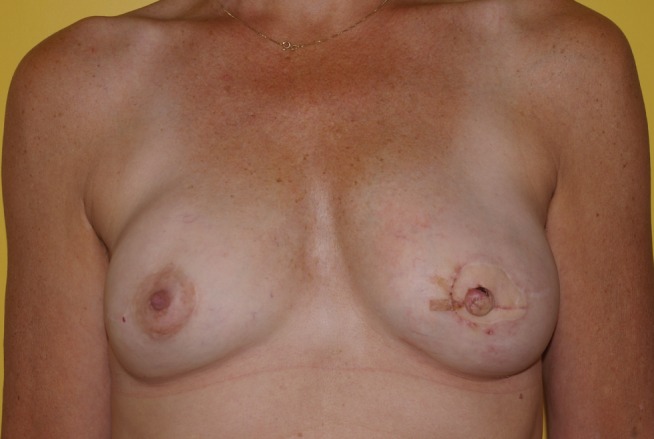
Bilateral transverse upper gracilis flaps to reconstruct bilateral breasts postoperatively. Right nipple-sparing – prophylactic mastectomy. Left skin-sparing mastectomy for cancer
Figure 15).
Bilateral transverse upper gracilis flaps to reconstruct bilateral breasts postoperatively. Right nipple-sparing – prophylactic mastectomy. Left skin-sparing mastectomy for cancer
REFERENCES
- 1.Yousif NJ, Matloub HS, Kolachalam R, Grunert BK, Sanger JR. The transverse gracilis musculocutaneous flap. Ann Plast Surg. 1992;29:482–90. doi: 10.1097/00000637-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Yousif NJ. The transverse gracilis musculocutaneous flap. Ann Plast Surg. 1993;31:382. doi: 10.1097/00000637-199310000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Wechselberger G, Scholler T, Bauer T, et al. Surgical technique and clinical application of the transverse gracilis myocutaneous free flap. Br J Plast Surg. 2001;54:423–7. doi: 10.1054/bjps.2001.3607. [DOI] [PubMed] [Google Scholar]
- 4.Schoeller T, Meirer R, Otto-Schoeller A, Wechselberger G, Piza-Katzer H. Medial thigh lift free flap for autologous breast augmentation after bariatric surgery. Obes Surg. 2002;12:831–4. doi: 10.1381/096089202320995655. [DOI] [PubMed] [Google Scholar]
- 5.Wechselberger G, Schoeller T. The transverse myocutaneous gracilis free flap: A valuable tissue source in autologous breast reconstruction. Plast Reconstr Surg. 2004;114:69–73. doi: 10.1097/01.prs.0000127797.62020.d4. [DOI] [PubMed] [Google Scholar]
- 6.Schoeller T, Huemer GM, Wechberger G. The transverse musculocutaneous gracilis flap for breast reconstruction: Guidelines for flap and patient selection. Plast Reconstr Surg. 2008;122:29–38. doi: 10.1097/PRS.0b013e318177436c. [DOI] [PubMed] [Google Scholar]
- 7.Pülzl P, Schoeller T, Kleenwein K, Wechselberger G. Donor-site morbidity of the transverse musculocutaneous gracilis flap in autologous breast reconstruction: Short-term results. Plast Reconstr Surg. 2011;128:233e–42e. doi: 10.1097/PRS.0b013e3182268a99. [DOI] [PubMed] [Google Scholar]
- 8.Buntic RF, Horton LM, Brooks D, Althubaiti GA. Transverse upper gracilis flap as an alternative to abdominal tissue breast reconstruction: Technique and modifications. Plast Reconstr Surg. 2011;128:607e–13e. doi: 10.1097/PRS.0b013e318230c2b6. [DOI] [PubMed] [Google Scholar]
- 9.Fattah A, Figus A, Mathur B, Ramakrishnan VV. The transverse myocutaneous gracilis flap: Technical refinements. J Plast Reconstr Aesthet Surg. 2010;63:305–13. doi: 10.1016/j.bjps.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Schoeller T, Huemer GM, Kolehmaninen M, Otto-Schoeller A, Wechselberger G. A new “Siamese” flap for breast reconstruction: The combined infragluteal-transverse myocutaneous gracilis muscle flap. Plast Reconstr Surg. 2005;115:1110–7. doi: 10.1097/01.prs.0000156213.68163.1b. [DOI] [PubMed] [Google Scholar]
- 11.Vega SJ, Sandeen SN, Bossert RP, Perrone A, Ortiz L, Herrera H. Gracilis myocutaneous free flap in autologous breast reconstruction. Plast Reconstr Surg. 2009;124:1400–9. doi: 10.1097/PRS.0b013e3181babb19. [DOI] [PubMed] [Google Scholar]
- 12.Fansa H, Schirmer S, Warnecke IC, Cervelli A, Frerichs O. The transverse myocutaneous gracilis muscle flap: A fast and reliable method for breast reconstruction. Plast Reonstr Surg. 2008;122:1326–33. doi: 10.1097/PRS.0b013e318188205f. [DOI] [PubMed] [Google Scholar]
- 13.Saint-Cyr M, Wong C, Oni G, et al. Modifications to extend the transverse upper gracilis flap in breast reconstruction: Clinical series and results. Plast Reconstr Surg. 2010;129:24e–36e. doi: 10.1097/PRS.0b013e31823620cb. [DOI] [PubMed] [Google Scholar]