Abstract
A pre-exposure to isoflurane reduces ischemic brain injury in rodents (isoflurane preconditioning). This neuroprotection has acute and delayed phases. Our previous in vitro studies suggest that the acute phase may involve excitatory amino acid transporters (EAAT). We determine whether this protection involves EAAT3, the major neuronal EAAT. Adult male EAAT3 knockout mice and their wild-type littermates were exposed or were not exposed to 1.5% isoflurane for 30 min. Sixty minutes later, they were subjected to a 90- or 60-min middle cerebral arterial occlusion (MCAO). Their neurological outcomes were evaluated 24 h after the MCAO. In another experiment, cerebral cortex was harvested for Western blotting at 30 min after animals were exposed to 1.5% isoflurane for 30 min. Here, we showed that isoflurane reduced brain infarct volumes and improved neurological functions of wild-type mice after a 90-min MCAO. However, isoflurane pre-exposure did not change the neurological outcome of EAAT3 knockout mice no matter whether the MCAO was for 90 min or 60 min. Isoflurane increased phospho-Akt, a survival-promoting protein, in the wild-type mice but not in the EAAT3 knockout mice. The isoflurane-induced neuroprotection in the wild-type mice was abolished by LY294004, an Akt activation inhibitor. LY294004 alone did not affect the neurological outcome of the wild-type or EAAT3 knockout mice after focal brain ischemia. These results suggest that the isoflurane preconditioning-induced acute phase of neuroprotection involves EAAT3. The downstream event includes Akt activation.
Keywords: Akt, glutamate transporter, isoflurane, neuroprotection, preconditioning
1. Introduction
A prior exposure to isoflurane has been shown to reduce ischemic brain injury in rodents (isoflurane preconditioning) (Kapinya, et al., 2002; Zheng and Zuo, 2003; Zheng and Zuo, 2004). This neuroprotection has two phases. The acute phase has an effective time-window from a few minutes to a few hours after the isoflurane exposure and has been shown mainly by using rat brain slice model (Wang, et al., 2007; Zheng and Zuo, 2003). The effective time-window of the delayed phase is from a few hours to a few days. This delayed phase of neuroprotection has been shown in various species under in vivo and in vitro conditions (Kapinya, et al., 2002; Zheng and Zuo, 2004; Zuo, 2012). It has been suggested that the acute phase of preconditioning-induced neuroprotection may involve mechanisms different from those for the delayed phase (Dirnagl, et al., 2003; Gidday, 2006). Our previous studies suggest that the acute phase of isoflurane preconditioning-induced neuroprotection may involve glutamate transporters (also called excitatory amino acid transporters, EAAT) in rats because EAAT inhibitors attenuate this neuroprotection (Wang, et al., 2007; Zheng and Zuo, 2003).
The major function identified so far for EAATs is to uptake glutamate and other related amino acids into cells (Danbolt, 2001). Glutamate is the major excitatory neurotransmitter. Extracellular enzyme to break down glutamate has not been found yet. Thus, binding and uptake of glutamate by EAATs have been proposed to be a major mechanism to regulate extracellular glutamate concentrations. EAATs via this function can regulate glutamate neurotransmission (Danbolt, 2001). Five EAATs have been identified. EAAT1 and EAAT2 are glial. EAAT3 and EAAT4 are mainly expressed in the neurons. EAAT5 is mostly in the retina. EAAT2 and EAAT3 are widely expressed in the central nervous system and are the major EAATs in the glia and neurons, respectively (Danbolt, 2001).
Our previous studies have shown that isoflurane increases EAAT3 activity and does not affect EAAT2 activity (Fang, et al., 2002; Huang and Zuo, 2005). Thus, we hypothesize that EAAT3 is involved in isoflurane preconditioning-induced acute phase of neuroprotection. We test this hypothesis by using EAAT3 knockout mice. Additional experiments are performed to determine if EAAT3 is needed for isoflurane to induce activation of proteins that promote cell survival.
2. Methods and Materials
2.1. Animal groups
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 1996.
EAAT3 knockout mice and their wild-type littermates were used in this study. EAAT3 knockout mice were descendants of mice used in a previous study (Peghini, et al., 1997). They were initially backcrossed with wild-type CD-1 mice for more than 10 generations to produce a strain of EAAT3 knockout mice before they were used in any of our studies. Additional backcrossing of the EAAT3 knockout mice with wild-type CD-1 mice at least once every 8 generations to prevent genetic drift has been performed in our laboratory. These CD-1 wild-type mice were from Charles River Laboratories (Wilmington, MA). It has been confirmed that the EAAT3 knockout mice after those times of backcrossing did not express EAAT3 proteins in our previous study (Li and Zuo, 2011). The wild-type littermates of these EAAT3 knockout mice were used in the current study.
Eight-week old male wild-type mice were randomly divided into the following groups: 1) middle cerebral arterial occlusion (MCAO), 2) MCAO plus pretreatment with isoflurane, 3) MCAO plus pretreatment with LY294004 (5 mg/kg, intraperitoneal injection at 120 min before the MCAO), and 4) MCAO plus pretreatment with isoflurane and LY294004 (5 mg/kg, intraperitoneal injection at 30 min before isoflurane exposure). Eight-week old male EAAT3 knockout mice were randomly divided into two groups: MCAO group and MCAO pretreated with isoflurane. The isoflurane pretreatment was performed by exposing mice to 1.5% isoflurane for 30 min at 60 min before a 90-min right MCAO.
In the second experiment, 8-week old wild-type mice were subjected to a 90-min MCAO but the EAAT3 knockout mice were exposed to a 60 min MCAO. EAAT3 knockout mice were randomly distributed to three groups: 1) MCAO, 2) MCAO plus pretreatment with isoflurane, and 3) MCAO plus pretreatment with LY294004. The isoflurane and LY294004 pretreatments were the same as in the first experiment.
In the third experiment, male wild-type or EAAT3 knockout mice were randomly assigned to be exposed to or not exposed to 1.5% isoflurane for 30 min. The brain cortices of these mice were harvested at 30 min after the exposure to isoflurane for Western analysis.
2.2. Isoflurane pretreatment and middle cerebral artery occlusion
Isoflurane pretreatment was performed in the following way. Anesthesia was induced with 4% isoflurane and then maintained through a mask with 1.5% isoflurane. The exact isoflurane concentrations in the mask were delivered by an isoflurane-specific vaporizer and were continuously monitored by a Datex infrared analyzer (Capnomac, Helsinki, Finland). Body temperature was maintained at 37°C using a thermostat-controlled heating blanket during isoflurane exposure. Heart rate, breathing rate and pulse oximeter oxygen saturation (SpO2) of mice were monitored continuously and noninvasively using a MouseOX Murine Plus Oximeter System (Starr Life Sciences Corporation, Oakmont, PA, USA).
MCAO was performed using the monofilament suture method (Li and Zuo, 2011) under anesthesia with 1.5% isoflurane. The procedure started at 30 min after isoflurane pretreatment (isoflurane-free period) and timed to take 30 min to achieve MCAO. After a midline incision was made at the ventral surface of the neck skin, an 8-0 monofilament nylon surgical suture with a rounded tip (Beijing Sunbio Biotech Co. Ltd, Beijing, China) was introduced through the right external carotid artery to the internal carotid artery until resistance was felt. Anesthesia was stopped once the right MCAO was achieved. Body temperature was maintained at 37°C. Heart rate, breathing rate and SpO2 were monitored continuously and noninvasively during the surgery. Mice were reanesthetized by isoflurane at 60 min or 90 min after the onset of MCAO to remove the suture. This reanesthesia lasted for ~2 min. After recovery from anesthesia, mice were placed back in their cages with ad libitum access to food and water.
2.3. Evaluation of motor coordination, neurological deficit scores and infarct volumes
Neurological deficit scores, motor coordination and infarct volumes of animals were evaluated at 24 h after transient MCAO as we previously described (Li and Zuo, 2011). Neurological deficit scores were evaluated based on an eight-point scale by an individual blinded to the group assignment with a scale described before (Rogers, et al., 1997).
Motor coordination was evaluated just before and at 24 h after the MCAO. Mice were placed on an accelerating rotarod. The speed of the rotarod was increased from 4 to 40 r.p.m. in 5 min. The latency and speed of the rotarod at which a mouse fell off the rotarod were recorded. Each mouse was tested five times. The speed–latency index (latency in seconds × speed in r.p.m.) of each of the five tests was calculated and the mean index of the five trials was used to reflect the motor coordination function of each mouse before or after MCAO. All mice were trained for two continuous days before the formal tests. They were placed on the rotarod five times each day. This training occurred just before they were subjected to the MCAO.
The assessment of infarct volumes was performed after 2,3,5-triphenyltetrazolium chloride staining. In brief, mice were killed by 5% isoflurane. The brains were removed rapidly and coronally cut into eight 1-mm-thick slices, except for the first and last slices that were 2 mm thick. The slices were incubated with 2% 2,3,5-triphenyltetrazolium chloride solution. The infarct areas were quantified using NIH Image 1.60 (NIH, Bethesda, MD, USA). The percentage of infarct volumes in ipsilateral hemisphere volumes was calculated to account for cerebral edema and differential shrinkage resulting from brain ischemia and tissue processing and to correct for the individual difference in brain volumes.
2.4. Western blot analysis
Total lysates of the cortex (100 μg protein per lane) were subjected to Western blot analysis. Primary antibodies used were rabbit polyclonal anti-phospho-Akt antibody (1:500 dilution; Catalog number: 9271), rabbit polyclonal anti-Akt antibody (1:1000 dilution; Catalog number: 9272), rabbit polyclonal anti-phospho-extracellular signal-regulated kinase 1/2 (ERK1/2) (1:500 dilution; Catalog number: 4377), rabbit polyclonal anti-Erk1/2 antibody (1:1000 dilution; Catalog number: 4695), rabbit polyclonal anti-phospho (Ser9)-glycogen synthase kinase 3β (GSK3β) antibody (1:500 dilution; Catalog number: 9336), rabbit monoclonal anti-GSK-3β antibody (1:1000 dilution; Catalog number: 9315) and rabbit polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:2000 dilution; Catalog number: G9545). The anti-GAPDH antibody was from Sigma-Aldrich (St Louise, MO). All other antibodies were from Cell Signaling Technology Inc. (Danvers, MA). The protein bands were visualized using enhanced chemiluminescence methods. The densities of phospho-Akt, Akt, phospho-ERK1/2, ERK1/2, phospho-GSK3β and GSK3β protein bands were normalized to those of GAPDH to control for errors in protein sample loading and transferring during western blotting. The results of mice in various groups were then normalized to the mean values of the wild-type control mice.
2.5. Statistical Analysis
Parametrical data are presented as means ± S.D. Comparison of the results of physiologic parameters, speed–latency index ratio, infarct volume and western blotting between the two EAAT3 knockout mouse groups in the first set of experiment or between the wild-type and EAAT3 knockout mice subjected to MCAO only were performed by Student’s t-test. Comparison of the neurological deficit scores between the two EAAT3 knockout mouse groups in the first set of experiment or between the wild-type and EAAT3 knockout mice subjected to MCAO only were analyzed by the Mann-Whitney rank-sum test. The results of physiologic parameters, speed–latency index ratio, infarct volume and western blotting in the wild-type and EAAT3 knockout mouse groups were analyzed by one-way analysis of variance, followed by the Student-Newman-Keuls test. Neurological deficit scores in the wild-type and EAAT3 knockout mouse groups were analyzed by one-way analysis of variance on ranks, followed by the Student-Newman-Keuls test. A P < 0.05 was accepted as significant. All statistical analyses were performed using the SigmaStat program (SYSTAT Software Inc., Point Richmond, CA, USA).
3. Results
No animals had an episode of hypoxia (SpO2 < 90%) during isoflurane pretreatment and the procedure to create MCAO. The heart rates and respiratory rates were stable during the procedure (Table 1).
Table 1.
Physiological data of mice during transient middle cerebral arterial occlusion (MCAO).
| Heart rate (beat/min) | Respiratory rate (time/min) | SpO2 (%) | |
|---|---|---|---|
| First study (MCAO was 90 min) | |||
| Wild-type MCAO | 412 ± 53 | 91 ± 27 | 98.9 ± 0.4 |
| Wild-type Iso + MCAO | 445 ± 77 | 81 ± 27 | 99.2 ± 0.4 |
| Wild-type LY + MCAO | 419 ± 40 | 119 ± 14* | 98.9 ± 0.5 |
| Wild-type LY + Iso + MCAO | 437 ± 40 | 92 ± 17 | 98.9 ± 0.3 |
| EAAT3−/− MCAO | 404 ± 47 | 92 ± 45 | 98.6 ± 1.4 |
| EAAT3−/− Iso + MCAO | 437 ± 64 | 83 ± 20 | 98.6 ± 0.7 |
| Second study (MCAO was 90 min for wild-type mice and 60 min for EAAT3−/− mice) | |||
| Wild-type MCAO | 505 ± 68 | 82 ± 25 | 97.1 ± 3.1 |
| EAAT3−/− MCAO | 402 ± 56 | 85 ± 24 | 98.4 ± 1.2 |
| EAAT3−/− Iso + MCAO | 400 ± 18 | 92 ± 8 | 99.2 ± 0.2 |
| EAAT3−/− LY + MCAO | 401 ± 55 | 88 ± 17 | 98.9 ± 0.4 |
Data are means ± S.D. (n = 7 – 10). The data were obtained at the time when the MCAO was achieved.
P < 0.05 compared with wild-type mice subjected to MCAO only group. Iso: 1.5% isoflurane for 30 min; LY: LY294004; SpO2: pulse oximeter oxygen saturation.
In the first experiment in which a 90-min MCAO was applied to all mice, the total number of mice used in each study group was 10 except that 11 EAAT3 knockout mice were used in the MCAO only group. One mouse from each group except for 3 mice in the wild-type LY294004 plus isoflurane preconditioning group and 2 mice in the EAAT3 knockout MCAO only group died during the observation period. There was no difference among the mortality rates by Chi-square. These dead mice had significant brain infarction. Their data were included in the analysis of neurological function outcome.
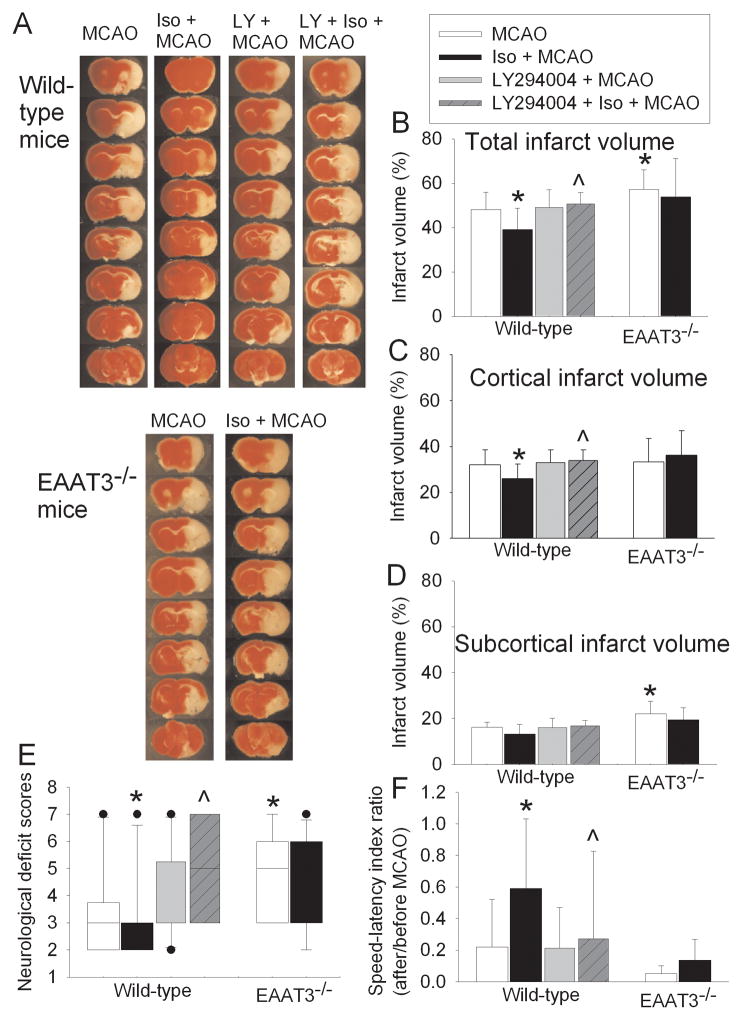
Wild-type mice with a prior isoflurane exposure had smaller infarct volumes (37.3 ± 10.2% vs. 48.2 ± 7.8% of control group, P < 0.05) and better neurological functions as assessed by neurological deficit scores and performance on rotarod than wild-type mice without a prior isoflurane exposure at 24 h after a 90-min MCAO. This isoflurane effect was abolished by LY294004, a phosphoinositide 3 kinase (PI3K) inhibitor. LY294004 did not affect the brain infarct volumes and neurological functions in wild-type mice subjected to the MCAO only. Consistent with our previous findings (Li and Zuo, 2011), EAAT3 knockout mice had bigger infarct volumes (57.2 ± 8.9% vs. 48.2 ± 7.8% of wild-type mice, P < 0.05) and worse neurological functions than wild-type mice after the 90-min MCAO. Unlike the case of wild-type mice, a prior isoflurane exposure did not reduce the brain infarct volumes and improve the neurological outcome in the EAAT3 knockout mice (Fig. 1).
Fig. 1. Isoflurane preconditioning-induced neuroprotection.
Mice were pretreated with or without 1.5% isoflurane for 30 min at 60 min before a 90-min middle cerebral arterial occlusion (MCAO). The results were evaluated at 24 h after the MCAO. A: Brain slices stained with 2,3,5-triphenyltetrazolium chloride from representative mice. B, C and D: Percentage of total, cortical and subcortical infarct volume in ipsilateral hemisphere volume. Results are the means ± S.D. (n = 10 – 11). E: Neurological deficit scores evaluated immediately before the animals were euthanized for the assessment of infarct sizes (data are presented in panels B, C and D) or assigned 7 to the animals that died before the end of observation period. Results are presented in a box plot format (n = 10 – 11). ●: lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25–75% interval including the median of the data. F: The performance on rotarod. Rats were tested before and 24 h after the MCAO and the speed-latency index ratio of these two tests are presented. Results are the means ± S.D. (n = 10 – 11). * P < 0.05 compared with the wildtype mice subjected to MCAO only. ^ P < 0.05 compared with wild-type mice subjected to isoflurane preconditioning plus MCAO. Iso: isoflurane; LY: LY294004; EAAT3−/−: EAAT3 knockout.
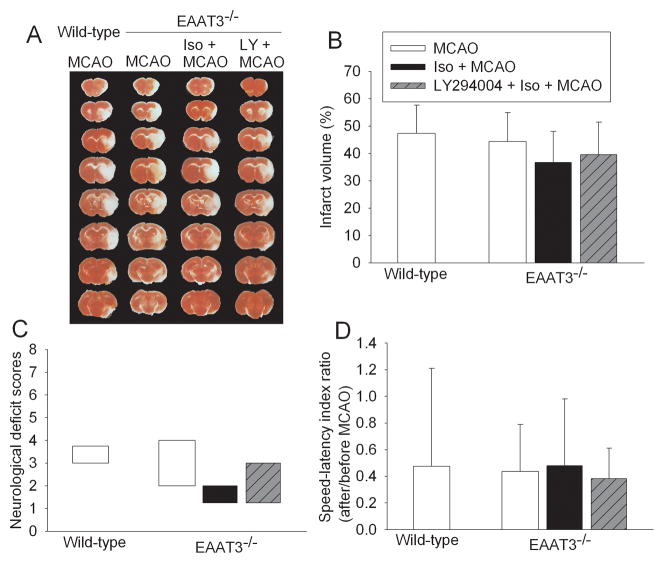
In the second experiment in which wild-type mice had a 90-min MCAO and EAAT3 knockout mice had a 60-min MCAO, the total number of mice used in each study group was 8 except that 7 EAAT3 knockout mice were used in the MCAO only group. One mouse from the wild type mouse group and the EAAT3 knockout LY294004 group died during the observation period. There was no difference among the mortality rates by Chi-square. These two dead mice had significant brain infarction. Their data were included in the analysis of neurological outcome. The infarct volumes and neurological functions of wild-type mice after a 90-min MCAO were similar to those of the EAAT3 knockout mice after a 60-min MCAO. However, isoflurane pretreatment still did not provide neuroprotection in the EAAT3 knockout mice after a 60-min MCAO. Pretreatment with LY294004 also did not affect the neurological outcome in these mice (Fig. 2).
Fig. 2. Failure for isoflurane to induce neuroprotection in EAAT3 knockout mice.
Wild-type mice had a 90-min middle cerebral arterial occlusion (MCAO). EAAT3 knockout mice were pre-treated with or without 1.5% isoflurane for 30 min at 60 min before a 60-min MCAO. The results were evaluated at 24 h after the MCAO. A: Brain slices stained with 2,3,5-triphenyltetrazolium chloride from representative mice. B: Percentage of total infarct volume in ipsilateral hemisphere volume. Results are the means ± S.D. (n = 7 – 8). C: Neurological deficit scores evaluated immediately before the animals were euthanized for the assessment of infarct sizes (data are presented in panel B) or assigned 7 to the animals that died before the end of observation period. Results are presented in a box plot format (n = 7 – 8). ●: lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25–75% interval including the median of the data. D: The performance on rotarod. Rats were tested before and 24 h after the MCAO and the speed-latency index ratio of these two tests are presented. Results are the means ± S.D. (n = 7 – 8). Iso: isoflurane; LY: LY294004; EAAT3−/−: EAAT3 knockout.
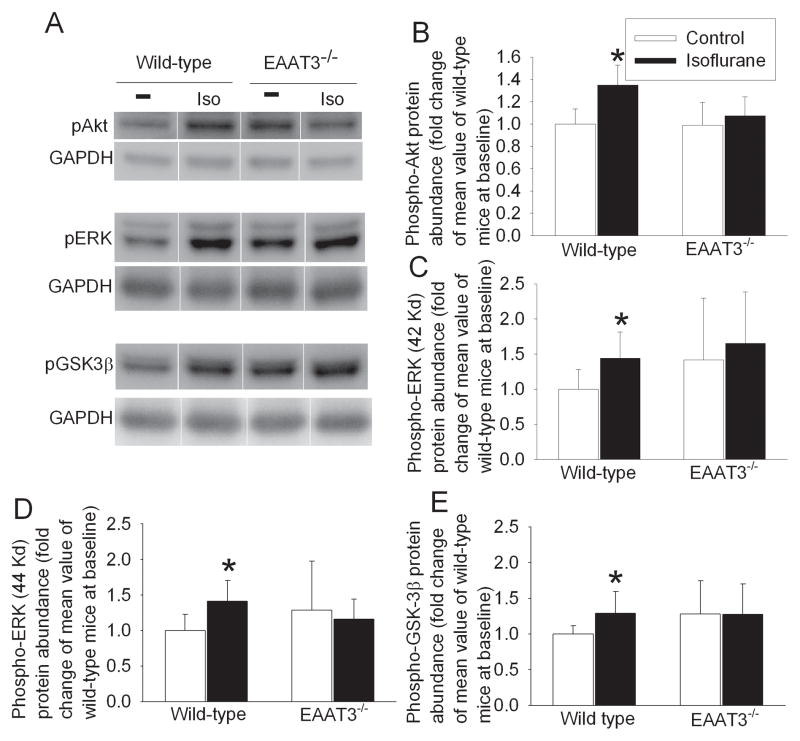
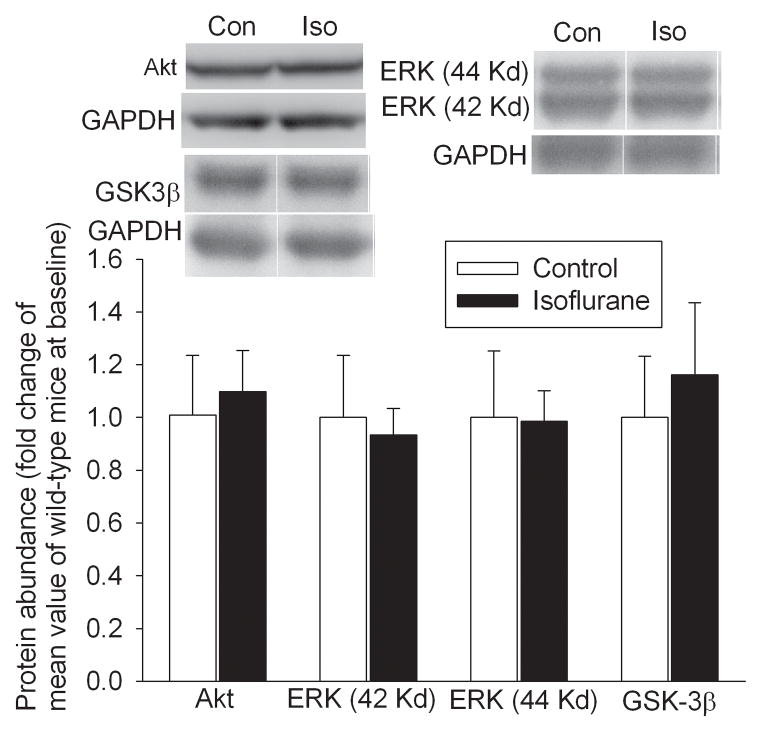
Isoflurane exposure significantly increased the expression of phospho-Akt (1.35 ± 0.18 vs. 1.00 ± 0.14 of control group, P < 0.05) and phospho-ERK in the cerebral cortices of wild-type mice. Phosphorylation of these protein kinases represents activated enzymes. Isoflurane exposure also increased the phosphorylation of GSK3β at Ser9. Phosphorylation at this site inhibits GSK3β. However, isoflurane exposure did not affect the expression of phospho-Akt, phospho-ERK and phospho-GSK3β in the EAAT3 knockout mouse brains (Fig. 3). Isoflurane exposure did not affect the total amount of Akt, ERK and GSK3β in the cerebral cortices of wild-type mice (Fig. 4).
Fig. 3. Isoflurane-induced phosphorylation of protein kinases.
The cerebral cortex was harvested at 30 min after mice were exposed to 1.5% isoflurane for 30 min. Total cell lysates were prepared for Western blotting. A: Representative Western blots. B – E: The graphic presentation of the phospho-Akt, phospho-ERK and Phospho-GSK3β protein abundance quantified by integrating the volume of autoradiograms from 6 – 8 mice for each experimental condition is shown. Values in graphs are the means ± S.D. * P < 0.05 compared with wild-type mice under control condition. EAAT3−/−: EAAT3 knockout.
Fig. 4. No effect of isoflurane on the expression of protein kinases in wild-type mice.
The cerebral cortex was harvested at 30 min after mice were exposed to 1.5% isoflurane for 30 min. Total cell lysates were prepared for Western blotting of total Akt, ERK and GSK3β. Representative Western blots are presented in the top panels. The graphic presentation of the Akt, ERK and GSK3β protein abundance quantified by integrating the volume of autoradiograms from 4 – 8 mice for each experimental condition is shown in the bottom pane. Values in graphs are the means ± S.D. Con: control; Iso: isoflurane.
4. Discussion
Protection induced by various preconditioning stimuli has been proposed to have two temporal phases based on the time at which the detrimental insult, such as ischemia, occurs in relationship to the preconditioning stimuli. The acute phase starts a few minutes and lasts for a few hours after the preconditioning stimuli. The delayed phase begins a few hours and can last for a few days after the preconditioning stimuli. It has been generally accepted that the mechanisms for the acute phase of protection involve alteration of the functions of existing proteins and that the delayed phase may be mediated by increased expression/synthesis of protective proteins (Dirnagl, et al., 2003; Gidday, 2006). In our study, there was a 60-min interval between the prior isoflurane exposure and the MCAO. The isoflurane exposure significantly improved neurological outcome after the MCAO in the wild-type mice. This result provides initial evidence that isoflurane preconditioning induces an acute phase of neuroprotection under in vivo condition in mice. Previous studies using rat brain slices under in vitro condition have shown this effect. Our previous studies also showed that this isoflurane effect was attenuated by EAAT inhibitors (Wang, et al., 2007; Zheng and Zuo, 2003), suggesting a role of EAATs in this protection. However, the subtype of EAATs involved in this effect was not identified due to the lack of selective subtype EAAT inhibitors. Our current study showed that isoflurane did not induce neuroprotection in the EAAT3 knockout mice, even when the severity of brain ischemia was reduced in these mice to produce a similar degree of ischemic brain injury at which isoflurane preconditioning induced neuroprotection in the wild-type mice. The failure for isoflurane to induce a preconditioning effect in the brains of EAAT3 knockout mice may not be due to over-activation of Akt, a protective protein, under baseline condition in these mice because LY294004 alone did not change their neurological outcome after a 60-min MCAO. Thus, our result strongly suggests the involvement of EAAT3 in the isoflurane preconditioning-induced acute phase of neuroprotection. Consistent with this finding, our previous studies have shown that isoflurane significantly increases EAAT3 activity in cultured cells and rodent brains (Huang, et al., 2006; Huang and Zuo, 2005). In addition, isoflurane can preserve EAAT3 activity under oxidative stress (Lee, et al., 2009).
The isoflurane preconditioning-induced neuroprotection can be due to activation of proteins that promote cell survival. Akt is a well-known survival protein (Downward, 1998). Various extracellular signals/ligands by working on corresponding receptors can activate PI3K, which then activates Akt (Downward, 1998). Akt can activate ERK (Ding, et al., 2009; Zhuang, et al., 2004). The activation of these protein kinases has been shown to provide neuroprotection in some studies (Irving and Bamford, 2002). Akt also phosphorylates Ser9 in GSK3β to inhibit GSK3β (Juhaszova, et al., 2009). Inhibition of GSK3β is known to provide neuroprotection (Li and Zuo, 2011; Lin, et al., 2011). We showed here that isoflurane increased the activated/phosphorylated Akt and ERK as well as inactivated/phosphorylated GSK3β in the wild-type mice, in which isoflurane induced a preconditioning effect, but did not affect the phosphorylation of these kinases in the EAAT3 knockout mice, in which isoflurane did not induce a protective effect. These results suggest the involvement of these protein kinases in the isoflurane preconditioning-induced acute phase of neuroprotection. Strongly supporting this suggestion, we showed that the isoflurane-induced protection was abolished by LY294004, a PI3K inhibitor and that LY294004 alone did not affect the neurological outcome after MCAO.
It is not clear from our study how EAAT3 affects intracellular survival signaling activation after the isoflurane exposure. If EAAT3 works only as a transporter, the increased EAAT3 activity by isoflurane should potentially cause a small reduction in the extracellular glutamate levels. This reduction may not cause any change in the activated/phosphorylated Akt and ERK or may reduce the activated/phosphorylated Akt and ERK because a small increase of extracellular glutamate levels has been shown to increase the activation of Akt and ERK (Abe and Saito, 2001; Wick, et al., 2002; Yin, et al., 2005). The result of this reasoning is opposite to what we observed in the wild-type and EAAT3 knockout mice. Thus, EAAT3 may regulate intracellular cell survival signaling through functions other than transporting glutamate. In supporting this possibility, a previous study showed that application of ligands/substrates of EAATs to astrocyte cultures increases the expression of phospho-ERK in the cells (Abe and Saito, 2001).
In addition to the mechanisms discussed above, multiple other mechanisms may be involved in the isoflurane preconditioning-induced neuroprotection. Isoflurane can enhance GABA neurotransmission (Kotani and Akaike, 2013), which may counteract the increased glutamate neurotransmission in the ischemic brain tissues. Isoflurane can also increase microRNA, such as microRNA-203, and inhibit neuroinflammation in ischemic brain tissues to provide neuroprotection (Cao, et al., 2012; Li, et al., 2013). The possible contribution of these mechanisms to the isoflurane preconditioning-induced acute phase of neuroprotection needs further investigation.
There are many studies on anesthetic preconditioning-induced delayed phase of neuroprotection and very few studies on the acute phase (Zuo, 2012), possibly due to the fact that the effective time-window of the delayed phase is much longer and is perceived to be more useful than that of the acute phase. Although volatile anesthetic preconditioning-induced neuroprotection has not been determined in humans, there are many clinical situations where anesthetic preconditioning-induced acute phase of neuroprotection may be useful and practical, such as during the brain and cardiac surgeries. This current study extends our previous in vitro findings to an in vivo condition using a transient focal brain ischemia in mice. This study also provides initial evidence for the involvement of EAAT3 and the downstream Akt activation in the isoflurane preconditioning-induced acute phase of neuroprotection. These findings improve our understanding of this form of neuroprotection and the biological functions of EAAT3.
Research highlights.
Isoflurane preconditioning induces an acute phase of neuroprotection in mice
Isoflurane preconditioning-induced acute phase of neuroprotection may be mediated by Akt
Isoflurane preconditioning-induced acute phase of neuroprotection may depend on glutamate transporter type 3
Acknowledgments
Grant support: This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, MD, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, OH, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, MD, and the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville, VA.
Abbreviations
- ERK1/2
extracellular signal-regulated kinase 1/2
- EAAT
excitatory amino acid transporters
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSK3β
glycogen synthase kinase 3β
- MCAO
middle cerebral arterial occlusion
- SpO2
pulse oximeter oxygen saturation
Footnotes
The research work was performed in and should be attributed to the Department of Anesthesiology, University of Virginia, Charlottesville, VA22908, USA.
Disclosure/conflict of interest: The authors declare no other financial supports for this study except for those grants stated above from funding agencies for non-profit. The authors also declare no conflict of interest in the content of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Saito H. Possible linkage between glutamate transporter and mitogen-activated protein kinase cascade in cultured rat cortical astrocytes. Journal of Neurochemistry. 2001;76:216–224. doi: 10.1046/j.1471-4159.2001.00062.x. [DOI] [PubMed] [Google Scholar]
- Cao L, Feng C, Li L, Zuo Z. Contribution of microRNA-203 to the isoflurane preconditioning-induced neuroprotection. Brain Research Bulletin. 2012;88:525–528. doi: 10.1016/j.brainresbull.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Ding J, Ning B, Huang Y, Zhang D, Li J, Chen CY, Huang C. PI3K/Akt/JNK/c-Jun signaling pathway is a mediator for arsenite-induced cyclin D1 expression and cell growth in human bronchial epithelial cells. Curr Cancer Drug Targets. 2009;9:500–509. doi: 10.2174/156800909788486740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends in Neurosciences. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Current Opinion in Cell Biology. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Fang H, Huang Y, Zuo Z. The different responses of rat glutamate transporter type 2 and its mutant (tyrosine 403 to histidine) activity to volatile anesthetics and activation of protein kinase C. Brain Research. 2002;953:255–264. doi: 10.1016/s0006-8993(02)03299-7. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Huang Y, Feng X, Sando JJ, Zuo Z. Critical Role of Serine 465 in Isoflurane-induced Increase of Cell-surface Redistribution and Activity of Glutamate Transporter Type 3. Journal of Biological Chemistry. 2006;281:38133–38138. doi: 10.1074/jbc.M603885200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zuo Z. Isoflurane induces a protein kinase C alpha-dependent increase in cell surface protein level and activity of glutamate transporter type 3. Molecular Pharmacology. 2005;67:1522–1533. doi: 10.1124/mol.104.007443. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. Journal of Cerebral Blood Flow & Metabolism. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circulation Research. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke K, Isaev NK, Dirnagl U. Tolerance Against Ischemic Neuronal Injury Can Be Induced by Volatile Anesthetics and Is Inducible NO Synthase Dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- Kotani N, Akaike N. The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission. Brain Research Bulletin. 2013;93:69–79. doi: 10.1016/j.brainresbull.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Lee SA, Choi JG, Zuo Z. Volatile anesthetics attenuate oxidative stress-reduced activity of glutamate transporter type 3. Anesthesia and Analgesia. 2009;109:1506–1510. doi: 10.1213/ANE.0b013e3181b6709a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yin J, Li L, Deng J, Feng C, Zuo Z. Isoflurane postconditioning reduces ischemia-induced nuclear factor-kappaB activation and interleukin 1beta production to provide neuroprotection in rats and mice. Neurobiology of Disease. 2013;54:216–224. doi: 10.1016/j.nbd.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neuroscience. 2011;199:44–50. doi: 10.1016/j.neuroscience.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Li G, Zuo Z. Volatile anesthetic post-treatment induces protection via inhibition of glycogen synthase kinase 3beta in human neuron-like cells. Neuroscience. 2011;179:73–79. doi: 10.1016/j.neuroscience.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO Journal. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- Wang C, Lee J, Jung H, Zuo Z. Pretreatment with volatile anesthetics, but not with the nonimmobilizer 1,2-dichlorohexafluorocyclobutane, reduced cell injury in rat cerebellar slices after an in vitro simulated ischemia. Brain Research. 2007;1152:201–208. doi: 10.1016/j.brainres.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. Journal of Neuroscience. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XH, Zhang QG, Miao B, Zhang GY. Neuroprotective effects of preconditioning ischaemia on ischaemic brain injury through inhibition of mixed-lineage kinase 3 via NMDA receptor-mediated Akt1 activation. Journal of Neurochemistry. 2005;93:1021–1029. doi: 10.1111/j.1471-4159.2005.03096.x. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning reduces Purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of p38 mitogen-activated protein kinase. Molecular Pharmacology. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. Journal of Neuroscience. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z. Are volatile anesthetics neuroprotective or neurotoxic? Medical Gas Research. 2012;2:10. doi: 10.1186/2045-9912-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]