Abstract
Rationale
A number of preclinical and clinical studies suggest ketamine, a glutamate NMDA (N-methyl-D-aspartate) receptor antagonist, has a rapid and lasting antidepressant effect when administered either acutely or chronically. It has been postulated that this effect is due to stimulation of AMPA (alpha-amino-3-hydroxy-5-methyl–4-isoxazolepropionic acid) receptors.
Objective
In this study, we tested whether AMPA alone has an antidepressant effect and if the combination of AMPA and ketamine provides added benefit in Wistar-Kyoto (WKY) rats, a putative animal model of depression.
Results
Chronic AMPA treatment resulted in a dose dependent antidepressant effect in both the forced swim test (FST) and sucrose preference test. Moreover, chronic administration (10–11d) of combinations of AMPA and ketamine, at doses that were ineffective on their own, resulted in a significant antidepressant effect. The behavioral effects were associated with increases in hippocampal brain derived neurotrophic factor (BDNF), synapsin, and mammalian target of rapamycin (mTOR).
Conclusion
These findings are the first to provide evidence for an antidepressant effect of AMPA, and suggest the usefulness of AMPA-ketamine combination in treatment of depression. Furthermore, these effects appear to be associated with increases in markers of hippocampal neurogenesis and synaptogenesis, suggesting a mechanism of their action.
Keywords: Ketamine, AMPA, depression, neurotrophic factor, mTOR, synaptic protein, BDNF, hippocampus, antidepressant, WKY rats
Introduction
Current antidepressants have latency to and are limited in their effectiveness. Plus, they are often associated with various side effects. This calls for investigation and development of new pharmacological approaches, especially ones with rapid onset, lasting duration, and fewer side effects (Penn and Tracy, 2012). Recent evidence suggests a role for the glutamatergic system in the pathophysiology and pharmacotherapy of mood disorders. For example, ketamine, a glutamatergic NMDA receptor antagonist, has been shown to have a rapid and lasting antidepressant effect in both preclinical and clinical studies (Sen and Sancora 2008; Zarate et al. 2006; Berman et al. 2000; Tizabi et al. 2012). To date, the numbers of clinical trials on ketamine are relatively low. Fewer studies have provided reports on ketamine’s antidepressant effect following chronic administration (aan het Rot et al. 2010; Messer and Haller, 2010). The exact mechanism of action of ketamine’s rapid and lasting effects are still unknown. The most common hypothesis centers on a functional interplay between AMPA and NMDA receptors, as the antidepressant effect of ketamine is blocked by NBQX, an AMPA receptor antagonist (Meang et al. 2008; Koike et al. 2011). Moreover, some AMPA potentiators have been shown to have an antidepressant-like effect (Alt et al. 2006; Li et al. 2001).
Guided by these findings, we investigated whether AMPA, a selective AMPA receptor agonist, also has an antidepressant effect when given chronically. Then we tested whether a combination of AMPA and ketamine provides an additive or synergistic antidepressant effect. Moreover, since studies have shown a role for neurogenesis and synaptogenesis in effectiveness of some antidepressants, including ketamine (Malberg 2000; Cornwell et al. 2012; Kodama et al. 2004, Freitas et al. 2013; Banasr et al. 2006; Garcia et al. 2008; Zarate et al. 2009), we investigated the effects of our drug treatments on these processes. To do this we measured the levels of brain derived neurotrophic factor (BDNF), synapsin, and mammalian target of rapamycin (mTOR) in hippocampus, an area directly implicated in mood regulation and effectiveness of antidepressants (Nestler et al. 2002; Campbell and MacQueen 2004).
In the studies presented, we used Wistar Kyoto (WKY) rats, which have been used extensively to investigate the neurobiological substrates of depression and the effectiveness of novel antidepressants (Tizabi et al. 2010; Tejani-Butt et al. 2003). Recently we have demonstrated that chronic administration of ketamine at very low doses has a significant and lasting antidepressant effect in these rats (Tizabi et al. 2012). WKY rats, derived from Wistar stock, were originally used as normotensive control for the spontaneously hypertensive rats, but were later shown to exhibit exaggerated immobility in the forced swim test (FST), which is indicative of their helplessness, a hallmark of depression (Paré 1994). These rats are also prone to develop stressinduced anxiety-like characteristics (Söderpalm 1989; Paré and Redei 1993; Pini et al. 1997). In addition, similar to what is seen in human depressed population, these rats show pattern of sleep disruption including an increase in total REM sleep and increased sleep fragmentation (Dugovic et al. 2000). Furthermore, it has been suggested that these rats may be a suitable model for some treatment resistant patients, since treatment of WKY rats with tricyclic antidepressants (e.g. desipramine), but not selective serotonin reuptake inhibitors (e.g. fluoxetine or paroxetine), result in a reduction of their immobility in the FST (Griebel et al. 1994; Lahmame et al. 1997; Lopez-Rubalcaava and Lucki 2000; Tejani-Butt et al. 2003; Tizabi et al. 2010). In these studies, we relate the central changes seen in the hippocampus to behavioral effects by evaluating the drugs effect on FST and sucrose preference (SPT), which tests another hallmark of depression, anhedonia (Treadway and Zald 2011).
Materials and Methods
Animals
Age matched adult male WKY rats (Harlan Laboratories, Indianapolis, IN) were used throughout the study. Animals were housed in pairs in standard polypropylene shoebox cages (42×20.5×20 cm) on hardwood chip bedding (alpha-dry). Animals had free access to food (Harlan Tek Lab) and water. The room was maintained at 24–26 °C at 51 – 66% relative humidity, on a 12-h reversed light/dark cycle (lights on at 19.00 h). The reversal of light cycle allows for convenient measurement of behavior in active (dark) phase. A red light source was used for illumination when working with the animals during this time. All experiments were carried out in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee.
Drug Treatment and Study Design
For AMPA studies, groups of rats (8/group) were injected (intraperitoneally, ip) once daily for 11 days with AMPA (0.25, 0.5 and 1.0 mg/kg) or saline (control). Twenty minutes after the last injection they were tested in open field locomotor activity (LMA) followed by the forced swim test (FST). Other studies have also shown antidepressant effect of AMPA-kines during this time point (Lindholm et al, 2012, Li et al, 2001). For combination studies, the animals were injected with ketamine alone (0.25 mg/kg or 0.5 mg/kg ip), AMPA alone (0.25 mg/kg or 0.5 mg/kg ip), or in combination (0.25 mg/kg each or 0.5 mg/kg each). The dose and regimen for ketamine were based on our previous study (Tizabi et al, 2012). The behavioral tests for LMA and FST were conducted 20–22 hr after the last ketamine injection on day 10 and twenty minutes after the last AMPA injection on day 11. Sucrose preference test (SPT) was carried out the day after the FST. Ketamine HCl was purchased from Henry Schein (Melville, NY) and AMPA was purchased for Torcis Bioscience (Ellsville, MO).
Locomotor Activity (LMA) Monitoring
The behavior of the rats in the open field locomotor activity test was assessed to determine if drug treatment affected general locomotor behavior, which might impinge on FST immobility assessment. Locomotor activity was measured first for each animal during a 10 minute period in an open-field activity monitoring cage (27×27×20.3 cm, Med Associates, Inc., St. Albans, VT), which automatically records movement as the number of infrared beam interruptions.
Forced Swim Test (FST)
A modified FST (Detke et al. 1995) was used to measure immobility of the rats, as described previously (Tizabi et al. 2012). Briefly, the rats were individually placed into a Pyrex cylinder filled with room temperature water (25 ± 1 °C) at a height of 30cm to ensure that animals could not touch the bottom of the container with their hind paws or tails. A time-sampling scoring technique was used, whereby the predominant behavior – immobility or swimming (including climbing) – in each 5-s period of the 300-s test was recorded. The observer was blind to the treatment given to the animal. We included the climbing score with the swimming as an active behavior, because very few such incidences were observed with these rats. It is of relevance to note that WKY rats exhibit spontaneous immobility in the forced swim test; hence, there is no need to have a pretest exposure to forced swimming the day before, as is customary in other strains, to induce helplessness (Tejani-Butt et al. 2003; Tizabi et al. 2012).
Sucrose Preference Test (SPT)
The animals were conditioned to sucrose solution for four days prior to initiation of drug treatments. SPT was performed using the Grippo et al. method (2002) with some modification. On the day of testing, the animals were housed individually in a clean standard polypropylene shoebox cage (42 × 20.5 × 20 cm) on hardwood chip bedding (alpha-dry). Each rat was given free access to two bottles for 6 h: one bottle was filled with a 1% (w/v) sucrose solution, and the other bottle was filled with water. Each bottle was weighed before and after the 6 h testing period. The two bottles were swapped three hours into the testing to prevent side preference. The % sucrose preference was calculated as: SP (%) = (sucrose solution consumed/ total liquid consumed) × 100.
Tissue preparation
The rats were treated chronically with ketamine (0.25mg/kg or 0.5 mg/kg ip), AMPA (0.25 mg/kg or 0.5 mg/kg ip), or a combination of the two as described above. In this case, however, the animals were sacrificed by decapitation without any behavioral test. The brains were rapidly removed, frozen on dry ice and stored at −80° C. Each frozen brain was later thawed on ice and hippocampus was dissected out (Tizabi et al. 2012) for western blot analysis.
Western blot
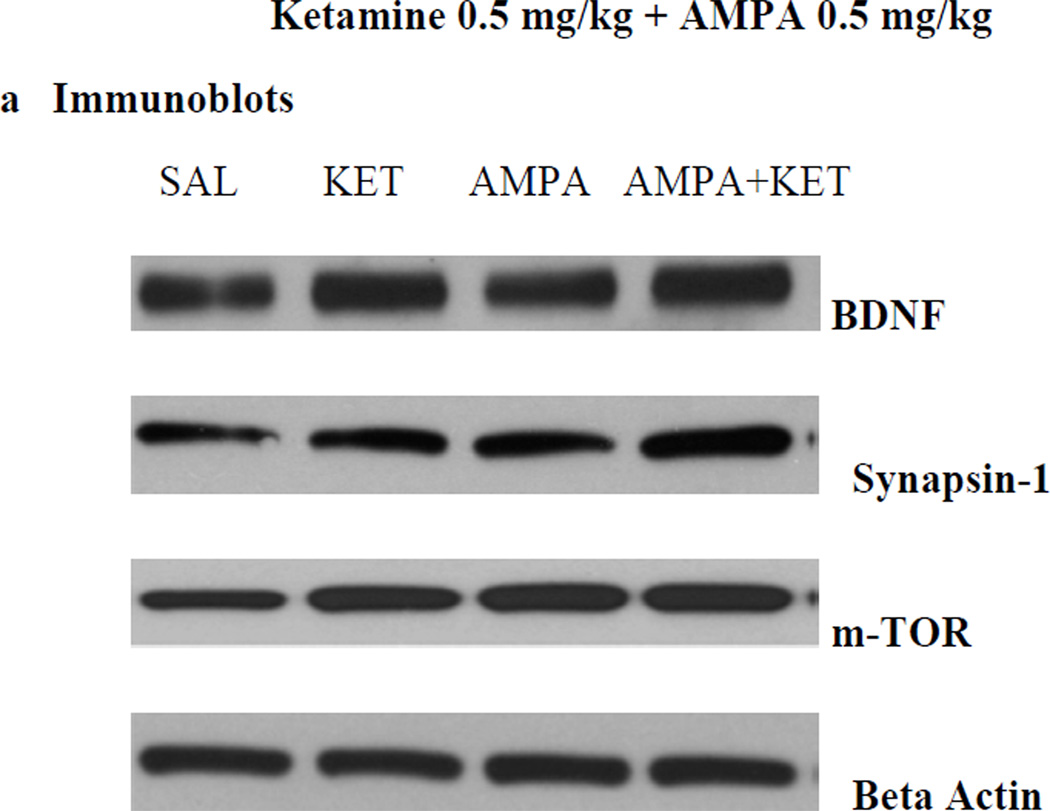
Homogenate of the dissected tissues were made in lysis buffer (10 mM Tris-buffer, 5 mM EDTA, 150 mM NaCl, 0.5% Triton X-100 (v/v) with protease inhibitors (Sigma-Aldrich, St. Louis, MO). The protein concentration in each sample was determined using a BCA protein Assay Kit (Pierce Biotechnology Inc., IL), and equal protein amount (as confirmed by β-actin) was loaded in each immunoblot. The proteins were separated using 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membranes were blocked with a blocking reagent (5% nonfat milk in TBS buffer) for ½ h and incubated at 4°C overnight with the primary antibody against BDNF, synapsin 1 (1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, CA) and m-TOR (1:500, abcam). The membranes were washed with TBST (TBS buffer with 1% Tween-20) and blocked with the blocking reagent. Membranes were then incubated for 1 h at room temperature in Goat Anti-Rabbit-HRP conjugated secondary antibody (1:3000 in TBS, Bio-Rad Laboratories, CA). The membranes were then washed in the TBST washing solution and then visualized using enhanced chemiluminescent kits (Bio-Rad Laboratoies, CA). The intensity of the protein bands on the gel was quantified using ChemiDoc XRS system (Bio-Rad Laboratories, CA).
Statistical Analysis
Statistical differences between treatment groups were determined by one-way ANOVA followed by post-hoc Newman-Keuls Multiple comparison test to determine which groups differed. Significant difference was considered a priori at p < 0.05. Data were analyzed using Graphpad Prism 3 (Graphpad Software, Inc, San Diego, CA, USA).
Results
Antidepressant-like effect of AMPA in WKY rats
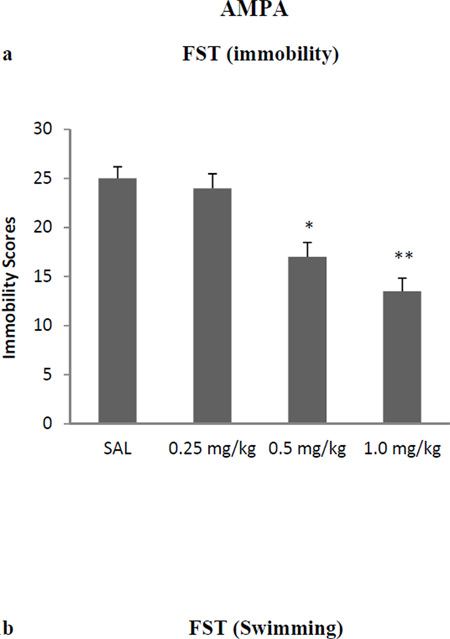
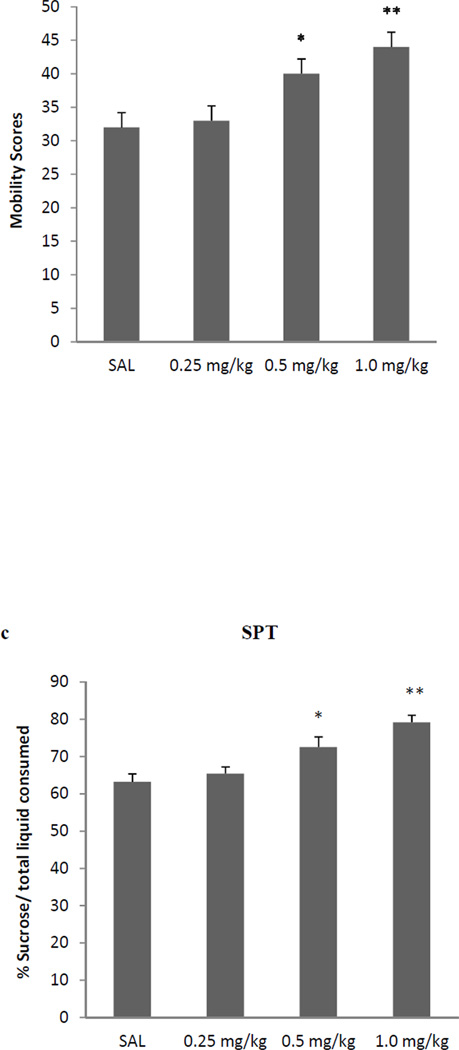
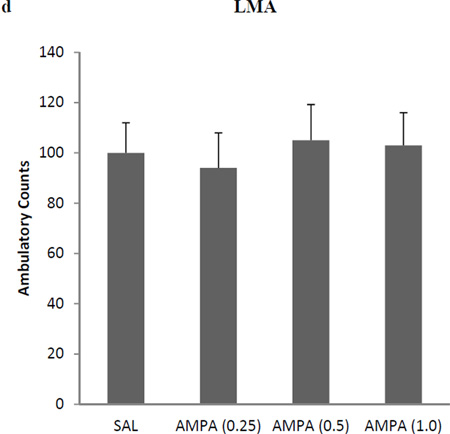
AMPA treatment resulted in a dose-dependent antidepressant-like effect in FST immobility [F (3,25)=33.3, p<0.01 (Fig 1a), and swimming F(3, 25) = 34.5, p<0.01 (Fig 1b)]. Because of very limited climbing activity statistical analysis could not be performed on this parameter. Thus, 0.25 mg/kg AMPA had no effect on either immobility or swimming in the FST or SPT, but higher doses did. AMPA at 0.5 mg/kg and 1.0 mg/kg resulted in about 30% reduction (p<0.05) and 44% reduction (p<0.01) in FST immobility, respectively. Similarly, AMPA at 0.5 mg/kg and 1.0 mg/kg resulted in about 20% increase (p<0.05) and 28% increase (p<0.01) in FST swimming, respectively. AMPA treatment resulted in a dose-dependent increase in sucrose preference [F (3,25)=26.6, p<0.01] (Fig 1c). Again, AMPA at 0.25 mg/kg had no effect on sucrose preference, but 0.5 mg/kg caused about 15% increase (p<0.05) and the 1.0 mg/kg, caused about 25% increase in sucrose preference (p<0.01). AMPA at any dose did not affect the locomotor activity of the rats (Fig 1d), suggesting that the immobility/swimming in the FST is independent of general locomotor activity.
Fig 1.
Effects of various doses of AMPA on (a) FST immobility, (b) FST swimming, (c) sucrose preference, and (d) locomotor activity in WKY rats. Animals were treated once daily for 11 consecutive days. Values are mean ± SEM, *p<0.05 **p<0.01 compared to SAL, n=7–8.
Antidepressant-like effect of AMPA-Ketamine in WKY rats
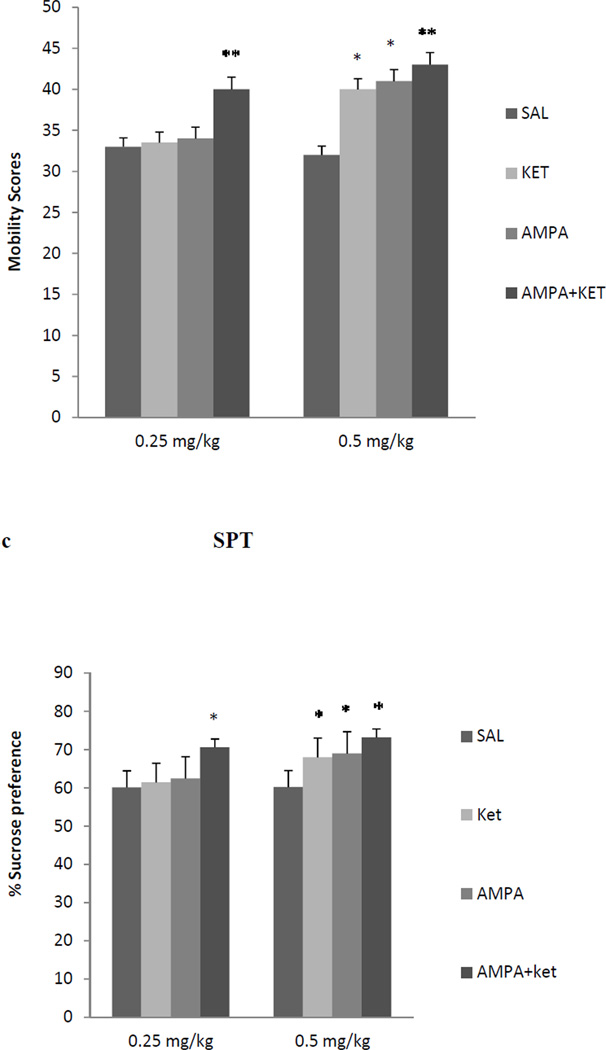
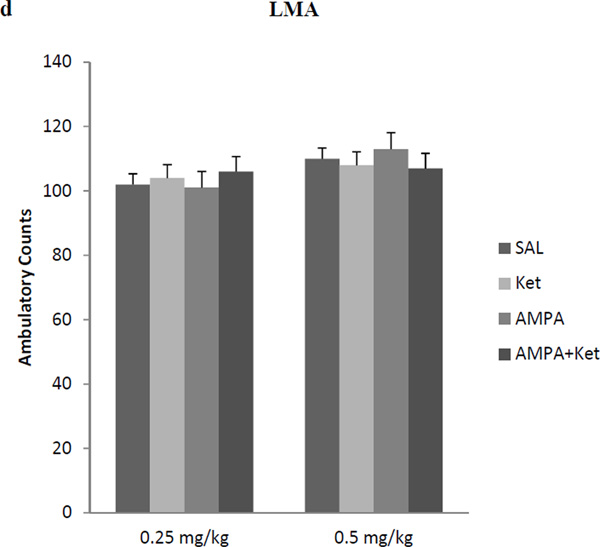
Neither 0.25 mg/kg ketamine nor 0.25 mg/kg AMPA alone had any significant effect on either FST or SPT (Fig 2). However, the combination treatment of the two at these doses resulted in a reduction (approx. 38%) in immobility in the FST [F(3,26)=36.4, p<0.01)] (Fig 2a). Similarly, the combination treatment of the two at these doses resulted in an increase (approx. 35%) in swimming in FST [F (3,25) = 37.2, p<0.01)] (Fig 2b). The combination treatment of the two at these doses resulted in an increase (approx. 16%) in sucrose preference [F (3,26)=15.2, p<0.05)] (Fig 2b). Both, 0.5 mg/kg ketamine and AMPA alone, as well as their combination resulted in significant reduction in FST immobility [F (3,26)=41.4, p<0.01)] (Fig 2a) and an increase in FST swimming F(3,26)=38.2, p<0.01)] (Fig 2b), and increase in sucrose preference [F(3,26)=14.8, p<0.05)] (Fig 2c). Thus alone, ketamine at 0.5 mg/kg resulted in approximately 28% reduction (p<0.05) in immobility and AMPA at 0.5 mg/kg resulted in approximately 30% reduction (p<0.05) in immobility. Their combination, however, resulted in approximately 40% reduction (p<0.01) in immobility in FST (Fig 2a). Also, ketamine at 0.5 mg/kg resulted in approximately 20% increase (p<0.05) in swimming and AMPA at 0.5 mg/kg resulted in approximately 21% increase (p<0.05) in swimming. Their combination, however, resulted in approximately 26% increase (p<0.01) in swimming in FST (Fig 2b). Similarly, ketamine at 0.5 mg/kg resulted in approximately 13% increase in sucrose preference (p<0.05), and AMPA at 0.5 mg/kg resulted in approximately 15% increase in sucrose preference when given alone. Their combination at this dose, however, resulted in approximately 22% increase in sucrose preference (p<0.05). Although the combination of 0.5 mg/kg of ketamine and AMPA resulted in higher reduction in immobility or further increase in swimming, these changes were not significantly different from individual effects.
Fig 2.
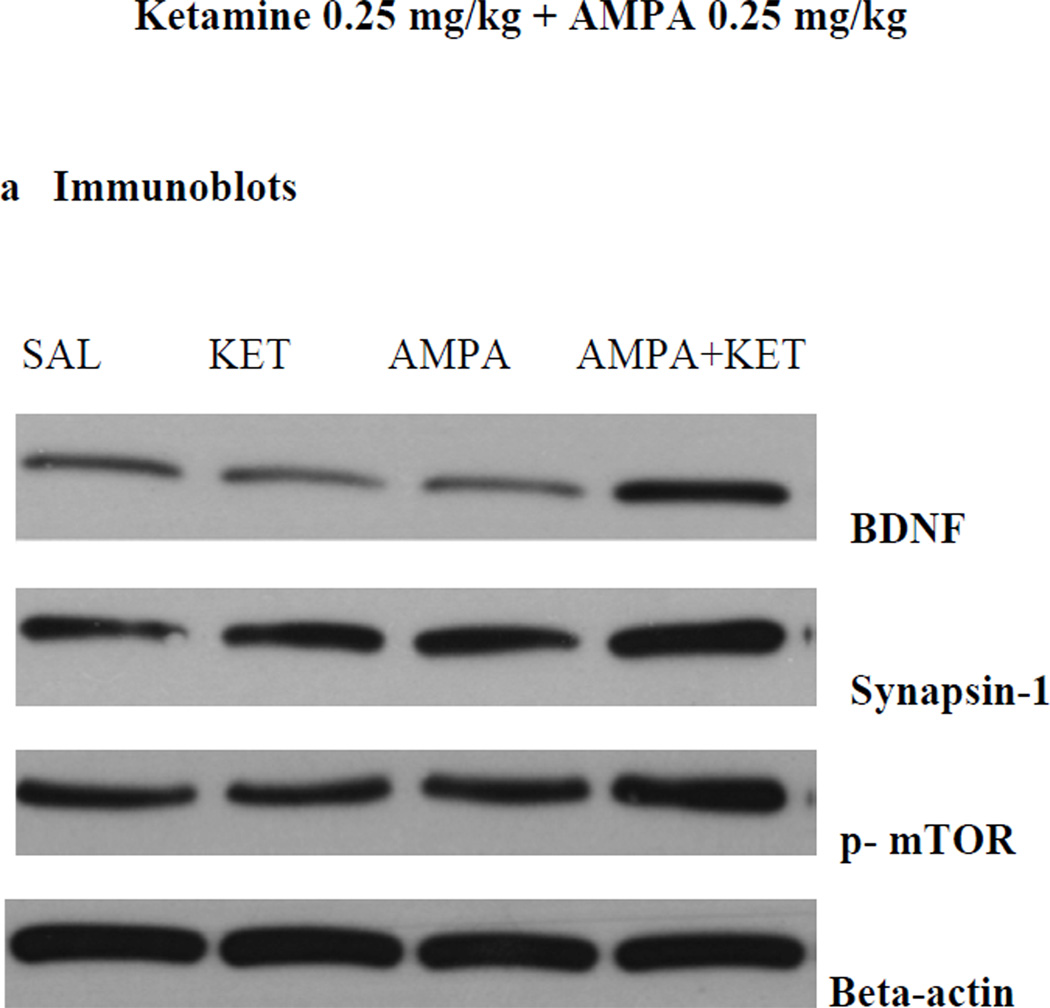
Effect of low doses of AMPA (0.25 mg/kg or 0.5 mg/kg), ketamine (0.25 mg/kg or 0.5 mg/kg), and their combination on (a) FST immobility, (b) FST swimming, (c) sucrose preference, and (d) locomotor activity in WKY rats. Animals were treated once daily for 11 consecutive days. Values are mean ± SEM, *p<0.05 **p<0.01 compared to SAL, n=7–8
Locomotor activity was not significantly affected by any treatment (Fig 2d). When the animals were tested one week after the last injection, no effect on any behavioral parameter was noted with any single or combination treatment (data not shown), suggesting that the regimens used did not result in a lingering effect.
Western Blot Analysis
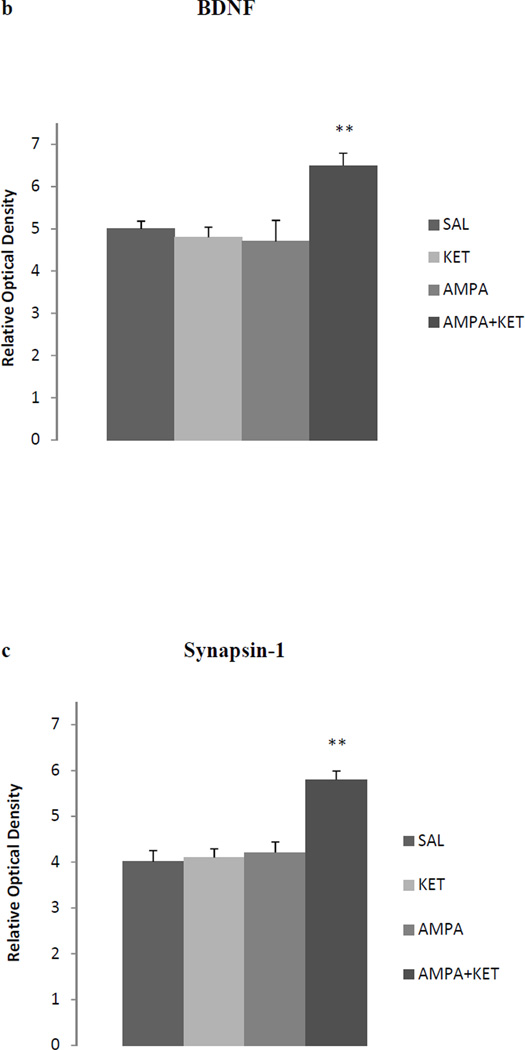
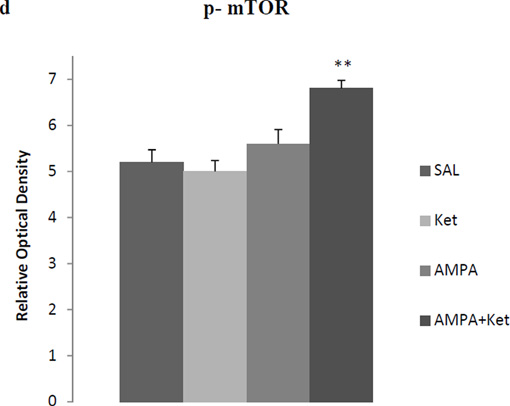
Figure 3 depicts the effect of 0.25 mg/kg ketamine, 0.25 mg/kg AMPA or their combination on hippocampal BDNF (Fig 3b), synapsin (Fig 3c), and p-mTOR (Fig 3d). Fig 3a represents the immunoblots of a representative assay. There was a significant main effect for BDNF [F(3,26)=31.8, p<0.01)], synapsin [F(3,26)=33.2, p<0.01)], and p-mTOR [F(3,26)=29.8, p<0.01)]. At the lower doses (0.25 mg/kg), neither ketamine nor AMPA alone had any significant effect on hippocampal BDNF, synapsin, and p-mTOR. However, their combination did result in a significant effect in all three markers in the hippocampus. Thus, there was approximately 18% increase in BDNF (p<0.01) (Fig 3b), approximately 27% increase in synapsin p (<0.01) (Fig 3c), and approximately 18% increase in p-mTOR (p< 0.01) (Fig 3d).
Fig 3.
Effect of very low doses of AMPA (0.25 mg/kg), ketamine (0.25 mg/kg), and their combination on markers of neurogenesis (BDNF), synaptogenesis (synapsin) and the signal transduction protein (mTOR) in the hippocampus. (a) Immunoblots, (b) BDNF, (c) synapsin, (d) mTOR. Values are mean ± SEM, **p<0.01 compared to SAL, n=7–8.
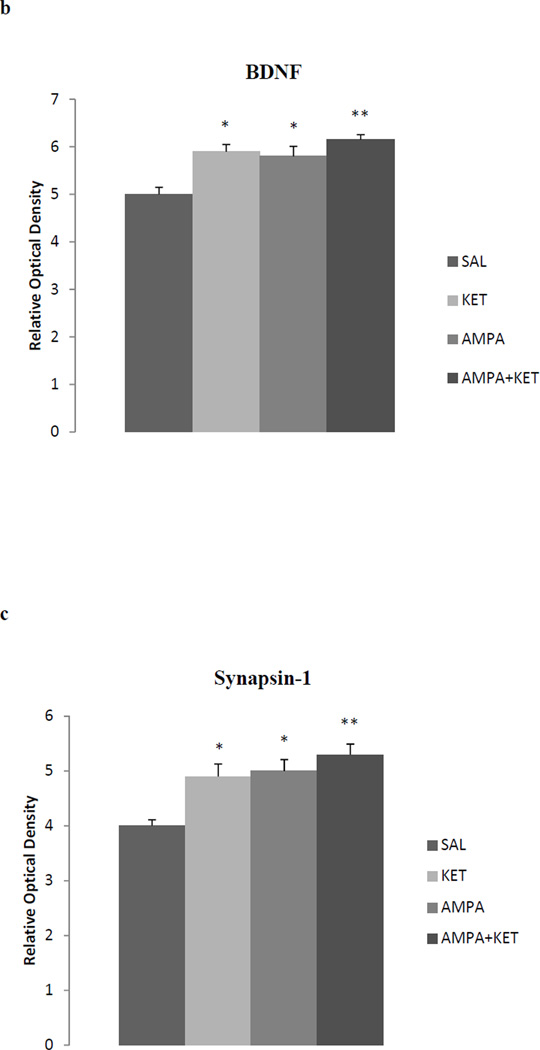
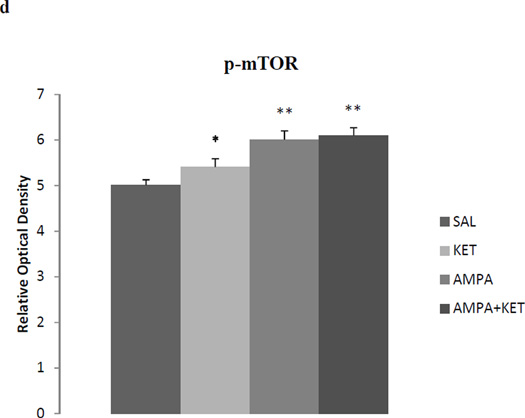
Figure 4 depicts the effect of 0.5 mg/kg ketamine, 0.5 mg/kg AMPA, or their combination on hippocampal BDNF (Fig 4b), synapsin (Fig 4c), and p-mTOR (Fig 4d). Fig 4a represents the immunoblots of a representative assay. There was a significant main effect of ketamine on hippocampal BDNF [F(3,26)=31.6, p<0.01)], synapsin [F(3,26)=34.1, p<0.01)], and p-mTOR [F(3,26)=30.5, p<0.01)]. Thus, ketamine caused approximately 18% increase (p<0.05), AMPA caused approximately 16% increase (p<0.05), and their combination caused approximately 23% increase (p<0.01) in hippocampal BDNF (Fig 4b). Hippocampal synapsin was increased by approximately 22% with ketamine (p<0.05), 25% by AMPA (p<0.05), and 32% by their combination (p<0.01) (Fig 4c). Hippocampal p-mTOR was increased by approximately 12% with ketamine (p<0.05), 20% by AMPA (p<0.01), and 22% by their combination (p<0.01) (Fig 4d).
Fig 4.
Effect of low doses of AMPA (0.5 mg/kg), ketamine (0.5 mg/kg), and their combination on markers of neurogenesis (BDNF), synaptogenesis (synapsin), and the signal transduction protein (mTOR) in the hippocampus. (a) Immunoblots, (b) BDNF, (c) synapsin, (d) mTOR. Values are mean ± SEM, *p<0.05 **p<0.01 compared to SAL, n=7–8.
Discussion
The results of the current studies provide evidence for an antidepressant effect of AMPA, as seen by a reduction in forced swim test immobility and an increase in sucrose preference in WKY rats. This finding is in line with previous studies where antidepressant effects of AMPA-kines in stress-induced model of depression have been reported (Li et al. 2001; Knapp et al. 2002; Mackowiak et al. 2002; Fumagalli et al. 2012).
The antidepressant effect of ketamine is well established in both human and animal models, including the WKY rats (Tizabi et al. 2012). Although previous studies have used relatively higher doses of acute ketamine, in the current study administration of a much lower chronic dose (0.5 mg /kg) had an antidepressant effect. Although it is possible that sensitization may occur following repeated administration of ketamine, we did not observe this effect in locomotor activity. Moreover, the few other studies that use a chronic paradigm (e.g. Cho et al. 2005) also did not observe any behavioral sensitization to ketamine. Perhaps, more importantly, our results indicate that a combination of AMPA and ketamine may provide further relief of depression. Indeed, combining very low and ineffective doses – when given alone – of each resulted in significant antidepressant effect as assessed by both measures of helplessness (reduced immobility in the FST) and anhedonia (increased sucrose preference). The 0.5 mg/kg ketamine and AMPA (low effective doses) were used as these doses were in the mid-point of the dose response curve and their combination could result in a higher effect. Although such increases in effect were seen with the combination treatment, the differences in general were not different from individual treatments, likely due to a ceiling effect. The question of dosing, particularly with ketamine is a crucial one since at higher concentrations ketamine may induce severe side effects including psychotic symptoms (Miyamoto et al. 2000; Tizabi, 2007). It has to be noted that the doses of AMPA and ketamine used are such low doses, that their combination might actually mitigate any possible adverse effects imposed by the drugs individually. That very low doses of AMPA and ketamine, when combined, can provide almost a maximal antidepressant effect could be of significant clinical relevance.
It is also noteworthy that a recent study by Carrier and Kabbaj (2013) demonstrated greater sensitivity to acute ketamine in female, compared to male, rats that were subjected to FST the day before (i.e., stress-induced model of depression paradigm). Our previous findings with female WKY yielded similar responses to those observed here with chronic ketamine. Nonetheless, it will be of importance to delineate possible sex differences in response to AMPA-ketamine combination. In addition, delineation of specific AMPA/NMDA receptor functions in such behavioral observations could lead to novel glutamatergic-based intervention in mood disorders.
Our finding of p-mTOR up-regulation is consistent with recent reports where antidepressant effects of ketamine were shown to be associated with stimulation of mTOR pathway (Li et al. 2010; Voleti and Duman 2012, Dazert and Hall 2011). mTOR signaling is directly linked to regulation of protein translation and synaptic plasticity (Li et al. 2010, Duman et al. 2012). Other investigators have also observed involvements of neurogenesis and synaptogenesis in effectiveness of ketamine (Ricci et al. 2011; Jernigan et al. 2011; Denk et al. 2011; Duman et al. 2012; Cornwell et al. 2012; Liu et al. 2012). However, to date a specific mechanism for antidepressant effect of AMPA or AMPA-kines have not been investigated. Our findings of AMPA effect on markers of synaptogenesis and neurogenesis provide a possible role for the same mechanisms as those involved in ketamine’s effect.
The importance of hippocampal neurogenesis in the effectiveness of many antidepressants has been well established (Russo-Neustadt et al. 2004; Machado-Vieira et al. 2009; Larsen et al. 2010), but our results cannot rule out the possibility of involvement of other brain regions in the antidepressant effects of AMPA and/or ketamine particularly via synaptic plasticity. A recent study indicates involvement of prefrontal cortical region in antidepressant effect of ketamine (Li et al. 2010). Changes in synaptic proteins (e.g., synapsin) or the signal transduction mediated via mTOR can occur rather quickly, which may contribute to the acute effectiveness of antidepressants such as ketamine (Duman et al. 2012; Li et al. 2010). However, changes in neuronal survival and/or de-novo induction of neuronal cells in critical area such as hippocampus may require longer time and may be essential in long term effects of the treatments.
In summary, these findings provide evidence for an antidepressant effect of low AMPA doses, and justification for combining AMPA with ketamine as novel intervention in depression, particularly in treatment resistant patients. Moreover, the results indicate interactions between AMPA and ketamine with hippocampal markers of neurogenesis and synaptogenesis, as a possible mechanism for their antidepressant actions.
Acknowledgement
Supported by NIH/NIGMS (2 SO6 GM08016-39)
Footnotes
Conflict of Interest: The authors declare no conflict of interest
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Alt A, Nisenbaum ES, Bleakman D, Witkin JM. A role for AMPA receptors in mood disorders. Biochem. Pharmacology. 2006;71:1273–1288. doi: 10.1016/j.bcp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Banasr M, Soumier A, Hery M, Mocaër E, Daszuta A. Agomelatine, a New Antidepressant, Induces Regional Changes in Hippocampal Neurogenesis. Biol Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, et al. Synaptic Potentiation Is Critical for Rapid Antidepressant Response to Ketamine in Treatment-Resistant Major Depression. Biol Psychiatry. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, D’Souza DC, Gueorguieva R, Perry EB, Madonick S, Karper LP, et al. Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology. 2005;179:136–143. doi: 10.1007/s00213-004-2066-5. [DOI] [PubMed] [Google Scholar]
- Denk MC, Rewerts C, Holsboer F, Erhardt-Lehmann A, Turck C. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am J Psychiatry. 2011;168:751–752. doi: 10.1176/appi.ajp.2011.11010128. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Diniz L, dos Santos TB, Britto LRG, Céspedes IC, Garcia MC, Spadari-Bratfisch RC, et al. Effects of chronic treatment with corticosterone and imipramine on fos immunoreactivity and adult hippocampal neurogenesis. Behav Brain Res. 2013;238:170–177. doi: 10.1016/j.bbr.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Solberg LC, Redei E, Van Reeth O, Turek FW. Sleep in the Wistar-Kyoto rat, a putative genetic animal model for depression. NeuroReport. 2000;11:627–631. doi: 10.1097/00001756-200002280-00038. [DOI] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu R-J, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas AE, Machado DG, Budni J, Neis VB, Balen GO, Lopes MW, et al. Fluoxetine modulates hippocampal cell signaling pathways implicated in neuroplasticity in olfactory bulbectomized mice. Behav Brain Research. 2013;237:176–184. doi: 10.1016/j.bbr.2012.09.035. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Calabrese F, Luoni A, Shahid M, Racagni G, Riva MA. The AMPA receptor potentiator Org 26576 modulates stress-induced transcription of BDNF isoforms in rat hippocampus. Pharmacol. Res. 2012;65:176–181. doi: 10.1016/j.phrs.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT uptake inhibitors differentially modulates emotional responses in anxiety models in rodents. Psychopharmacology. 1994;113:463–470. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Mofitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Progress in Neuro-Psychopharmacol and Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, et al. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur J Pharmacology. 2002;440:27–35. doi: 10.1016/s0014-2999(02)01338-9. [DOI] [PubMed] [Google Scholar]
- Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry. 2004;56:570–580. doi: 10.1016/j.biopsych.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Lahmame A, del Arco C, Pazos A, Yritia M, Amario A. Are Wistar-Kyoto rats a genetic model of depression resistant to antidepressants? Eur J Pharmacol. 1997;337:115–123. doi: 10.1016/s0014-2999(97)01276-4. [DOI] [PubMed] [Google Scholar]
- Larsen MH, Mikkelsen JD, Hay-Schmidt A, Sandi C. Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J Psychiat Research. 2010;44:808–816. doi: 10.1016/j.jpsychires.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;29:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm JSO, Autio H, Vesa L, Antila H, Lindemann L, Hoener MC, et al. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf+/− heterozygous null mice. Neuropharmacology. 2012;62:391–397. doi: 10.1016/j.neuropharm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Liu R-J, Lee FS, Li X-Y, Bambico F, Duman RS, Aghajanian GK. Brain-Derived Neurotrophic Factor Val66Met Allele Impairs Basal and Ketamine-Stimulated Synaptogenesis in Prefrontal Cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubalcaava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, O’Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology. 2002;43:1–10. doi: 10.1016/s0028-3908(02)00066-7. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-Derived Neurotrophic Factor and Initial Antidepressant Response to an N-Methyl-D-Aspartate Antagonist. J Clin. Psychiatry. 2009;70:662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of α-Amino-3- Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eische AJ, Nestler EJ, Duman RS. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. The J of Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer MM, Haller IV. Maintenance Ketamine Treatment Produces Long-term Recovery from Depression. Primary Psychiatry. 2010;17:48–50. [Google Scholar]
- Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE. Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology. 2000;22:400–412. doi: 10.1016/S0893-133X(99)00127-X. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiol of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Paré WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Paré WP, Redei E. Sex differences and stress response of WKY rats. Physiol Behav. 1993;54:1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Penn E, Tracy DK. The drugs don’t work? antidepressants and the current and future pharmacological management of depression. Ther Adv Psychopharmacol. 2012;5:179–188. doi: 10.1177/2045125312445469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini S, Cassano GB, Simonini E, Savino M, Russo A, Montgomery SA. Prevalence of anxiety disorders comorbidity in bipolar depression, unipolar depression, and dysthymia. J Affect Disord. 1997;42:145–153. doi: 10.1016/s0165-0327(96)01405-x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le PM, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1997;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Ricci V, Martinotti G, Gelfo F, Tonioni F, Caltagirone C, Bria P, et al. Chronic ketamine use increases serum levels of brain-derived neurotrophic factor. Psychopharmcol. 2011;215:143–148. doi: 10.1007/s00213-010-2121-3. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AM, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal Brain-Derived Neurotrophic Factor Expression Following Treatment with Reboxetine, Citalopram, and Physical Exercise. Neuropsychopharmacology. 2004;29:2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Sen S, Sanacora G. Major depression: emerging therapeutics. Mt Sinai J Med. 2008;75:204–225. doi: 10.1002/msj.20043. [DOI] [PubMed] [Google Scholar]
- Söderpalm B. The SHR exhibits less"anxiety" but increased sensitivity to the anticonflict effect of clonidine compared to normotensive controls. Pharmacol Toxicol. 1989;65:381–386. doi: 10.1111/j.1600-0773.1989.tb01193.x. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt S, Kluczynski J, Paré WP. Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Tizabi Y. Nicotine and nicotinic system in hypoglutamatergic models of schizophrenia. Neurotox Res. 2007;12:233–246. doi: 10.1007/BF03033907. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Hauser SR, Tyler KY, Getachew B, Madani R, Sharma Y, et al. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:62–69. doi: 10.1016/j.pnpbp.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]