Abstract
Mono-hydroxy methoxychlor (mono-OH MXC) is a metabolite of the pesticide, methoxychlor (MXC). Although MXC is known to decrease antral follicle numbers, and increase follicle death in rodents, not much is known about the ovarian effects of mono-OH MXC. Previous studies indicate that mono-OH MXC inhibits mouse antral follicle growth, increases follicle death, and inhibits steroidogenesis in vitro. Further, previous studies indicate that CYP11A1 expression and production of progesterone (P4) may be the early targets of mono-OH MXC in the steroidogenic pathway. Thus, this study tested whether supplementing pregnenolone, the precursor of progesterone and the substrate for CYP11A1, would prevent decreased steroidogenesis, inhibited follicle growth, and increased follicle atresia in mono-OH MXC-treated follicles. Mouse antral follicles were exposed to vehicle (dimethylsulfoxide), mono-OH MXC (10μg/mL), pregnenolone (1μg/mL), or mono-OH MXC and pregnenolone together for 96 h. Levels of P4, androstenedione (A), testosterone (T), estrone (E1), and 17β-estradiol (E2) in media were determined, and follicles were processed for histological evaluation of atresia. Pregnenolone treatment alone stimulated production of all steroid hormones except E2. Mono-OH MXC-treated follicles had decreased sex steroids, but when given pregnenolone, produced levels of P4, A, T, and E1 that were comparable to those in vehicle-treated follicles. Pregnenolone treatment did not prevent growth inhibition and increased atresia in mono-OH MXC-treated follicles. Collectively, these data support the idea that the most upstream effect of mono-OH MXC on steroidogenesis is by reducing the availability of pregnenolone, and that adding pregnenolone may not be sufficient to prevent inhibited follicle growth and survival.
Keywords: methoxychlor, metabolites, antral follicles, ovary, pregnenolone, steroidogenesis
INTRODUCTION
The ovary contains a finite number of ovarian follicles. Throughout a female’s life, ovarian follicles mature through various stages of development, produce ovarian sex steroids, become ovulated or die by a programmed cell death process known as follicular atresia. In fact, ovarian follicular development and ovarian follicle atresia exist in a very delicate balance (Hirshfield 1991). Any stimulus that alters this balance has the potential to cause serious disruptions to ovarian function, which can result in female infertility.
The ovarian follicles responsible for the majority of ovarian sex steroid hormone production are the antral follicles. Antral follicles consist of an oocyte (egg) surrounded by various layers of granulosa cells, which in turn are surrounded by a basal membrane and two layers of another somatic cell type, the theca cells. Antral follicles receive their name from the fluid-filled cavity that forms within them as they mature (Hirshfield 1991). The main product of antral follicle steroidogenesis is 17β-estradiol (E2) and its production is achieved by interactions between the granulosa and theca cell compartments of the follicle. Theca cells express receptors for luteinizing hormone (LH), which stimulates the expression of the enzymes necessary for the stepwise conversion of cholesterol to androgens. The granulosa cells express receptors for follicle-stimulating hormone (FSH), which stimulates the expression of the enzymes necessary for the conversion of androgens to estrogens. Specifically, cholesterol is transported in the inner mitochondrial membrane by steroidogenic acute regulatory protein (StAR) where cytochrome P450 11A1 also known as cholesterol side chain cleavage (CYP11A1) will convert it to pregnenolone. Pregnenolone diffuses to the smooth endoplasmic reticulum where it will be converted to progesterone by the enzyme 3β-hydroxysteroid dehydrogenase (HSD3B) or to dehydroepiandrosterone (DHEA) by cytochrome P450 17A1 (also known as 17α-hydroxylase/17,20-lyase). Both progesterone and DHEA can be converted into androstenedione by the action of CYP17A1 or HSD3B1, respectively. Androstenedione can be converted to testosterone by the enzyme 17β-hydroxysteroid dehydrogenase (HSD17B) or to estrone by aromatase within the granulosa cells. Finally, both estrone and testosterone can be converted to E2 via the action of HSD17B and aromatase, respectively. Appropriate levels of the ovarian sex steroid hormones, specifically of E2, are required for normal ovarian function (Britt, et al. 2004, Hirshfield 1991) as well as for skeletal, cardiovascular, and brain health as evidenced by increased risk for infertility (Levi and Widra 1994), osteoporosis (Bagur and Mautzlen 1992), cardiovascular disease (Hu, et al. 1999), and depression (Dennerstein, et al. 1999) in estrogen-deficient women.
Although biomonitoring data on concentrations of MXC in women are scare, some studies have reported concentrations of MXC in breast milk, placenta, adipose tissues, and serum of Finnish, Danish, and Spanish women. Specifically, levels of MXC in breast milk have been estimated to range between 0.19–0.27 μM, while for placenta they have been estimated to range from 0.13 to 0.76 μM (Shen, et al. 2007). MXC levels in women from Southern Spain were estimated at approximately 79.6 μM in adipose tissue and 1.10 μM in serum Botella, et al. 2004). This is of concern because a large number of studies have established the organochlorine pesticide methoxychlor (MXC) as a model endocrine disruptor and environmental estrogen. MXC has been shown to reduce fertility, cause ovarian atrophy, decrease antral follicle numbers, and increase antral follicle atresia in mice (Borgeest, et al. 2002, Cummings and Gray 1989, Eroschenko, et al. 1995, Swartz and Corkern 1992). Also, in vitro experiments have shown that MXC inhibits growth, increases atresia, and reduces sex steroid hormone production by mouse antral follicles (Basavarajappa, et al. 2011, Miller, et al. 2005).
MXC is metabolized by hepatic cytochrome P450 enzymes to form a mono-hydroxylated metabolite commonly known as mono-OH methoxychlor (mono-OH MXC) and a bis-hydroxylated metabolite commonly referred to as HPTE (Kapoor, et al. 1970). Both metabolites have been shown to inhibit mouse antral follicle growth and increase atresia at lower doses than their parent compound and are considered to be more toxic than the parent compound (Miller, et al. 2006). Previous studies have evaluated the ability and mechanisms by which MXC and HPTE disrupt ovarian steroidogenesis using primary and stable granulosa (Chedrese and Feyles 2001, Crellin, et al. 2001, Harvey, et al. 2009, Tiemann, et al. 1996, Zachow and Uzumcu 2006) and theca cell cultures (Akgul, et al. 2008), but no studies have evaluated in detail the mechanisms by which mono-OH MXC alters the levels of sex steroids in mouse antral follicles, which contain both granulosa and theca cells. However, we have shown previously that both MXC and mono-OH MXC decrease production of progesterone, androstenedione, testosterone, and E2 potentially by decreasing the expression of various key enzymes in the steroidogenic pathway (Basavarajappa, et al. 2011, Craig, et al. 2010). In our previous studies with mouse ovarian antral follicles, Cyp11a1 mRNA expression and progesterone levels were the earliest steps of the steroidogenic pathway that were decreased by mono-OH MXC treatment. Further, to our knowledge, no previous studies have supplemented E2 precursors to determine whether steroidogenesis can be restored in mono-OH MXC treated follicles. Therefore, this study was designed to evaluate whether co-treating with pregnenolone would prevent the decreases in sex steroid hormones observed in mono-OH MXC-treated follicles. We also set out to determine whether co-treating with pregnenolone would affect the ability of mono-OH MXC to induce follicle growth inhibition and increase antral follicle atresia.
MATERIALS AND METHODS
Chemicals
Mono-OH MXC (purity 99%) was synthesized by the laboratory of Dr. Vincent Njar (University of Maryland, Baltimore, MD), dimethylsulfoxide (DMSO), ITS (insulin, transferrin, selenium), penicillin and streptomycin, and pregnenolone were obtained from Sigma-Aldrich (St. Louis, MO). Alpha-minimal essential medium (α-MEM) was obtained from Invitrogen (Carlsbad, CA). Charcoal-stripped fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA), and human recombinant follicle stimulating hormone (rFSH) was obtained from Dr. A.F. Parlow (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA).
Animals
Cycling female CD-1 mice were purchased from Charles River Laboratories (Charles River, CA) and housed four animals per cage at the University of Illinois College of Veterinary Medicine Central Animal Facility. Food and water were provided ad libitum. Temperature was maintained at 22 ± 1°C and animals were subjected to 12L:12D cycles. Animals were euthanized at 35–39 days old by carbon dioxide (CO2) inhalation followed by cervical dislocation. The ovaries were removed and antral follicles were isolated as previously described (Craig, et al. 2010). All experiments and methods involving animals were approved by the University of Illinois Institutional Animal Care and Use Committee (IACUC) and conformed to the Guide for the Care and Use of Experimental Animals (Institute of Laboratory 1996).
Experimental dosages
A stock solution of mono-OH MXC was prepared as previously described (Craig, et al. 2010) using dimethylsulfoxide (DMSO) as a solvent. A dose of mono-OH MXC at 10 μg/mL (~28.9 μM) was selected for this study based on previous reports showing that this concentration of mono-OH MXC reduces antral follicle growth and sex steroid hormone synthesis (Craig, et al. 2010) and is comparable to doses of the parent compound, MXC, which were previously shown to result in decreased antral follicle growth and increased atresia in vitro, and an increased number of atretic antral follicles in vivo (Agency for Toxic Substances and, Disease Registry 2002, Borgeest, et al. 2002, Borgeest, et al. 2004, Miller, et al. 2005). Although this dose is high relative to levels reported in breast milk, placenta and serum by others (Shen, et al. 2007, Botella, et al. 2004), this concentration of MXC may model levels observed in the adipose tissue of women (Botella, et al. 2004).
Antral follicle culture
Mice were euthanized by CO2 inhalation followed by cervical dislocation. Ovaries were removed, trimmed of fat, and antral follicles were mechanically isolated based on relative size (diameter > 200 μm) and placed in culture as previously described (Craig, et al. 2010). Follicles were isolated from 3–4 mice per culture, with approximately 15–25 antral follicles obtained from each mouse. Each follicle culture experiment contained 10–24 follicles per treatment. For treatment, antral follicles were placed in individual wells of 96-well culture plates with 75 μL of unsupplemented α-MEM.
Treatment groups included a vehicle control consisting of DMSO (≤ 0.075%), mono-OH MXC (10 μg/mL), pregnenolone (1 μg/mL), or mono-OH MXC and pregnenolone added together. All dosing solutions were prepared individually in supplemented α-MEM with an equal volume of chemical added for each dose to control for the amount of vehicle in each preparation. Supplemented α-MEM contained 5% FBS, 1% ITS, 100 U/mL penicillin, 100 mg/mL streptomycin, and 5 IU/mL human rFSH. Follicles were treated by replacing the unsupplemented media with 150 μL supplemented α-MEM containing the treatment and incubated for 96 h at 37°C in 5% CO2.
Hormone measurements
The medium from each culture well containing an individual follicle was collected and stored at −80°C. When at least three separate culture experiments were completed, media samples from all experiments were randomly selected and subjected to enzyme-linked immunosorbent assays (ELISA). Levels of progesterone, androstenedione, testosterone, estrone, and E2 in media were measured using kits from DRG International (Mountainside, NJ). Some samples were diluted to match the dynamic range of each ELISA kit. The analytical sensitivity of each kit was 9.71 pg/mL for E2, 6.3 pg/mL for estrone, 0.083 ng/mL for testosterone, 0.019 ng/mL for androstenedione, and 0.1 ng/mL for progesterone. The intra-assay coefficients of variation (CVs) were 2.8% for E2, 2.2% for estrone, 1.8% for testosterone, 1.9% for androstenedione, and 3.5% for progesterone. The inter-assay CVs were 7.8% for E2, 9.2% for estrone, 3.6% for testosterone, 6.5% for androstenedione, and 10.4% for progesterone. Lack of significant cross-reactivity with pregnenolone was verified by running samples containing media supplemented with pregnenolone alone and by consulting the cross-reactivity information provided by the manufacturer of each kit. Cross-reactivity with pregnenolone was zero or insignificant in all cases. Overall, sex steroid hormone data consisted of values from 11–26 individual follicles obtained from three separate culture experiments.
Analysis of antral follicle atresia
At the end of the 96 h cultures with pregnenolone co-treatment, some follicles were randomly selected and fixed in Dietrich’s fixative for subsequent histological evaluation of atresia as previously described (Craig, et al. 2013). Stained sections were evaluated for presence of apoptotic bodies (cell death debris) and rated according to the following scale: 1 for healthy, 2 for 1–10% apoptotic bodies, 3 for 11–30% apoptotic bodies, and 4 for >40% apoptotic bodies. Each follicle received an overall rating calculated from the average of all sections evaluated for that follicle. All ratings were assigned without knowledge of treatment by two researchers and averaged. Atresia ratings consisted of values obtained from 3–4 individual follicles randomly selected from three different experiments.
Statistical analysis
All data were analyzed using SPSS statistical software (SPSS Inc., Chicago, IL). For all comparisons, statistical significance was assigned at p<0.05. Comparisons between treatments were conducted on data obtained from 3–4 experiments using one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc tests (antral follicle growth data), or Kruskal-Wallis followed by Mann-Whitney tests for non-parametric data (antral follicle atresia and sex steroid hormone data). Testosterone values were log transformed prior to analysis to meet statistical test assumptions.
RESULTS
Effect of pregnenolone and mono-OH MXC co-treatment on production of progesterone
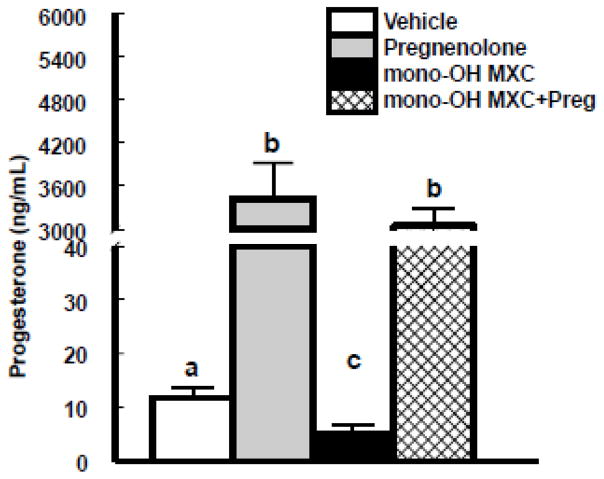
We have previously shown that mono-OH MXC reduces antral follicle steroidogenesis in vitro, and that progesterone is the earliest hormone affected in the pathway (Craig, et al. 2010). Therefore, we hypothesized that co-treating with pregnenolone, the immediate precursor of progesterone, would prevent the decrease in progesterone synthesis observed in antral follicles treated with mono-OH MXC. To test this hypothesis, we compared the levels of progesterone accumulated in the media from follicles treated with vehicle and mono-OH MXC with and without pregnenolone supplementation (Figure 1). Both mono-OH MXC and pregnenolone significantly affected progesterone levels after 96 h (p<0.05). Specifically, addition of pregnenolone treatment significantly increased the levels of progesterone in the media from vehicle-treated follicles (vehicle: 11.87 ± 1.97 ng/mL versus pregnenolone: 3417.9 ± 503.8, p<0.05). As previously reported, mono-OH MXC treatment decreased the accumulation of progesterone in the media from antral follicles relative to vehicle-treated follicles (mono-OH MXC: 5.63 ± 1.15, p<0.05). Finally, levels of progesterone in the media of follicles treated with mono-OH MXC and pregnenolone together did not differ from those observed in media from follicles treated with pregnenolone alone (mono-OH MXC + pregnenolone: 3051.8 ± 234.6; p>0.05), but were significantly higher than those observed in follicles treated with mono-OH MXC alone.
Figure 1. Effect of pregnenolone and mono-OH MXC co-treatment on levels of progesterone in media from mouse antral follicles.
Isolated mouse antral follicles were cultured for 96 h with DMSO as a vehicle control, mono-OH MXC (10 μg/mL), pregnenolone (1 μg/mL), or mono-OH MXC and pregnenolone together. Following culture, media samples from individual follicles were subjected to measurement of progesterone levels as described in Materials and Methods. Data were expressed as mean ± SE calculated from follicles in three separate experiments. Different letters indicate differences between treatment groups (p<0.05).
Effect of pregnenolone and mono-OH MXC co-treatment on production of androgens
We previously reported that decreased accumulation of progesterone in the media of mono-OH MXC treated follicles is accompanied by decreases in the levels of the androgens androstenedione and testosterone. We therefore hypothesized that co-treatment with pregnenolone would prevent the decrease in androgen levels observed following mono-OH MXC treatment because their upstream precursor progesterone is restored. To test this hypothesis, we compared the levels of androstenedione (Figure 2A) and testosterone (Figure 2B) accumulated in the media of follicles treated with vehicle and mono-OH MXC with and without pregnenolone supplementation for 96 h. As with progesterone, both mono-OH MXC and pregnenolone treatment resulted in significant differences in androstenedione and testosterone levels (p<0.05). Specifically, mono-OH MXC treatment significantly reduced the levels of both androstenedione (vehicle: 0.66 ± 0.20 ng/mL; mono-OH MXC: 0.23 ± 0.08 ng/mL, p<0.05) and testosterone (vehicle: 0.95 ± 0.14 ng/mL; mono-OH MXC: 0.24 ± 0.08 ng/mL, p<0.05) in the media of antral follicles compared to vehicle treatment. Similar to our observations with progesterone, pregnenolone treatment increased production of both androstenedione (pregnenolone: 1.44 ± 0.24 ng/mL; p<0.05) and testosterone (pregnenolone: 1.46 ± 0.12 ng/mL; p<0.05) by antral follicles compared to vehicle controls. Furthermore, when follicles were treated with mono-OH MXC and pregnenolone together, levels of androstenedione were increased to levels similar to those observed in vehicle treated follicles (0.77 ± 0.06 ng/mL); however, these levels were significantly lower than those observed in follicles treated with pregnenolone alone. Interestingly, follicles co-treated with pregnenolone and mono-OH MXC together showed a trend (p=0.057) for increased testosterone levels compared to mono-OH alone (mono-OH MXC + pregnenolone: 0.56 ± 0.13 ng/mL) and were no different than those observed in samples from vehicle-treated follicles.
Figure 2. Effect of pregnenolone and mono-OH MXC co-treatment on levels of androstenedione and testosterone in media from mouse antral follicles.
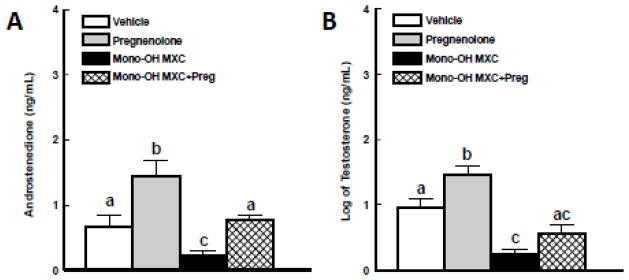
Isolated mouse antral follicles were cultured for 96 h with DMSO as a vehicle control, mono-OH MXC (10 μg/mL), pregnenolone (1 μg/mL), or mono-OH MXC and pregnenolone together. Following culture, media samples from individual follicles were subjected to measurement of androstenedione and testosterone levels as described in Materials and Methods. Data were expressed as mean ± SE calculated from follicles in three separate experiments. Data for testosterone levels were log-transformed to achieve normality. Different letters indicate differences between treatment groups (p<0.05).
Effect of pregnenolone and mono-OH MXC co-treatment on production of estrogens
We hypothesized that co-treatment with pregnenolone would prevent the mono-OH MXC-induced decrease in estrone levels in the media due to its ability to recover androstenedione, a precursor of estrone. To test this hypothesis, we compared the levels of estrone (Figure 3A) accumulated in the media of follicles treated with vehicle and mono-OH MXC with and without pregnenolone supplementation for 96 h. Both mono-OH MXC and pregnenolone altered estrone levels produced by antral follicles (p<0.05). Specifically, treatment with mono-OH MXC significantly reduced the level of estrone accumulated in the media from antral follicles (vehicle: 495.2 ± 105.1 ng/mL; mono-OH MXC: 26.5 ± 5.4 ng/mL; p<0.05). Treatment with pregnenolone alone caused an increase in estrone levels (pregnenolone: 956.9 ± 143.8 ng/mL), while treatment with both pregnenolone and mono-OH MXC together resulted in estrone levels that were higher than those observed in mono-OH MXC follicles, but comparable to those observed in vehicle-treated follicles (mono-OH MXC + pregnenolone: 381.8 ± 40.9 ng/mL; p>0.05).
Figure 3. Effect of pregnenolone and mono-OH MXC co-treatment on levels of estrone and E2 in media from mouse antral follicles.
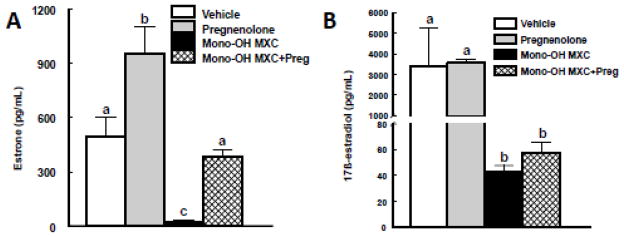
Isolated mouse antral follicles were cultured for 96 h with DMSO as a vehicle control, mono-OH MXC (10 μg/mL), pregnenolone (1 μg/mL), or mono-OH MXC and pregnenolone together. Following culture, media samples from individual follicles were subjected to measurement of estrone and E2 levels as described in Materials and Methods. Data were expressed as mean ± SE calculated from follicles in three separate experiments. Different letters indicate differences between treatment groups (p<0.05).
We also hypothesized that co-treatment with pregnenolone would prevent the decrease in E2 production associated with mono-OH MXC treatment (Figure 3B). Mono-OH MXC treatment decreased the production of E2 by antral follicles (vehicle: 3098.8 ± 996.6 ng/mL; mono-OH: 43.2 ± 5.7 ng/ml; p<0.05) compared to vehicle. Contrary to what we observed with upstream sex steroid levels, co-treatment with pregnenolone alone did not increase E2 production by control antral follicles (pregnenolone: 3578.9 ± 986.9 ng/mL) nor did it increase E2 production by mono-OH MXC-treated follicles (mono-OH MXC + pregnenolone: 53.7 ± 5.2 ng/mL).
Effect of pregnenolone and mono-OH MXC co-treatment on antral follicle growth
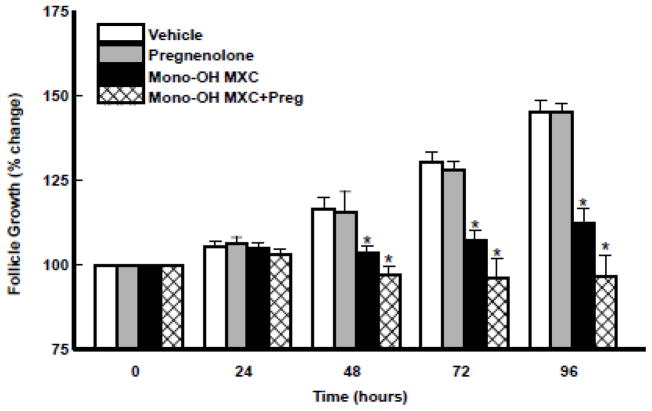
The growth of antral follicles was monitored and compared among treatment groups over time to determine whether pregnenolone co-treatment affected the ability of mono-OH MXC to inhibit antral follicle growth (Figure 4). Follicles treated with vehicle or pregnenolone increased their diameter significantly overtime. Compared to vehicle, mono-OH MXC treatment resulted in growth inhibition starting at 48 h and continued until the end of the culture period at 96 h (p<0.05). Interestingly, co-treating with pregnenolone did not prevent inhibition of follicle growth by mono-OH MXC, and the inhibition of follicle growth observed in follicles co-treated with mono-OH MXC and pregnenolone together was not significantly different (p>0.05) from that observed in follicles treated with mono-OH MXC alone.
Figure 4. Effect of pregnenolone and mono-OH MXC co-treatment on in vitro growth of mouse antral follicles.
Isolated mouse antral follicles were cultured for 96 h with DMSO as a vehicle control, mono-OH MXC (10 μg/mL), pregnenolone (1 μg/mL), or mono-OH MXC and pregnenolone together. Antral follicle growth was assessed as described in Materials and Methods. Data represent means ± SE from three separate experiments. Asterisks (*) indicate statistically significant differences (p<0.05) between treatments and vehicle.
Effect of pregnenolone and mono-OH co-treatment on antral follicle atresia
Follicles were processed for histological analysis of follicle atresia following culture with vehicle and mono-OH with and without pregnenolone supplementation to determine whether pregnenolone co-treatment affected the ability of mono-OH MXC to cause antral follicle atresia (Figure 5). Mono-OH MXC treatment significantly increased the percentage of apoptotic bodies present in antral follicles, an indication of increased follicle atresia. Interestingly, co-treating with pregnenolone did not prevent mono-OH MXC from increasing the number of apoptotic bodies present in treated antral follicles.
Figure 5. Effect of pregnenolone and mono-OH MXC co-treatment on mouse antral follicle atresia.
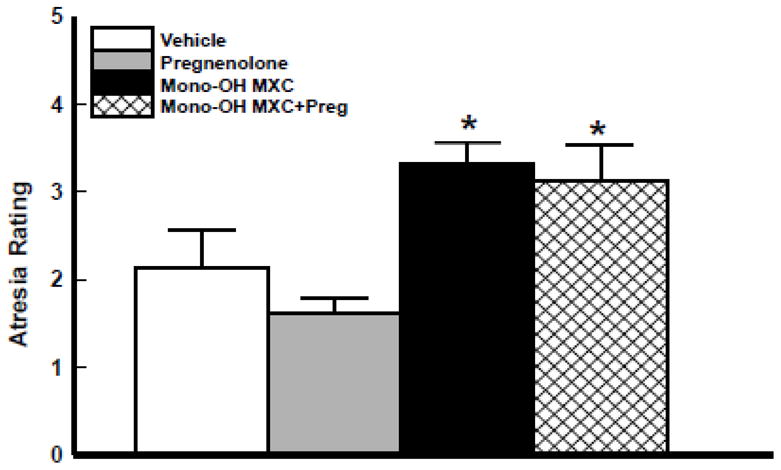
Isolated mouse antral follicles were cultured for 96 h with DMSO as a vehicle control, mono-OH MXC (10 μg/mL), pregnenolone (1 μg/mL), or mono-OH MXC and pregnenolone together. Following culture, follicles were processed for histological evaluation of follicular atresia as described in Materials and Methods. Data represent means ± SE from 3 separate experiments. Asterisks (*) indicate statistically significant differences (p<0.05) between treatments and vehicle.
DISCUSSION
We used an isolated mouse antral follicle culture system to investigate whether replacing pregnenolone, the rate-limiting substrate in ovarian steroidogenesis, would prevent the decreases in sex steroid hormone levels previously observed in the media of antral follicles exposed to mono-OH MXC. Our results show that pregnenolone treatment restores levels of progesterone, androstenedione, testosterone, and estrone to control levels in follicles treated with mono-OH MXC for 96 h. Interestingly, adding excess pregnenolone to the media did not restore E2 levels and did not prevent mono-OH MXC from inducing follicle growth inhibition and follicular atresia. Thus, our findings support the idea that decreased availability of pregnenolone may be the main cause for the decreases in E2 precursors observed in media samples from mono-OH MXC treated follicles. Our results also suggest that the mechanisms by which mono-OH MXC induces follicle growth inhibition and follicular atresia may be independent from its effects on the synthesis of E2 precursors.
We had previously identified CYP11A1 and pregnenolone as the most upstream targets of mono-OH MXC in the antral follicle steroidogenic pathway based on the observations that Cyp11a1 mRNA expression and progesterone levels are reduced in the absence of changes to Hsd3b1 mRNA expression. Thus, we hypothesized that adding excess pregnenolone to the media of mono-OH MXC-treated follicles would provide HSD3B1 with substrate to restore progesterone production. Our results for mono-OH MXC alone agree with previous reports of decreased progesterone levels in antral follicles treated with MXC (Basavarajappa, et al. 2011) and mono-OH MXC (Craig, et al. 2010), as well as those in rat whole ovary cultures (Cummings and Laskey 1993), and bovine (Tiemann, et al. 1996), porcine (Chedrese and Feyles 2001, Crellin, et al. 2001), and rat (Zachow and Uzumcu 2006) granulosa cell cultures. Here, we show that the mono-OH MXC-induced decrease in progesterone production by mouse antral follicles is prevented when follicles are treated with pregnenolone and mono-OH MXC together. No other studies have evaluated the effect of adding excess pregnenolone to follicles treated with MXC and its metabolites; however, the ability of pregnenolone supplementation to increase progesterone synthesis has been documented previously in cultured sheep follicles (Tambe and Nandedkar 1993), primary and stable porcine granulosa cells (Rodway, et al. 1999, Tiemann, et al. 2007), and in mouse ovarian antral follicles exposed in vitro to the endocrine disruptors bisphenol A (BPA; (Peretz, et al. 2011)) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; (Karman, et al. 2012)).
Mono-OH MXC treatment decreases the levels of the androgens produced by mouse antral follicles in vitro (Craig, et al. 2010). Therefore, we expanded on previous work by evaluating whether pregnenolone supplementation restores androstenedione and testosterone production by mono-OH MXC treated follicles. We hypothesized that pregnenolone co-treatment would increase androstenedione production in mono-OH MXC treated follicles based on the observation that levels of progesterone, the immediate precursor of androstenedione, were recovered. Likewise, we hypothesized that increased androstenedione production would result in recovery of testosterone levels since androstenedione is converted to testosterone by HSD17B1 which is not affected in mono-OH MXC treated follicles (Craig, et al. 2010). In mono-OH MXC-treated follicles, pregnenolone co-treatment resulted in androstenedione and testosterone levels that were similar to those observed in vehicle control follicles; however, these levels were still lower than those observed in follicles exposed to pregnenolone alone. This observation could be explained by the fact that mono-OH MXC has been shown to decrease the expression of Cyp17a1 mRNA, the enzyme responsible for the conversion of progesterone to androstenedione in mouse antral follicles (Craig, et al. 2010). Perhaps, the decreased availability of CYP17A1 enzyme, due to its decreased transcript levels following mono-OH MXC treatment, prevents antral follicles from converting progesterone to androstenedione as efficiently as their counterparts treated with pregnenolone alone. Furthermore, the observation that testosterone levels in mono-OH MXC + pregnenolone follicles were not comparable to those treated with pregnenolone alone was unexpected since Hsd17b1 mRNA has been reported to be unaffected by mono-OH MXC in mouse antral follicles (Craig, et al. 2010). Therefore, we think that the decreased availability of androstenedione in these follicles may be enough to result in lower testosterone levels in mono-OH MXC-treated follicles when compared to those observed in follicles treated with pregnenolone alone.
In this study, we showed that mono-OH MXC exposure resulted in decreased levels of estrone and E2 in mouse antral follicles. These observations are in agreement with previous studies exposing mouse antral follicles to MXC and its metabolites (Basavarajappa, et al. 2011, Craig, et al. 2010). When co-treated with pregnenolone, the levels of estrone in the media of mono-OH MXC treated antral follicles were significantly greater than in follicles treated with mono-OH MXC alone and similar to those observed in vehicle control follicles, suggesting the presence of aromatase activity. As with androstenedione and testosterone, estrone levels in the media of mono-OH MXC-treated follicles that received pregnenolone supplementation were significantly lower than those in follicles treated with pregnenolone alone. We think that this difference is due to the lower androgen substrate availability observed between these treatments and the decrease in aromatase expression reported previously (Basavarajappa, et al. 2011, Craig, et al. 2010). To our surprise, pregnenolone treatment alone was not sufficient to elicit an increase in E2 production by mouse antral follicles regardless of whether they were exposed to mono-OH MXC treatment. Although the reason why this occurs is not fully understood, it has been previously suggested that autocrine control mechanisms may exist between granulosa and theca cells (Tambe and Nandedkar 1993) based on observations that increased progesterone levels exert a specific and irreversible inhibitory effect on rat granulosa cell E2 production (Fortune and Vincent 1983). Another study evaluating the effects of the plasticizer BPA on antral follicle steroidogenesis has also reported no increase in E2 levels following pregnenolone supplementation (Peretz, et al. 2011). A similar phenomenon was also reported by Rodway et al., (Rodway, et al. 1999) in a stable porcine granulosa cell line where increased levels of androstenedione interfered with 8-Br-cAMP activation of aromatization, but this was not observed when androstenedione was added at low concentrations. In fact, estrogen receptor alpha signaling has been shown to mediate an intraovarian negative feedback loop by modulating the expression of CYP17A1, the enzyme that catalyzes the synthesis of the estrogen precursor androstenedione (Taniguchi, et al. 2007). Therefore, it is possible that the increases in upstream E2 precursors such as progesterone, androstenedione, and testosterone due to pregnenolone supplementation had a negative impact on aromatase expression and/or activity in mouse antral follicles. Taken together, these observations also support the idea of autocrine feedback loops that modulate steroidogenesis based on local sex steroid hormone levels within ovarian follicles. Although we have not evaluated protein levels for these enzymes here, other studies, in which mRNA and protein for steroidogenic enzymes have been measured simultaneously, have demonstrated that mRNA and protein expression are correlated (Conley, et al. 1994, Havelock, et al. 2006). Thus, we think that it is very likely that protein expression levels will show similar effects to those reported previously for mRNA expression (Craig, et al. 2010). Yet, future studies will be needed to completely understand the effects of mono-OH MXC and pregnenolone supplementation on steroidogenic enzyme expression and activity.
Finally, we determined whether the partial restoration of sex steroid levels by pregnenolone supplementation prevented inhibited follicle growth and increased atresia in mono-OH MXC treated follicles. As in previous reports with MXC and metabolites (Gupta, et al. 2006, Miller, et al. 2005, Miller, et al. 2006), mono-OH MXC treatment inhibited in vitro growth of mouse antral follicles. Despite restoring the levels of various sex steroid hormones in the pathway, pregnenolone supplementation failed to prevent inhibition of antral follicle growth and induction of atresia by mono-OH MXC. It is widely recognized that E2 stimulates granulosa cell proliferation, a known mechanism by which antral follicles develop and increase in diameter, and inhibits follicular atresia (reviewed in (Quirk, et al. 2004) and (Drummond and Findlay 1999)). Thus, future studies should be aimed at determining whether growth inhibition and increased atresia in follicles treated with mono-OH MXC and pregnenolone together is due to the inability of pregnenolone to recover E2 levels in these follicles.
In summary, we have shown that co-treating with pregnenolone as a substrate for steroidogenesis alleviates most, but not all the sex steroid hormone deficiencies associated with exposure to mono-OH MXC in mouse ovarian antral follicles. We have also shown that mono-OH MXC is still able to inhibit growth and increase atresia in follicles co-treated with pregnenolone. These findings are important because they expand on our understanding of the mechanisms by which MXC and its metabolites disrupt ovarian follicle steroidogenesis and highlight how these alterations may impact antral follicle development and health. Future studies should be aimed at determining the time course of, as well as, pathways involved in the ability of mono-OH MXC to decrease steroidogenic enzyme expression and sex steroid hormone production in ovarian follicles.
HIGHLIGHTS.
Mono-OH MXC inhibited antral follicle steroidogenesis, growth, and survival.
Pregnenolone partially restored steroidogenesis in mono-OH MXC-treated follicles.
Pregnenolone did not prevent mono-OH MXC-induced inhibition of growth and survival.
Acknowledgments
The authors thank Ayelet Ziv-Gal and Timothy DelValle for technical help, Lalji Gedia (Dr. Vincent Njar laboratory) for the synthesis of mono-OH MXC, and grant support provided by the National Institute of Environmental Health Sciences (NIEHS) grants R01ES019178 (JAF), K99ES021467 (ZRC), the University of Illinois Billie A. Field Fellowship in Reproductive Biology program (ZRC), and an Environmental Toxicology Scholarship (PRH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zelieann R. Craig, Email: zelieann@illinois.edu.
Patrick R. Hannon, Email: phannon2@illinois.edu.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- Agency for Toxic Substances and, Disease Registry. Toxicological Profile for Methoxychlor. U.S. Department of Health and Human Services Public Health Service; Atlanta, GA: 2002. [PubMed] [Google Scholar]
- Akgul Y, Derk R, Meighan T, Rao K, Murono E. The methoxychlor metabolite, HPTE, directly inhibits the catalytic activity of cholesterol side-chain cleavage (P450scc) in cultured rat ovarian cells. Reprod Toxicol. 2008;25:67–75. doi: 10.1016/j.reprotox.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bagur AC, Mautzlen C. Risk for developing osteoporosis in untreated premature menopause. Calcif Tiss Int. 1992;51:4–7. doi: 10.1007/BF00296207. [DOI] [PubMed] [Google Scholar]
- Basavarajappa M, Craig Z, Hern ndez-Ochoa I, Paulose T, Leslie T, Flaws J. Methoxychlor reduces estradiol levels by altering steroidogenesis and metabolism in mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2011;253:161–169. doi: 10.1016/j.taap.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeest C, Miller K, Gupta R, Greenfeld C, Hruska K, Hoyer P, Flaws J. Methoxychlor-induced atresia in the mouse involved Bcl-2 family member, but not gonadotropins or estradiol. Biol Reprod. 2004;70:1828–1835. doi: 10.1095/biolreprod.103.022889. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Symonds D, Mayer L, Hoyer P, Flaws J. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian surface epithelium in the mouse. Toxicol Sci. 2002;68:473–478. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, Olea N. Exposure of women to organochlorine pesticides in Southern Spain. Environ Res. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Britt K, Saunders M, McPherson S, Misso M, Simpson E, Findlay J. Estrogen actions on follicle formation and early follicle development. Biol Reprod. 2004;71:1712–1723. doi: 10.1095/biolreprod.104.028175. [DOI] [PubMed] [Google Scholar]
- Chedrese P, Feyles F. The diverse mechanism of action of dichlorodiphenyldichloroethylene (DDE) and methoxychlor in ovarian cells in vitro. Reprod Toxicol. 2001;15:693–698. doi: 10.1016/s0890-6238(01)00172-1. [DOI] [PubMed] [Google Scholar]
- Conley A, Howard H, Slanger W, Ford J. Steroidogenesis in the preovulatory porcine follicle. Biol Reprod. 1994;51:655–661. doi: 10.1095/biolreprod51.4.655. [DOI] [PubMed] [Google Scholar]
- Craig Z, Hannon P, Wang W, Ziv-Gal A, Flaws J. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod. 2013;88:23. doi: 10.1095/biolreprod.112.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig Z, Leslie T, Hatfield K, Gupta R, Flaws J. Mono-hydroxy methoxychlor alters levels of key sex steroids and steroidogenic enzymes in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2010;249:107–113. doi: 10.1016/j.taap.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellin N, Kang H, Swan C, Chedrese P. Inhibition of basal and stimulated progesterone synthesis by dichlorodiphenyldichloroethylene and methoxychlor in a stable pig granulosa cell line. Reproduction. 2001;121:485–492. [PubMed] [Google Scholar]
- Cummings A, Gray LJ. Antifertility effect of methoxychlor in female rats: Dose- and time-dependent blockade of pregnancy. Toxicol Appl Pharmacol. 1989;97:454–462. doi: 10.1016/0041-008x(89)90250-0. [DOI] [PubMed] [Google Scholar]
- Cummings A, Laskey J. Effect of methoxychlor on ovarian steroidogenesis: role in early pregnancy loss. Reprod Toxicol. 1993;7:17–23. doi: 10.1016/0890-6238(93)90005-r. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Lehert P, Burger H, Dudley E. Mood and the menopausal transition. J Nerv Ment Dis. 1999;187:685–691. doi: 10.1097/00005053-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Drummond A, Findlay J. The role of estrogen in folliculogenesis. Mol Cell Endocrinol. 1999;151:57–64. doi: 10.1016/s0303-7207(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Eroschenko V, Abuel-Atta A, Grober M. Neonatal exposures to technical methoxychlor alters ovaries in adult mice. Reprod Toxicol. 1995;9:379–387. doi: 10.1016/0890-6238(95)00025-6. [DOI] [PubMed] [Google Scholar]
- Fortune J, Vincent S. Progesterone inhibits the induction of aromatase activity in rat granulosa cells in vitro. Biol Reprod. 1983;28:1078–1089. doi: 10.1095/biolreprod28.5.1078. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Harvey C, Esmail M, Wang Q, Brooks A, Zachow R, Uzumcu M. Effect of the methoxychlor metabolite HPTE on the rat ovarian granulosa cell transcriptomein vitro. Toxicol Sci. 2009;110:95–106. doi: 10.1093/toxsci/kfp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelock J, Rainey W, Bradshaw K, Carr B. The post-menopausal ovary displays a unique pattern of steroidogenic enzyme expression. Human Reprod. 2006;21:317. doi: 10.1093/humrep/dei373. [DOI] [PubMed] [Google Scholar]
- Hirshfield A. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson J, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory, A.R. Guide for the Care and use of Laboratory Animals. National Academies Press; Washington, DC: 1996. [Google Scholar]
- Kapoor IP, Metcalf RL, Nystrom RF, Sangha GK. Comparative metabolism of methoxychlor, methiochlor, and DDT in mouse, insects, and in a model ecosystem. J Agric Food Chem. 1970;18:1145–1152. doi: 10.1021/jf60172a017. [DOI] [PubMed] [Google Scholar]
- Karman B, Basavarajappa M, Craig Z, Flaws J. TCDD activates the AHR pathway and alters estradiol levels without affecting growth in CD-1 mouse antral follicles in vitro. 2012 doi: 10.1016/j.taap.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AJ, Widra EA. In: Basic infertility: Etiology and Therapy. Seifer DB, Samuels P, Kniss DA, editors. Lippincott Williams & Wilkins; Philadelphia, PA: 1994. pp. 245–263. [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Peretz J, Gupta R, Singh J, Hern ndez-Ochoa I, Flaws J. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119:209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk S, Cowan R, Harman R, Hu C, Porter D. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci. 2004;82:E40–E52. doi: 10.2527/2004.8213_supplE40x. [DOI] [PubMed] [Google Scholar]
- Rodway M, Swan C, Gillio-Meina C, Crellin N, Flood P, Chedrese P. Regulation of steroidogenesis in jc-410, a stable cell line of porcine granulosa origin. Mol Cell Endocrinol. 1999;148:87–94. doi: 10.1016/s0303-7207(98)00233-0. [DOI] [PubMed] [Google Scholar]
- Shen H, Main KM, Virtanen HE, Damggard IN, Haavisto AM, Kaleva M, Boisen KA, Schmidt IM, Chellakooty M, Skakkebaek NE, Toppari J, Schramm KW. From mother to child: investigation of prenatal and postnatal exposure to persistent bioaccumulating toxicants using breast milk and placenta biomonitoring. Chemosphere. 2007;67:S256–62. doi: 10.1016/j.chemosphere.2006.05.106. [DOI] [PubMed] [Google Scholar]
- Swartz W, Corkern M. Effects of methoxychlor treatment of pregnant mice on female offspring of the treated and subsequent pregnancies. Reprod Toxicol. 1992;6:431–437. doi: 10.1016/0890-6238(92)90006-f. [DOI] [PubMed] [Google Scholar]
- Tambe S, Nandedkar T. Steroidogenesis in sheep ovarian antral follicles in culture: time course study and supplementation with a precursor. Steroids. 1993;58:379–383. doi: 10.1016/0039-128x(93)90041-k. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Couse J, Rodriguez K, Emmen J, Poirier D, Korach K. Estrogen receptor-alpha mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17alpha-hydroxylase/17,20 lyase) expression. FASEB J. 2007;21:586–595. doi: 10.1096/fj.06-6681com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann U, Pöhland R, Schneider F. Influence of organochlorine pesticides on physiological potency of cultured granulosa cells from bovine preovulatory follicles. Theriogenology. 1996;46:253–265. doi: 10.1016/0093-691x(96)00182-3. [DOI] [PubMed] [Google Scholar]
- Tiemann U, Schneider F, Vanselow J, Tomek W. In vitro exposure of porcine granulosa cells to the phytoestrogens genistein and daidzein: effects on the biosynthesis of reproductive steroid hormones. Reprod Toxicol. 2007;24:317–325. doi: 10.1016/j.reprotox.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Zachow R, Uzumcu M. The methoxychlor metabolite, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, inhibits steroidogenesis in rat ovarian granulosa cells in vitro. Reprod Toxicol. 2006;22:659–665. doi: 10.1016/j.reprotox.2006.04.018. [DOI] [PubMed] [Google Scholar]