Abstract
In urine specimens that were collected from pregnant women in a large cohort, 24% contained more than 10 ng/ml of total bisphenol A (BPA), suggesting external contamination. Therefore, we conducted an investigation of the source(s) of extraneous BPA in the specimens. We found that under the conditions used to collect urine specimens in the epidemiologic study, contamination with BPA occurred, and by two separate mechanisms.
Keywords: bisphenol A, urine specimen collection, contamination
1. Introduction
Over 65,000 urine specimens were collected from pregnant women in the Norwegian Mother and Child Cohort Study (MoBa) (Ronningen et al., 2006, Magnus et al., 2006). The specimens were obtained when the women attended their local clinic for a routine prenatal ultrasound examination. Within 4 hours of collection, urine was placed in a tube that contained a proprietary preservative which contained ethyl paraben, sodium propionate, and chlorhexidine. To keep the study costs manageable, the urine was shipped by mail at ambient temperature from the collection points throughout Norway to a central repository in Oslo, where the urine was frozen before any analyses were done.
Subsequently, while conducting analyses of MoBa urine specimens, where both total and unconjugated (“free BPA”) were measured, we encountered unexpectedly high concentrations of total and free BPA (data not shown), suggesting external contamination. Therefore, we conducted an investigation of the source of free BPA in the specimens. Here we report the findings from this investigation.
2. Methods
2.1 1st experiment: preservative and shipping effects
The goal of this experiment was to determine whether addition of the preservative and simulated shipping conditions influenced the concentration of free and conjugated BPA in urine.
Spot urine specimens were collected from 15 adults, 18 years of age or older, in Oslo, in 2010. Each subject provided urine in a polypropylene collection cup. All materials employed were of the same type used for the MoBa urine collection. The urine specimens were placed on ice immediately after collection and aliquoted within 30 minutes. Six aliquots were taken from each urine specimen. The common elements to the handling of the six aliquots were: a) placed in an 8 ml Vacutainer urine collection tube (Urinalysis Urine Tube, BD Diagnostics, Franklin Lakes, NJ), b) the urine was vortexed, and c) using a filtered-tip polypropylene pipette, 0.93 ml was put in a polypropylene tube and frozen at −80° C. The handling of the six aliquots varied as follows. The first aliquot was put in a urine collection tube that contained the preservative (Urinalysis Preservative Plus Urine Tube, BD Diagnostics), comprising ethyl paraben, 5.6%; sodium propionate, 94%; and chlorhexidine, 5.6%, applied by the manufacturer as a spray coating to the interior of the collection tube. The second aliquot was put in a collection tube without the preservative. The third aliquot was put in a collection tube with the preservative and left at room temperature for 24 hours before pipetting. The fourth aliquot was put in a collection tube but without the preservative and left at room temperature for 24 hours before pipetting. The fifth aliquot was put in a collection tube with the preservative and was left at room temperature for 24 hours and was gently agitated mechanically for the 24 hour period before pipetting. The sixth aliquot was put in a collection tube with the preservative and left at room temperature for 72 hours before pipetting. A new pipette was used for each aliquot.
Thus, in total, the preservative and shipping experiment involved 90 aliquots (15 original specimens with each treated 6 different ways). All 6 aliquots from each subject were analyzed sequentially in the same analytical batch. The analyzing laboratory was blinded to the identity of the samples.
2.2 2nd experiment: field blanks
The main goal of this experiment was to determine whether free BPA contaminated purified water when the water was handled so as to simulate the collection procedure for the MoBa urine specimens (field blanks). This experiment, however, was done using Vacutainers that did not contain the preservative.
At an arbitrarily-selected MoBa data collection center in Stavanger, Chromasolv water (LC-MS grade, Sigma-Aldrich, St. Louis, Mo.) was heated to 37°C in clean laboratory glassware and covered with aluminum foil. A midwife went to one of three restrooms between 1300-1400 h, and stayed there for 3-5 minutes with the opened collection cup and then filled it with the heated Chromasolv water. When leaving the restroom, the collection cup cover was replaced. The specimen remained in the collection cup for 10 minutes before being placed in the Vacutainer (no preservative). Thereafter, the sample was handled as normal MoBa urine specimen; it was refrigerated before shipping at ambient temperature to the biobank by mail at 1500 h, and at the biobank 0.93 was put in a polypropylene tube with a polypropylene pipette and frozen at −80°C. This procedure was repeated six times.
To create a group of “reference blanks” to compare with the field blanks, six reference blanks were prepared with Chromasolv water at the biobank in Oslo. Each aliquot of the water was poured into a clean glass container then transferred using a polypropylene pipette to a 0.93 polypropylene vial and frozen at −80°C.
2.3 Measurement of BPA
The free (unconjugated) BPA concentration was determined using a high-performance liquid chromatography–mass spectrometry method (on-line SPE HPLC–MS/MS) (Koch et al., 2003, Ye et al. 2005), with slight modifications (Koch et al., 2012). The limit of detection (LOD) was 0.05ng/mL (based on a signal-to-noise ratio of 3) and the limit of quantification (LOQ) was 0.1 ng/mL (based on a signal-to-noise ratio of 9). BPA is a ubiquitous contaminant in laboratories and other environments (Ye et al., 2013). With the on-line methodology, however, the laboratory blank value of BPA was kept below the LOD of 0.05 ng/mL. The relative SDs for the quality control (QC) samples were 6.5% (Qlow; 2.9 ng/mL) and 3.4% (Qhigh; 11.8 ng/mL) for the within-series imprecision and 5.6% (Qlow) and 3.4% (Qhigh) for the day-to-day imprecision. The relative recovery from spiked (10 μg BPA/l) individual urine samples (creatinine content between 0.17 and 2.4 g/L) was 96.8% (88.5–104%). To ensure data accuracy and precision we included internal QC samples at two concentration levels, standards, and reagent blanks in each batch of samples. The concentration of total BPA was determined according to the methodology described above but was preceded by a hydrolysis step (enzyme, incubation). The relative SDs and recovery were the same as without initial enzymatic deconjugation.
2.4 Statistical Methods
The concentration of conjugated BPA in each urine specimen was calculated as the difference between total and free BPA. The distributions of free and conjugated BPA were skewed, with the long tail to the right. Normalizing transformations were identified as the natural logarithm for free BPA and as the square root for conjugated BPA, and the statistics were performed on the transformed values. For the preservative and shipping experiment we used paired t-tests to evaluate differences between handling procedures (aliquots) and mixed effects ANOVA to evaluate overall differences among aliquots; for the field blank experiment we used an unpaired t-test.
3. Results
Preservative and shipping experiment
Among the 15 urine specimens from subjects in Oslo, in the aliquots that were frozen immediately, without the preservative, the median concentration of total BPA was 2.5 ng/ml; the median concentration of conjugated BPA was 1.4 ng/ml; and the median percent conjugated was 54. The median concentration of total BPA was similar to the median value of 2.7 ng/ml in the U.S. NHANES 2003-2004 (Calafat et al., 2008).
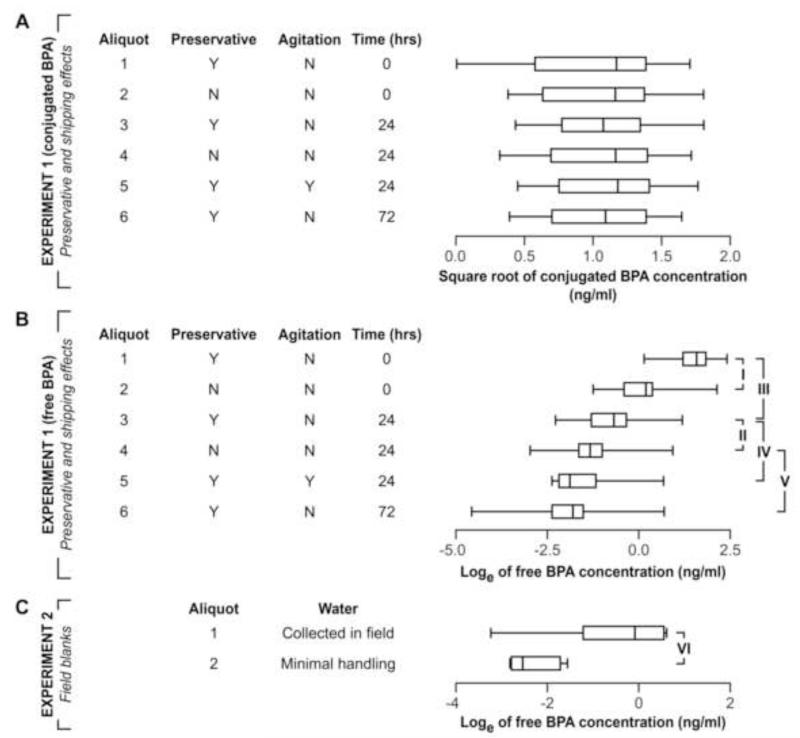
The concentration of conjugated BPA was similar regardless of hours the specimen was left at room temperature and whether agitated or not (figure, panel A; and Table 1), and the results were statistically homogeneous (ANOVA p for difference = 0.78; F=0.49, with 5 and 14 d.f.). For free BPA, however, a striking feature of the results was the nearly monotonic decrease in concentration across aliquots (figure, panel B; and Table 1). Compared with the no preservative specimens, the concentration of free BPA in the specimens containing the preservative was higher both among specimens that were frozen immediately and in the specimens that had been at room temperature for 24 hours before freezing. Among the specimens with preservative at 0 and 24 hours before freezing, free BPA was higher at 0 hours. Among the specimens that had been at room temperature for 24 hours and had preservative added, the concentration of free BPA was lower in the specimens that had been agitated. Among the specimens with the preservative, the specimens at room temperature for 72 hours had a lower free BPA than the specimens that were frozen after being at room temperature for 24 hours.
Field blank experiment
The water specimens that were handled as if they were urine specimens being collected for MoBa in Stavanger had higher free BPA (median 0.6 ng/ml) than in the specimens that were frozen after minimal handling in Oslo (0.1 ng/ml) (figure, panel C).
4. Discussion
The results suggest that the MoBa study urine specimens could have been contaminated by two different mechanisms. First, the field blanks experiment showed that about 0.5 ng/ml of free BPA was introduced into water specimens that were collected like MoBa urine specimens at an arbitrarily-selected MoBa specimen collection site. Second, the results show that the urine preservative contained free BPA. The concentration of conjugated BPA, however, was stable under the conditions studied.
In retrospect, two previous reports based on the analysis of urine specimens that were either from MoBa subjects (Ye et al. 2009), or of urine samples spiked with free/unconjugated BPA that contained the same preservative (Hoppin et al. 2006), presented information suggesting that the preservative influenced the measurements. Free BPA was not measured in either study. In Ye et al. (2009), the concentrations of total BPA were unusually high (mean, 4.5 ng/mL). In Hoppin et al. (2006), the concentration of total BPA in specimens without preservative was not reported, due to “instrumentation problems”. We speculate that these problems may have been due to some type of carry-over effect of the preservative on measurements of BPA in specimens that did not contain the preservative, such as was suggested by the results in the present study (see below).
Some of the results in the present study may have been due to a carry-over effect, with volatized BPA in one aliquot contaminating the next. For example, the decrease in free BPA concentration in specimens without preservative from 0 to 24 hours could be an artifact caused by carry-over increasing the free BPA in the 0 hour specimens. The nearly monotonic decrease in free BPA across aliquots in the figure (panel B) is consistent with carry-over. Carry-over, however, cannot explain the higher free BPA in the specimens with preservative.
In a previous study of the stability of conjugated BPA in 15 urine specimens at room temperature over several days or longer (Ye et al., 2007), the proportion of total BPA that was conjugated decreased after 48 hours. In our data, the concentration of conjugated BPA was stable after up to 72 hours at room temperature. The stability of the conjugated BPA concentration at 72 hours in our specimens may have been due to the presence of the preservative. In studies where both free and conjugated BPA have been measured in native urine, the percent conjugated is ~95% (Ye et al., 2007, Koch et al., 2012). On a related note, in the specimens with preservative, we observed a decrease in free BPA after 24 hours at room temperature, and a further decrease after agitation of the specimen at 24 hours. Perhaps free BPA was reacting with constituents of urine. If such a decrement in free BPA occurred in a biomonitoring study conducted under a contaminant-free protocol, it would likely have a negligible effect on total BPA, due to the small (~5%) contribution of free BPA to the total.
In our field blanks experiment, the Chromasolv water came in contact with glasswear, and was open to the air in the laboratory while heating, providing an opportunity for contamination. Thus, it is possible that the increase in free BPA in the water specimens handled in Stavanger were contaminated not by the urine collection procedure, but by the earlier manipulation of the water. Because absorbent underpads used in laboratories have been identified as a source of contamination of specimens with bisphenol A (Ye et al., 2013), we checked whether any such materials had been used in the Stavanger laboratory—none were. Other authors have either directly documented (Markaham et al., 2010, Vandentorren et al., 2011, Ye et al., 2013) or commented on (Twaddle et al., 2010, Ye et al., 2011) the susceptibility of urine specimens to contamination by free BPA.
We have shown that under typical conditions used to collect urine specimens in an epidemiologic study of outpatients, contamination with BPA occurred, and by two separate mechanisms, one known and one unknown. The known mechanism was via addition of the preservative; the other was possibly via dust or air in clinics. The effect of the preservative on the ability to analyze the urine samples is, we believe, new information. If the MoBa urine specimens are analyzed for total and free BPA, then the difference between the two, the concentration of conjugated BPA can be used to estimate exposure. Any analytical chemist who agrees to analyze MoBa urine specimens needs to be made aware of the potential for contamination of their laboratory with ethyl paraben and related compounds present in the preservative.
Figure 1.
A: Concentration of conjugated BPA from Experiment 1. B: Concentration of free BPA from Experiment 1. C: Concentration of free BPA in Experiment 2. P values for contrasts indicated by brackets: I, <0.0001; II, 0.003; III, <0.0001; IV, 0.0009; V, 0.0001; and VI, <0.0001.
Table 1.
Median concentrations of free and conjugated BPA (ng/ml) among specimens in the 6 experiments on preservation and shipping effects
| Aliquot | Preservative | Agitation | Time (hours) | Free BPA |
Conjugated BPA |
|---|---|---|---|---|---|
| 1 | Y | N | 0 | 4.65 | 1.37 |
| 2 | N | N | 0 | 1.19 | 1.35 |
| 3 | Y | N | 24 | 0.50 | 1.15 |
| 4 | N | N | 24 | 0.26 | 1.36 |
| 5 | Y | Y | 24 | 0.15 | 1.39 |
| 6 | Y | N | 72 | 0.16 | 1.19 |
Acknowledgments
Funding: This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS).
Glossary
Abbreviations
- BPA
bisphenol A
- LOD
limit of detection
- LOQ
limit of quantitation
- MoBa
Norwegian Mother and Child Cohort Study
- QC
quality control
- SD
standard deviation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics: This research was approved by the Southeast Regional Medical Ethics Committee of Norway and the NIH Office for Human Subjects Research.
References
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Ulmer R, Calafat AM, Barr DB, Baker SV, Meltzer HM, Rønningen KS. Impact of urine preservation methods and duration of storage on measured levels of environmental contaminants. J. Expo. Sci. Environ. Epidemiol. 2006;16:39–48. doi: 10.1038/sj.jea.7500435. [DOI] [PubMed] [Google Scholar]
- Koch HM, Reche LM, Angerer J. On-line clean-up by multidimensional liquid chromatography-electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary phthalate metabolites in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;784:169–182. doi: 10.1016/s1570-0232(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Koch HM, Kolossa-Gehring M, Schröter-Kermani C, Angerer J, Brüning T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: A retrospective exposure evaluation. J. Expo. Sci. Environ. Epidemiol. 2012;22:610–616. doi: 10.1038/jes.2012.39. [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, MoBa Study Group Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int. J. Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Markham DA, Waechter JM, Jr., Wimber M, Rao N, Connolly P, Chuang JC, Hentges S, Shiotsuka RN, Dimond S, Chappelle AH. Development of a method for the determination of bisphenol A at trace concentrations in human blood and urine and elucidation of factors influencing method accuracy and sensitivity. J. Anal. Toxicol. 2010;34:293–303. doi: 10.1093/jat/34.6.293. [DOI] [PubMed] [Google Scholar]
- Rønningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, Haugen M, Nystad W, Magnus P, Hoppin JA. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur. J. Epidemiol. 2006;21:619–625. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaddle NC, Churchwell MI, Vanlandingham M, Doerge DR. Quantification of deuterated bisphenol A in serum, tissues, and excreta from adult Sprague-Dawley rats using liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2010;24:3011–3020. doi: 10.1002/rcm.4733. [DOI] [PubMed] [Google Scholar]
- Vandentorren S, Zeman F, Morin L, Sarter H, Bidondo ML, Oleko A, Leridon H. Bisphenol-A and phthalates contamination of urine samples by catheters in the Elfe pilot study: implications for large-scale biomonitoring studies. Environ. Res. 2011;111:761–764. doi: 10.1016/j.envres.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Angerer J, Meltzer HM, Jaddoe VW, Tiemeier H, Hoppin JA, Longnecker MP. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) Int. J. Hyg. Environ. Health. 2009;212:481–491. doi: 10.1016/j.ijheh.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XY, Kuklenyik Z, Needham LL, Calafat AM. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2005;383:638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. Temporal stability of the conjugated species of bisphenol A, parabens, and other environmental phenols in human urine. J. Expo. Sci. Environ. Epidemiol. 2007;17:567–572. doi: 10.1038/sj.jes.7500566. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhou X, Needham LL, Calafat AM. In-vitro oxidation of bisphenol A: Is bisphenol A catechol a suitable biomarker for human exposure to bisphenol A? Anal. Bioanal. Chem. 2011;399:1071–1079. doi: 10.1007/s00216-010-4344-x. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential External Contamination with Bisphenol A and Other Ubiquitous Organic Environmental Chemicals during Biomonitoring Analysis: An Elusive Laboratory Challenge. Environ. Health Perspect. 2013;121:283–286. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]