Abstract
Background
Fetal Alcohol Spectrum Disorder (FASD) is characterized by neurodevelopmental anomalies manifesting in cognitive and behavioral deficits in the offspring with diverse severities. Social behavior is affected in FASD and these deficits overlap with those of autism spectrum disorder (ASD). Identifying some of the molecular characteristics related to ASD in an animal model of FASD could ultimately provide details on the underlying molecular mechanisms of both disorders that could lead to novel treatments.
Methods
Pregnant Sprague-Dawley rats received the following diets: control (C, ad libitum standard lab chow), nutritional control pair-fed (PF), ethanol (E) or an E diet supplemented with 0.3, 1.5, or 7.5 mg T4/L in the diet. Social behavior and memory were tested in the adult offspring. Plasma total T4, free T3 and TSH levels were measured. Hippocampal expression of Gabrb3, Ube3a, Nr2b, Rasgrf1 and Dio3 were measured by RT-qPCR and protein levels of Mecp2 and Slc25a12 by western blotting.
Results
Adult male offspring of E dams showed elevated free T3 and low TSH levels. Adult male, but not female offspring of E dams exhibited social behavior and memory deficits. Expression of autism candidates, Gabrb3, Ube3a, Mecp2 and Slc25a12 were significantly increased in the hippocampus of male offspring of E dams. Hippocampal Nr2b and Dio3 were also increased while Rasgrf1 were decreased in the same population. Peripheral thyroid function, social behavioral deficits and altered expression of the above genes were normalized by simultaneous administration of 0.3mg/L T4 in the E diet.
Conclusions
Our data suggest that social interaction deficits of FASD share molecular mechanism with ASD by showing altered hippocampal expression of several ASD candidate genes. Social interaction deficits as well as the gene expression changes in the offspring of ethanol consuming dams can be reversed by low dose of thyroid hormone supplementation to the mothers.
Keywords: Fetal Alcohol Spectrum Disorders, Autism Spectrum Disorders, Social Interaction, Social Memory, Thyroxine
Introduction
Fetal Alcohol Spectrum Disorders (FASD) encompasses a continuum of disabilities known to be caused by fetal alcohol exposure (FAE). In the United States, FASD may affect as many as two to five percent of school age children (May et al., 2009). This makes prenatal alcohol exposure the leading non-genetic cause of mental retardation in the U.S. (Mizejewski, 2010). FASD manifests in a variety of behavioral, cognitive, and physical abnormalities; however some of the most debilitating effects of FAE are its effects on social behavior (Bishop et al., 2007, Kully-Martens et al., 2012). FASD patients have difficulty interpreting social cues and considering the consequences of their actions (Kelly et al., 2000, Kully-Martens et al., 2012) and show deficits in social information processing (Timler et al., 2005).
Animal models of FASD show deficits in social interaction, (Hamilton et al., 2010, Sittig et al., 2011b, Kelly and Dillingham, 1994), and reduced social memory, particularly in males (Kelly et al., 2009, Kelly and Dillingham, 1994). Moreover, animal models of FASD have identified specific brain regions that are affected and possibly related to poor social behavior such as amygdala (Balaszczuk et al., 2011, Kelly and Dillingham, 1994), septal area and hippocampus (Gil-Mohapel et al., 2010, Kelly, 1996, Valenzuela et al., 2012).
Many of the social impairments caused by prenatal exposure to alcohol overlap with symptoms of autism spectrum disorders (ASD). Autism spectrum disorders are a group of neurocognitive developmental disorders with higher prevalence in males. ASD primarily affects social behavior, often causing developmental delays and mental retardation; symptoms that are similar to those of FASD (Bishop et al., 2007). ASD and FASD may share similar etiologies as well; there is significant evidence from both human and animal studies that autism is caused by insults to the developing fetus in utero (Bishop et al., 2007, Arndt et al., 2005, Rinaldi et al., 2007). The fetus is most sensitive to the majority of these insults during the first trimester, which corresponds to the peak period of ethanol vulnerability (Arndt et al., 2005). Despite the similarities in the etiologies and presentations of ASD and FASD, Bishop and colleagues (2007) demonstrated that in humans there are subtle, yet key differences between the typical phenotypes of the two families of disorders. Therefore, identifying some of the molecular characteristics related to ASD in an animal model of FASD, could ultimately provide details on the underlying molecular mechanisms of both disorders that could lead to novel treatments.
In this study, we aimed to examine the expression of hippocampal genes associated with the genetics and pathophysiology of ASD in the offspring of ethanol consuming dams (E). These genes include ubiquitin protein ligase E3A (Ube3a) and Gabrb3, the gene coding for the β3 subunit of the GABAA receptor (Samaco et al., 2005), which are mapped to chromosome 15q11-13 that is the most commonly reported genetic loci implicated in autism in human (Cook et al., 1997, Hogart et al., 2007). Expression of the ASD related methyl CpG binding protein 2 (Mecp2) (Samaco et al., 2005) and Slc25a12, a mitochondrial glutamate/aspartate carrier protein (Lepagnol-Bestel et al., 2008), was also explored in the hippocampus of E offspring. Additionally, we investigated the changes of NMDA receptor subunit 2b (Nr2b) and ras guanine nucleotide releasing factor 1 (Rasgrf1) expression in response to prenatal ethanol exposure. Nr2b has been implicated in the pathophysiology of ASD (Choudhury et al., 2012) as well as of FASD (Samudio-Ruiz et al., 2010), and Rasgrf1 has been shown to be involved in Nr2b-dependent processes that are important for hippocampal dependent memory (Giese et al., 2001). Additionally, a recent study reports that structural variation in deiodinase-3 opposite strand, Dio3os, which is co-regulated with Dio3 (Dietz et al., 2012), is involved in ASD (Matsunami et al., 2013). Since, we have shown that hippocampal expression of this thyroid hormone metabolizing enzyme, Dio3, is functionally associated with decreased olfactory investigation of the E male offspring in the social interaction test (Sittig et al., 2011b, Sittig and Redei, 2011), we also investigated whether T4 administration would affect expression differences of Dio3 in this study.
In our previous study, we reported that administration of a pharmacological dose of thyroxine (T4) to the ethanol consuming pregnant dams reversed some of the cognitive deficits seen in E offspring (Wilcoxon et al., 2005), but suppressed plasma TSH and free T3 in the female E offspring (Wilcoxon and Redei, 2004). Since, intrauterine thyroid dysfunction has also been hypothesized to contribute to autism (Sadamatsu et al., 2006), in the present study, we examined the effects of lower doses of T4 supplementation to the ethanol consuming dams on the social deficits of their offspring and on the selected hippocampal mediators of ASD.
Materials and Methods
Animals
All animal procedures were approved by the Northwestern University Animal Care and Use Committee. Maternal diet and animal procedures were performed as described previously (Wilcoxon et al., 2005). Shortly, adult female Sprague-Dawley (SD) rats (Harlan, Indianapolis, IN, USA) were mated with SD males overnight and gestational day 1 (G1) was assigned by the presence of sperm in vaginal smears. Pregnant females were assigned to one of six diet groups on G4, control (C, ad libitum standard lab chow), pair-fed (PF), ethanol (E), or an ethanol diet supplemented with one of three doses of thyroxine (T4). All pregnant rats except those assigned to the control group received ad libitum isocaloric liquid diet (Lieber-DeCarli ‘82) from G4 to G8. On G8, rats started their assigned diet. E dams received a diet consisting of 5% w/v, 35% ethanol-derived calories. PF dams received an amount of isocaloric liquid diet that matched the paired E dam’s diet consumption in the previous day. The three T4 groups received the E diet that was additionally supplemented with 0.3 [E+T4 (0.3)], 1.5 [E+T4 (1.5)], or 7.5 [E+T4 (7.5)] mg T4 per liter diet. The ethanol diet was introduced in stages starting on G8 with one third ethanol diet and two thirds standard liquid diet, followed by two thirds ethanol and one third standard diet, and from G10–G21 ethanol rats received a full 5% (w/v) ethanol diet. The three T4 groups received the T4 supplemented ethanol diet starting on G8 as described above. Liquid diets were replaced with lab chow on G21. Number of litters for each group; C=5, PF=5, E=5, E+T4 (0.3)=4, E+T4 (1.5)=4, and E+T4 (7.5)=4. Blood alcohol concentrations (BAC) were not measured in this study, but ethanol diet consumption was similar to those observed in the same animal model previously (Sittig and Redei, 2010) and resulted in blood alcohol levels of 126 mg/dl.
Pups were weaned at 24 days of age and housed separately by sex and treatment. All adult rat offspring underwent behavioral testing at approximately the same age. Only 1–2 animal/sex/litter were used for the different testing.
Two weeks following the completion of behavioral testing, adult rat offspring were sacrificed by decapitation between 10:00 and 12:00 h. Trunk blood was collected into EDTA-coated tubes on ice, and plasma was obtained by centrifugation. Frontal cortex (A/P 5.2-2.7; M/L 0–3.3; D/V 9.0-5.0; Paxinos Brain Atlas) and whole hippocampus were immediately dissected and collected directly into RNAlater reagent (Ambion, Austin, TX, USA) or on dry ice and stored at −80°C.
Social Interaction Test
Social interaction test was performed as described previously (Sittig et al., 2011b). At approximately 70 days of age rats were isolated in a clean cage for 24 hours. Following the isolation period, an unrelated and unfamiliar male rat pup (pup 1) aged about 28 days was placed into the cage. The animals’ behavior was recorded on video for four minutes, after which time pup 1 was removed from the cage, and the adult rat allowed to rest for one hour. Following the hour-long break, pup 1 was reintroduced into the cage along with a second, unfamiliar pup (pup 2). The animals’ behavior was again recorded for four minutes before the pups were removed. Following the experiment the adult rats were returned to their original cages with their cage mates. At a later date, a trained observer scored olfactory investigation of the adult towards each pup, defined as seconds spent in sniffing the familiar or novel pup.
RNA isolation and Quantitative RT-PCR
Total RNA extraction, reverse transcription and quantitative PCR (qPCR) were performed as described previously (Shukla et al., 2011). Briefly, RNA was isolated using Trizol reagent according to the manufacturer’s instructions (Life Technologies, Grand Island, NY, USA). Reverse transcription of 1ug total RNA was performed by using the TaqMan Reverse Transcription kit (Applied Biosystems, Branchburg, NJ, USA). Real-time PCR was conducted with the ABI 7300 system using SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). Reactions were performed in triplicate and reached threshold amplification within 32 PCR cycles. Relative levels of gene expressions were determined relative to 18S (primers commercially available from ABI, Foster City, CA, USA) and a general calibrator using the 2−ΔΔCt method. The primer pairs used for each gene are listed in Table 1.
Table 1.
Quantitative RT-PCR primer sequences.
| Gene | Sequence 5′ - 3′ |
|---|---|
| Nr2b | F: GGACCCTGGATATTCCCAACA R: CAGCTAGTCGGCTCTCTTGGTT |
| Ube3a | F: TGAAAACAGGAAGGAATTTGTCAGT R: CGTCGAAATGCCTTGAATTGT |
| Gabrb3 | F: AGCAAGATATTGGAGGCCTCTCT R: CCGATCCAAGATTCTAGTGAAGATAGT |
| Rasgrf1 | F: AGAGGAGCTGTCCAGGGTCAT R: CCTCGGTGATCATAGCTCAA |
| Dio3 | F: CTGTTCCCGCGCTTCCTA R: GTCCCTTGTGCGTAGTCGAG |
Protein Isolation and Western Blot
Hippocampus of adult offspring was individually homogenized in ice-cold lysis buffer as described previously (Shukla et al., 2010). Samples containing 60ug protein were electrophoresed on either 12% or 7.5% (w/v) SDS polyacrylamide gels and transferred onto polyvinylidene difluoride membranes for detection with Mecp2 and Slc25a12 primary antibodies (Abcam, Cambridge, MA, USA). Membranes were incubated overnight at 4°C in a primary antibody concentration of 1:1,000 in 5% milk (w/v) in blocking buffer. The membranes were then incubated in 1:1,000 secondary antibody solutions for two and a half hours, and then developed using Amersham ECL Plus Western Blotting System (GE Healthcare, Little Chalfont, UK). β-actin protein levels were measured in the same membranes using a monoclonal antibody (1:10,000, Sigma, St. Louis, MO, USA). The optical density of each protein was normalized to the corresponding β-actin signal using ImageJ software (NIH).
Radioimmunoassays
Total T4 and free T3 (fT3) as well as TSH in plasma were measured by RIA as previously described (Sittig and Redei, 2010). A rat TSH RIA manufactured by Alpco Diagnostics (Salem, NH, USA) was used according to manufacturer’s instructions with assay sensitivity 1.6 ng/ml and intra-assay coefficients of variation (CV) 3.9%. Total T4 and fT3 assays were manufactured by MP Biomedicals, LLC (Irvine, CA, USA) and assay sensitivity/CVs were T4: 0.8 ug/dl; 3.3% ; fT3: 0.6 pg/ml, 8%.
Results
Morphometric Measurements
Based on the diet consumption, the three T4 groups received the E diet that was additionally supplemented with 0.3 [E+T4 (0.3)], 1.5 [E+T4 (1.5)], or 7.5 [E+T4 (7.5)] mg T4 per liter of diet received increasing doses of T4 (Table 2). Dams on the two higher T4 doses (E+T4 (1.5) and E+T4 (7.5)) consumed significantly less liquid diet than all the other diet-fed animals (F(4,21)=25.8, p<0.01; Table 2). Subsequently, they did not receive the same dose of ethanol as the dams fed an E or E+T4 (0.3) diet (F(4,16)=9.0, p<0.01; Table 2). Although the thyroid functions of these offspring were tested, they were excluded from subsequent studies.
Table 2.
Morphometric measurement and proportion of liquid diet, ethanol and T4 consumption by maternal diet.
| Maternal Diet | G21 Maternal Weight (g) | Average Liquid Consumed (ml/day) | Ethanol Consumption (mg/100g BW/day) | T4 Dose (mg/100g BW/day) |
|---|---|---|---|---|
| C | 315 ± 3.9 | - | - | - |
| PF | 313 ± 3.9 | 78.4 ± 0.4 | - | - |
| E | 314 ± 1.6 | 76.6 ± 0.6 | 135.8 ± 2.3 | - |
| E+T4 (0.3) | 322 ± 2.7 | 78.2 ± 1.2 | 135.2 ± 2.3 | 0.8 ± 0.0 |
| E+T4 (1.5) | 327 ± 7.9 | 70.1 ± 0.7^^ | 113.7 ± 5.2## | 3.4 ± 0.2 |
| E+T4 (7.5) | 309 ± 3.1 | 69.3 ± 0.3^^ | 120.9 ± 4.5## | 18.0 ± 0.8 |
Values are shown as mean ± SEM.
p < 0.01 compared to E and E+T4 (0.3).
p < 0.01 compared to liquid diet consumed by all other groups.
All mothers, regardless of diet, gave birth to litters of approximately the same size and sex ratio (Table 3). There were no visible morphological changes in the newborns, as expected from this moderate ethanol consumption paradigm. Pup weights were recorded at weaning to not disturb animals while nursing. Pup weights did not vary by sex or by prenatal diet, except that pups of the E+T4 (0.3) mothers weighed less than pups of all other dams (F(5,42)=3.29, p<0.05; Table 3).
Table 3.
Litter sizes, male/female ratios and weaning weights by sex obtained from different diet groups.
| Maternal Diet | Litter Size | Male/Female Ratio | Male Pups Weight (g) | Female Pups Weight (g) |
|---|---|---|---|---|
| C | 10.4 ± 0.5 | 1.0 ± 0.19 | 34.4 ± 0.8 | 34.3 ± 1.4 |
| PF | 14.2 ± 0.4 | 1.0 ± 0.16 | 31.8 ± 1.0 | 33.6 ± 0.9 |
| E | 11.0 ± 1.6 | 1.5 ± 0.63 | 36.3 ± 2.8 | 36.6 ± 3.4 |
| E+T4 (0.3) | 12.8 ± 1.7 | 1.0 ± 0.31 | 28.5 ± 0.2^ | 30.3 ± 1.0^ |
| E+T4 (1.5) | 9.3 ± 1.9 | 1.4 ± 0.85 | 33.1 ± 1.2 | 34.2 ± 2.4 |
| E+T4 (7.5) | 9.5 ± 2.5 | 2.0 ± 0.56 | 32.8 ± 1.2 | 32.8 ± 1.1 |
Values are shown as mean ± SEM.
p < 0.05 compared to all other groups.
As expected, adults males weight significantly more than females (F(1,85)=1613.0, p<0.01; Table 4). The only adult weight difference due to prenatal diet was that male offspring of E+T4 (1.5) dams weighed significantly more than rats from diet groups C, PF and E+T4 (0.3) (F(5,85)=2.33, p<0.05; Table 4). Adrenal weights were normalized to body weight and showed a significant difference by diet (F(5,79)=6.48, p<0.001), sex (F(1,79)=262.64, p<0.001) and sex X diet (F(5,79)=3.79, p<0.01) (Table 4). Specifically, maternal PF and E diet led to enlarged adrenal glands of the females, but not the males.
Table 4.
Body and adrenal weights of adult offspring by maternal diet and sex.
| Maternal Diet | Sex | Body Weight (g) | Adrenal Weight (mg)/BW(g) |
|---|---|---|---|
| C | M | 394 ± 10.0 | 0.194 ± 0.017 |
| F | 238 ± 3.4 | 0.294 ± 0.015 | |
| PF | M | 389 ± 5.2 | 0.167 ± 0.009# |
| F | 250 ± 5.2 | 0.388 ± 0.022** | |
| E | M | 406 ± 5.1 | 0.215 ± 0.014 |
| F | 243 ± 4.2 | 0.349 ± 0.006* | |
| E+T4 (0.3) | M | 384 ± 11.8 | 0.207 ± 0.011 |
| F | 249 ± 4.7 | 0.315 ± 0.020 | |
| E+T4 (1.5) | M | 420±10.1*+& | 0.226 ± 0.011 |
| F | 251 ± 6.8 | 0.361 ± 0.012** | |
| E+T4 (7.5) | M | 408 ± 6.9 | 0.165 ± 0.012# |
| F | 246 ± 4.7 | 0.286 ± 0.017# |
Values are shown as mean ± SEM.
p < 0.05,
p < 0.01 compared to C.
p < 0.05 compared to E.
p < 0.05 compared to PF.
p < 0.05 compared to E+T4 (0.3).
Thyroid Status
Plasma free T3 levels differed only in the male offspring by the different prenatal diets (Figure 1A, sex F(1,64)=11.9, p<0.01; diet F(5,64)=5.68, p<0.01; diet X sex F(5,64)=9.04, p<0.01). Free T3 levels were significantly higher in E male offspring compared to C (p<0.01). This increase was normalized in the offspring by simultaneous administration of either 0.3 or 1.5 mg/L T4 in the E diet (E vs E+T4 (0.3), p<0.01; E vs E+T4 (1.5), p<0.05; Figure 1A). Plasma TSH levels were similarly only affected in the male offspring by the maternal diet (Figure 1B, sex F(1,51)=51.0, p<0.01; sex X diet F(5,51)=3.66, p<0.01). Plasma TSH was significantly lower in E male offspring compared to C (p<0.01). This suppressed TSH was normalized by simultaneous administration of 0.3 mg/L T4 in the E diet (E vs E+T (0.3), p<0.05). No effects of prenatal treatment on plasma free T3 and TSH were found in the female offspring (Supplemental Figures S1A and S1B). There was no significant difference in total T4 levels by prenatal treatment in either sex (data not shown).
Figure 1. Adult male offspring of ethanol consuming mothers (E) show peripheral hyperthyroid profile that is normalized by administration of T4 in the ethanol containing liquid diet.
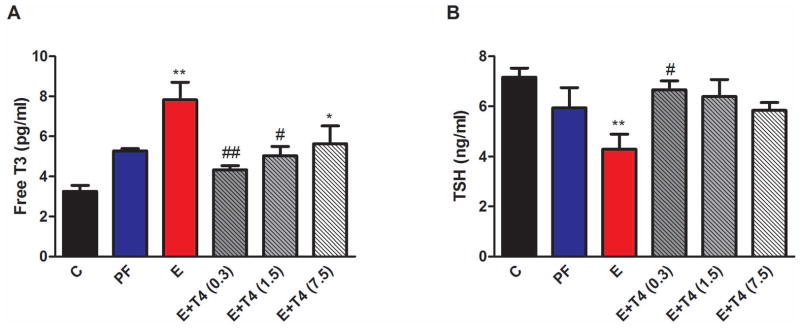
A) Plasma free T3 levels, B) Plasma TSH levels. ** p<0.01, * p<0.05 compared to C; ## p<0.01, # p<0.05 compared to E by Bonferroni post-hoc test. Values are shown as mean ± SEM. N=4–7/group.
Ethanol Exposure in utero Decreases Male Social Investigation and Recognition
The first trial of the social interaction test measures the social interest and curiosity of the test animal, by how much time the adult rat spends in olfactory investigation of the pup. Male and female offspring showed significantly different level of social interaction, and prenatal treatment had a significant, but differential effect on male and female offspring (Figure 2A, diet F(3,43)=3.10, p<0.05, sex F(1,43)=10.29, p<0.01, sex X diet F(3,43)=45.52, p<0.01). Specifically, male rats prenatally exposed to ethanol showed significantly decreased olfactory investigation compared to rats whose mothers consumed a C diet (Figure 2A, p<0.05). However, when ethanol consuming mothers’ diets were supplemented with 0.3 mg T4/L diet, this social deficit disappeared which was displayed as a significant (p<0.01) increase in olfactory investigation compared to E animals. In contrast, adult female offspring of dams on different diets exhibit significantly increased social interaction compared to controls (Figure S2A).
Figure 2. Adult male offspring of ethanol consuming mothers (E) show social interaction (A and B), and social memory deficit (C), these deficits are attenuated by administration of 0.3 mg T4/L in the ethanol containing liquid diet (E+T4 (0.3)).
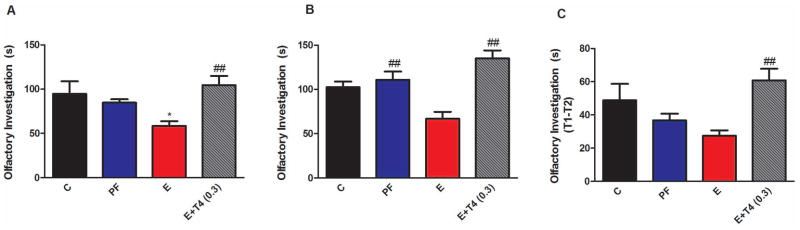
A) Social interaction is measured by time spent (in seconds) with olfactory investigation of an unfamiliar test pup in the first trial of the social interaction test. B) Social interaction is measured by total time spent (in seconds) with olfactory investigation of the familiar test pup from the first trial and an unfamiliar test pup introduced in second part of the social interaction test. C) Social memory is characterized by the time difference between investigating the same test pup in the first and the second trials (T1–T2 in seconds). Details are as in Figure 1. N=7/group.
The second half of the social interaction test employs the familiar pup from trial one (T1) and a novel pup. The total time spent with olfactory investigation of these two pups presented a profile similar to the first test; E offspring exhibited decreased olfactory investigation compared to PF (Figure 2B, p<0.01), which was reversed by prenatal T4 treatment (F(3, 23)=10.32, p<0.01; E vs E+T4, p<0.01). Since, total olfactory investigation did not differ between C and PF offspring, these controls were combined. Olfactory investigation of this combined control group is significantly higher than that of E offspring (F(2,23)=15.6, p<0.01). The second half of the social interaction test is also designed to test social memory and recognition. If the adult rat recognizes pup 1 from T1, then it spends less time investigating pup 1 in trial two (T2). Social recognition is presented as T1–T2 of pup 1 and prenatal treatment affected it in male and female offspring differently (sex X diet F(3,40)=5.28, p<0.01). Male offspring of E dams showed a trend toward decreased social recognition compared to the offspring of C dams (t(10)=2.06, p=0.07). However, when ethanol consuming mothers’ diet was supplemented with 0.3 mg T4/L diet, this trend in social memory deficit disappeared (F(3,22)=4.75, p<0.01; Figure 2C; E vs E+T4, p<0.01). In contrast, adult female offspring of dams on different diets exhibit significantly increased social memory compared to controls (Figure S2B).
Altered Expression of Hippocampal Genes after Prenatal Exposure to Ethanol
Hippocampal gene expression of selected genes was measured by RT-qPCR, shown in Figure 3. Transcript levels were measured in males only, because only male offspring of E mothers demonstrated a behavioral deficit in the social interaction test.
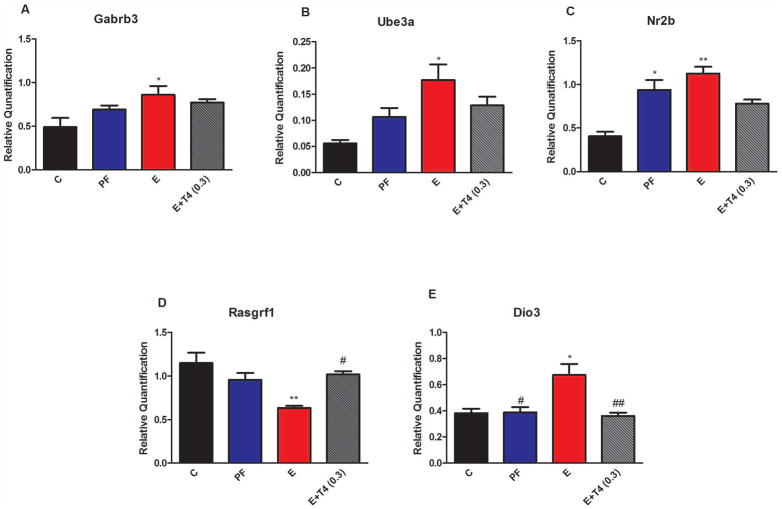
Figure 3. Relative Quantification measured by real time RT-PCR indicates an increase in Gabrb3 (A), Ube3a (B), Nr2b (C), and a decrease in Rasgrf1 (D), as well as an increase Dio3 (E) transcript levels in the hippocampus of adult male offspring of ethanol consuming mothers (E). Rasgrf1 and Dio3 expression levels are normalized by administration of 0.3 mg T4/L in the ethanol containing liquid diet.
Transcript levels were normalized to 18S and a general calibrator using the 2−ΔΔCt method. Details are as in Figure 1. N=4–8/group.
Levels of transcripts for autism candidate genes, Gabrb3 and Ube3a were significantly increased in the hippocampus of adult male offspring of E dams compared to those of C, but not PF (Gabrb3, F(3,12)=4.18, p<0.05, Ube3a, F(3,12)=6.81, p<0.05; C vs E, p<0.05) (Figure 3A and B). Transcript levels of NMDA receptor subunit Nr2b were similarly increased in the male E hippocampus as well as in the PF hippocampus compared to C (F(3,12)=14.58, p<0.01, Figure 3C; C vs E, p<0.01; C vs PF, p<0.05). In contrast, mRNA levels of Rasgrf1 were decreased by prenatal ethanol treatment (F(3,13)=6,17, p<0.01, Figure 3D). There was a similar effect of low dose T4 administration (E+T4 (0.3)) for the reversal of prenatal ethanol induced changes in the expression of these genes, which reached significance for Rasgrf1 (C vs E, p<0.01; E vs E+T4, p<0.05). Dio3 expression was significantly increased in the hippocampus of E animals compared to both C and PF groups (C vs E, p<0.05; PF vs E, p<0.05), which was normalized in the E+T4 (0.3) group (F(3,14)=7.74, p<0.01, Figure 3E; E vs E+T4, p<0.01). Frontal cortex Dio3 expression has been shown not to be altered by prenatal alcohol exposure (Sittig et al., 2011b). Nr2b expression was not measured in the frontal cortex. There were no significant differences in the transcript levels of Gabrb3, Ube3a and Rasgrf1 in frontal cortex of adult male offspring from different prenatal treatment groups (Figure S3).
Elevated Hippocampal Mecp2 and Slc25a12 Protein Levels after Prenatal Exposure to Ethanol
Protein levels of both Mecp2 and Slc25a12 increased significantly in the hippocampus of adult male offspring of E dams compared to C (Mecp2, F(3,16)=4.25, p<0.05; Slc25a12, F(3,18)=4.39, p<0.05) (Figure 4). The low dose T4 administration concomitantly with E attenuated this increase for both proteins (Mecp2, t(7)=1.97, p=0.08; Slc25a12, t(6)=1.47, p=0.09). There were no significant differences in the protein levels of Mecp2 and Slc25a12 in the frontal cortex of adult male offspring from different prenatal treatment groups (Figure S4).
Figure 4. Protein levels of Mecp2 (A) and Slc25a12 (B) are increased in the hippocampus of adult male offspring of ethanol consuming mothers (E).
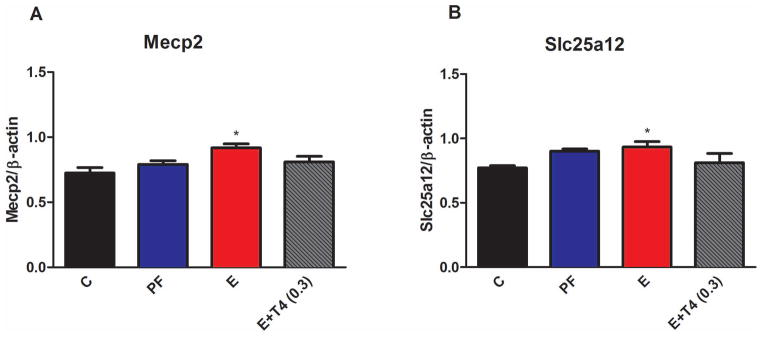
Protein levels were normalized to β-actin. Details are as in Figure 1. N=4–6/group.
Thyroid Hormone Responsive Elements (TREs) in Genes Associated with ASD
Since altered expressions of ASD related genes in the hippocampus of E offspring were reversed by prenatal T4 treatment of the alcohol consuming dams, we investigated the presence of TREs in the promoter regions (2000bp upstream of the start site) of these genes. TRE sites were predicted using Transcription Element Search System (http://www.cbil.upenn.edu/cgi-bin/tess/). TREs were found in all, except Dio3, of both human and rat genes as shown in Table 5.
Table 5.
Chromosomal locations of thyroid hormone responsive elements (TREs) within 2000bp from the transcriptional start site.
| Gene Name | Rat Chromosomal Location (bp) | TRE Location | Human Chromosomal Location (bp) | TRE Location |
|---|---|---|---|---|
| Nr2b | 4: 233,824,034–234,258,927 reverse | −1830 | 12: 13,714,144–14,133,053 reverse | −111, −1039, −1554, −1720 |
| Ube3a | 1: 117,746,227–117,834,011 forward | −1498, −1522 | 15: 25,582,381–25,684,128 reverse | −644, −1377 |
| Gabrb3 | 1: 114,022,939–114,275,043 forward | −454, −1982 | 15: 26,788,693–27,184,686 reverse | −1346 |
| Rasgrf1 | 8: 96,778,646–96,905,924 forward | −12, −470, −629 | 15: 79,252,289–79,383,115 reverse | −1445 |
| Dio3 | 6: 143,766,523–143,768,383 forward | Not found | 14: 102,027,688–102,029,789 forward | Not found |
| Mecp2 | 1: 152,450,808–152,452,884 forward | −1110, −1969 | X: 153,287,024–153,402,578 reverse | −274, −549, −738, −851, −1431 |
| Slc25a12 | 3: 64,378,093–64,485,534 reverse | −179, −1330 | 2: 172,640,880–172,864,766 reverse | −530, −926 |
Discussion
There are two major findings of this study. First, our data suggests that social interaction deficits caused by prenatal ethanol exposure share molecular mechanisms with ASD by showing altered hippocampal expression of several ASD candidate genes. Second, we demonstrated that social interaction deficits as well as prenatal ethanol exposure-induced changes in hippocampal gene expression in the offspring of ethanol consuming dams can be reversed by low dose of thyroid hormone supplementation to the ethanol consuming pregnant dams. Although these results are novel and can lead to interesting hypotheses, the behavioral and gene expression findings are correlational; therefore their causal connection will need to be investigated in the future.
Social behavior deficits
Fetal alcohol exposure caused decreased social interaction in the adult male, but not female, Sprague-Dawley rats compared to offspring of controls. This male-specific finding is robust as it is in agreement with previous reports across several laboratories and different paradigms (Kelly and Dillingham, 1994, Kelly, 1996, Hamilton et al., 2010, Sittig et al., 2011b, Kelly et al., 2000, Kelly et al., 2009). In contrast to the deficit in social behavior in males, female offspring of ethanol consuming dams showed increased social interaction and memory. This prenatal ethanol induced enhanced social interaction has been previously shown in female rats (Kelly and Dillingham, 1994, Hamilton et al., 2010). Although, there are no sex differences explicitly reported in the social behavior of children and adolescents with FASD, gender differences in other behavioral and cognitive outcomes of FASD are well known (Herman et al., 2008). It is interesting to note that there is also a sex bias in the incidence of ASD, such as autism is four times more common in males than in females (Baron-Cohen et al., 2009).
The cause of this profound sex difference in social interaction deficit of E offspring is not known. If this sex difference has similar molecular causality to the sex differences in the prevalence of ASD, changes in fetal or perinatal levels of testosterone could be involved. However, while some evidence links elevated fetal testosterone levels to autistic symptomatology (Auyeung et al., 2010), both fetal and neonatal testosterone levels are decreased in male offspring of ethanol consuming rat dams (McGivern et al., 1988). It is a possibility though, that positive or negative alterations from normal fetal testosterone levels could lead to the observed sex differences in the behavioral measures. A potential alternative explanation for these behavioral differences proposes that short periods of social isolation can alter the central mechanism of social behavior system through changes in social motivation and possibly through changes in social learning (Lugo et al., 2003). In our study rats were socially isolated for 24 hours that could be stressful and reported sex differences in social behavior may reflect the differential sensitivity to such a stressor between male and female rats (Palanza, 2001).
Gene expression changes
The findings that several ASD-related genes showed hippocampal expression changes in male E offspring is of great interest, as it poses the hypothesis of common molecular mechanism(s) between the autism- and prenatal ethanol-induced social interaction deficits (Olexova et al., 2012). However, expression of UBE3A and GABRB3 are decreased in autism brain samples (Hogart et al., 2007), in contrast to the increased expression found here in the E male hippocampus. The concurrence of increased protein levels of Mecp2 with the increased transcript levels of hippocampal Ube3a and Gabrb3 is exactly the opposite that was found in ASD (Samaco et al., 2005). These three opposite directional changes in E male hippocampus are in contrast to findings in ASD and are reminiscent of the fetal testosterone differences in FASD models and those proposed in ASD. Protein levels of another autism candidate gene, Slc25a12, were also increased in the E male hippocampus, but this increase is similar to what is found in autism brain samples (Palmieri et al., 2010). Aralar, which is another alias of Slc25a12, is an aspartate/glutamate carrier, and increased plasma levels of glutamate has been found in autism that correlates with levels of social impairment (Shinohe et al., 2006). Thus, increased protein levels of Slc25a12 in the E male hippocampus may contribute to the defined social behavioral deficit.
Our finding of increased Nr2b expression in the E male hippocampus has not resolved the contradiction in the literature regarding the direction of change in Nr2b expression levels by prenatal alcohol (Samudio-Ruiz et al., 2010). The elevated Nr2b expression in the PF hippocampus indicates that nutritional deficit in the E group may also contribute to the increased Nr2b expression in the E hippocampus. However, this increased Nr2b expression is similar to the significantly increased Nr2b expression in an animal model of autism; rats prenatally exposed to valproic acid (Rinaldi et al., 2007). Furthermore, a selective NMDA receptor antagonist with an Nr2b subunit specificity can attenuate the memory retention deficit in the water maze navigation caused by ethanol exposure in utero (Lewis et al., 2012), suggesting a true increase in Nr2b levels in some animal models of FASD. For the increased hippocampal Nr2b levels to have consequences in neuronal function and connectivity, the interaction between Nr2b and Rasgrf1 cannot be disrupted (Sepulveda et al., 2010). Thus, we believe that the opposite directional changes of Rasgrf1 and Nr2b levels in the E hippocampus may lead to abnormal neuronal function and subsequently results in social behavior deficits.
It is of particular interest that the altered expression of all of these genes in the E male hippocampus was reversed, or the difference reduced, by the low dose T4 treatment; in parallel with the behavioral outcome. Additionally, the elevated Dio3 expression in the hippocampus of male offspring of ethanol consuming dams was reversed by simultaneous administration of T4. The elevated hippocampal Dio3 expression in these offspring is in contrast to the decreased Dio3 expression described previously in the SD by Brown-Norway (BN) cross (Sittig et al., 2011b). The differences in these results are possibly due to strain differences, whereby the fetus that is exposed to ethanol is SD by BN hybrid in the previous study, but SD by SD in the present study.
The significance of prenatal T4 induced reversals in the altered expression of these genes in the E male hippocampus need to be determined. One appealing hypothesis is that prenatal ethanol induced changes in thyroid function can alter expression of these ASD related genes as most of them have thyroid responsive element (see Table 5). Additionally, there are indications in the literature that some of these TREs are functional. In the rat hippocampus, mRNA levels of NMDA receptor subunits Nr1 and Nr2b, are controlled by thyroid hormones (Lee et al., 2003). Binding and activity of the GABA-A receptor complexes of the brain are also modulated by thyroid hormones (Martin et al., 1996). Although, we found no TREs in the promoter region of Dio3, its expression in the brain is known to be regulated by thyroid hormones (Tu et al., 1999).
It is important to note that Ube3a, Rasgrf1 and Dio3 are imprinted genes. UBE3A (and GABRB3) are mapped to human chromosome 15q11-13, a genetic locus implicated in autism (Cook et al., 1997). Imprinting of mouse Ube3a is thought to be region-specific in the embryonic brain, and the sense transcript is expressed maternally in neurons (Yamasaki et al., 2003). As expected, the non-imprinted Gabrb3 shows biallelic expression; monoallelic expression of Gabrb3 does not occur in normal brain (Hogart et al., 2007). Additionally, Rasgrf1 is an imprinted gene that is only expressed from the paternal allele in neonatal mouse brain until postnatal day 21, at which time expression becomes biallelic (Drake et al., 2009). Finally, we have shown previously that the preferential paternal expression of Dio3 in the frontal cortex of SD x BN cross is switched to preferentially maternal expression in the hippocampus (Sittig et al., 2011a, Sittig et al., 2011b). Similar to the changes in the allele-specific expression of Dio3, it is likely that maternal ethanol exposure affects not only the total, but also the allele specific expression of one or more of these genes as well (Sittig et al., 2011b), and that such altered expression patterns contribute to the detrimental phenotypes associated with FASD.
Thyroxin supplementation and reversal of prenatal ethanol effects
It is well known that thyroid hormone is essential for normal brain development (Heindel and Zoeller, 2003). Even mild maternal hypothyroidism negatively affects a child’s neuropsychological development (Zoeller, 2004). It has been suggested that some of the prenatal ethanol-related deficits are due to the effects of ethanol on the disturbance of maternal-fetal thyroid homeostasis (Scott et al., 1998, Wilcoxon and Redei, 2004). Decreased levels of T4 have been found in alcoholics (Leggio et al., 2008), decreased serum TSH and T4 in alcohol consuming pregnant women (Herbstman et al., 2007), and in newborns exposed to alcohol in utero (Hernandez et al., 1992). Similar findings in animal models are reported with decreased peripheral free T4, free T3 and TSH in ethanol consuming pregnant animals at gestational day 18 (Wilcoxon and Redei, 2004). In a more recent study, although plasma T4 and TSH levels are reported to be low, free T3 levels are found to be elevated in ethanol consuming SD dams (Sittig and Redei, 2010). The thyroid function measures of the adult offspring of ethanol consuming dams are similarly discordant. In the present study, adult male, but not female, offspring of ethanol consuming dams showed decreased plasma TSH and increased free T3, which is a hyperthyroid profile. Prenatal ethanol-related changes in this hyperthyroid profile of plasma TSH/free T3 is the opposite of the hypothyroid profile of elevated TSH and decreased free T3 found previously in the offspring of ethanol-consuming dams (Wilcoxon and Redei, 2004), and the cause of this discrepancy is unknown. One potential explanation is within strain differences of Sprague-Dawley rats used in the current study compared to those used previously. A similar difference in other measures has been noted previously (Weinberg, J; personal communication).
Regardless of differences in the thyroid function of both the ethanol consuming dams and their adult offspring, administration of T4 reverses selected consequences of maternal ethanol exposure. Pharmacological dose of prenatal T4 treatment reverses the cognitive and motivational deficits (Wilcoxon et al., 2005), but suppresses plasma TSH and free T3 in the female E offspring (Wilcoxon and Redei, 2004). The approximately ten times lower dose of T4, administered in the current study, normalized the offspring’s thyroid function, eliminated their social interaction deficit and hippocampal expression differences induced by E in the adult male offspring. These findings support the indispensable role of appropriate maternal thyroid function in the normal neuropsychological development of the fetuses, and manipulations of it could lead potential treatment methods.
Using a rat model of fetal alcohol exposure, we found that male, but not female, offspring of ethanol consuming dams exhibited a significant impairment in both social interaction and memory. Significantly, these deficits were reversed by supplementation of the maternal ethanol diet with a low dose of T4 that has also restored thyroid function in the adult offspring. Since several autism-related genes showed altered expression profiles in the male E offspring that were normalized by T4 supplementation, ASD and FASD may share common affected molecular pathways.
Supplementary Material
Acknowledgments
This study was funded by NIH AA013452 and AA 017978 to EER.
References
- Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23:189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Taylor K, Hackett G, Baron-Cohen S. Foetal testosterone and autistic traits in 18 to 24-month-old children. Mol Autism. 2010;1:11. doi: 10.1186/2040-2392-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaszczuk V, Bender C, Pereno GL, Beltramino CA. Alcohol-induced neuronal death in central extended amygdala and pyriform cortex during the postnatal period of the rat. Int J Dev Neurosci. 2011;29:733–742. doi: 10.1016/j.ijdevneu.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, Brayne C. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194:500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- Bishop S, Gahagan S, Lord C. Re-examining the core features of autism: a comparison of autism spectrum disorder and fetal alcohol spectrum disorder. J Child Psychol Psychiatry. 2007;48:1111–1121. doi: 10.1111/j.1469-7610.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- Choudhury PR, Lahiri S, Rajamma U. Glutamate mediated signaling in the pathophysiology of autism spectrum disorders. Pharmacol Biochem Behav. 2012;100:841–849. doi: 10.1016/j.pbb.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Lindgren V, Leventhal BL, Courchesne R, Lincoln A, Shulman C, Lord C, Courchesne E. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am J Hum Genet. 1997;60:928–934. [PMC free article] [PubMed] [Google Scholar]
- Dietz WH, Masterson K, Sittig LJ, Redei EE, Herzing LB. Imprinting and expression of Dio3os mirrors Dio3 in rat. Front Genet. 2012;3:279. doi: 10.3389/fgene.2012.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake NM, Park YJ, Shirali AS, Cleland TA, Soloway PD. Imprint switch mutations at Rasgrf1 support conflict hypothesis of imprinting and define a growth control mechanism upstream of IGF1. Mamm Genome. 2009;20:654–663. doi: 10.1007/s00335-009-9192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Friedman E, Telliez JB, Fedorov NB, Wines M, Feig LA, Silva AJ. Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1) Neuropharmacology. 2001;41:791–800. doi: 10.1016/s0028-3908(01)00096-x. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Zoeller RT. Thyroid hormone and brain development: translating molecular mechanisms to population risk. Thyroid. 2003;13:1001–1004. doi: 10.1089/105072503770867165. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, Jones RS, Park A, Zhang Y, Heidler J, Needham LL, Goldman LR. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ Health Perspect. 2007;115:1794–1800. doi: 10.1289/ehp.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman LE, Acosta MC, Chang PN. Gender and attention deficits in children diagnosed with a Fetal Alcohol Spectrum Disorder. Can J Clin Pharmacol. 2008;15:e411–419. [PubMed] [Google Scholar]
- Hernandez JT, Hoffman L, Weavil S, Cvejin S, Prange AJ., Jr The effect of drug exposure on thyroid hormone levels of newborns. Biochem Med Metab Biol. 1992;48:255–262. doi: 10.1016/0885-4505(92)90072-7. [DOI] [PubMed] [Google Scholar]
- Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM. 15q11-13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Hum Mol Genet. 2007;16:691–703. doi: 10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ. Effects of alcohol exposure and artificial rearing during development on septal and hippocampal neurotransmitters in adult rats. Alcohol Clin Exp Res. 1996;20:670–676. doi: 10.1111/j.1530-0277.1996.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicol Teratol. 1994;16:377–384. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Leggett DC, Cronise K. Sexually dimorphic effects of alcohol exposure during development on the processing of social cues. Alcohol Alcohol. 2009;44:555–560. doi: 10.1093/alcalc/agp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kully-Martens K, Denys K, Treit S, Tamana S, Rasmussen C. A review of social skills deficits in individuals with fetal alcohol spectrum disorders and prenatal alcohol exposure: profiles, mechanisms, and interventions. Alcohol Clin Exp Res. 2012;36:568–576. doi: 10.1111/j.1530-0277.2011.01661.x. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady D, Koenig JI. Thyroid hormone regulation of N-methyl-D-aspartic acid receptor subunit mRNA expression in adult brain. J Neuroendocrinol. 2003;15:87–92. doi: 10.1046/j.1365-2826.2003.00959.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Malandrino N, Mirijello A, D’Angelo C, Vonghia L, Miceli A, Capristo E, Kenna GA, Gasbarrini G, Swift RM, Addolorato G. Relationship between the hypothalamic-pituitary-thyroid axis and alcohol craving in alcohol-dependent patients: a longitudinal study. Alcohol Clin Exp Res. 2008;32:2047–2053. doi: 10.1111/j.1530-0277.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- Lepagnol-Bestel AM, Maussion G, Boda B, Cardona A, Iwayama Y, Delezoide AL, Moalic JM, Muller D, Dean B, Yoshikawa T, Gorwood P, Buxbaum JD, Ramoz N, Simonneau M. SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry. 2008;13:385–397. doi: 10.1038/sj.mp.4002120. [DOI] [PubMed] [Google Scholar]
- Lewis B, Wellmann KA, Kehrberg AM, Carter ML, Baldwin T, Cohen M, Barron S. Behavioral deficits and cellular damage following developmental ethanol exposure in rats are attenuated by CP-101,606, an NMDAR antagonist with unique NR2B specificity. Pharmacol Biochem Behav. 2012;100:545–553. doi: 10.1016/j.pbb.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Jr, Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiol Behav. 2003;78:185–194. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- Martin JV, Williams DB, Fitzgerald RM, Im HK, Vonvoigtlander PF. Thyroid hormonal modulation of the binding and activity of the GABAA receptor complex of brain. Neuroscience. 1996;73:705–713. doi: 10.1016/0306-4522(96)00052-8. [DOI] [PubMed] [Google Scholar]
- Matsunami N, Hadley D, Hensel CH, Christensen GB, Kim C, Frackelton E, Thomas K, da Silva RP, Stevens J, Baird L, Otterud B, Ho K, Varvil T, Leppert T, Lambert CG, Leppert M, Hakonarson H. Identification of Rare Recurrent Copy Number Variants in High-Risk Autism Families and Their Prevalence in a Large ASD Population. PLoS One. 2013;8:e52239. doi: 10.1371/journal.pone.0052239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Raum WJ, Salido E, Redei E. Lack of prenatal testosterone surge in fetal rats exposed to alcohol: alterations in testicular morphology and physiology. Alcohol Clin Exp Res. 1988;12:243–247. doi: 10.1111/j.1530-0277.1988.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Mizejewski GJ. Can prenatal screening for fetal alcohol spectrum disorder be justified? A commentary. Gynecol Obstet Invest. 2010;69:128–130. doi: 10.1159/000263460. [DOI] [PubMed] [Google Scholar]
- Olexova L, Talarovicova A, Lewis-Evans B, Borbelyova V, Krskova L. Animal models of autism with a particular focus on the neural basis of changes in social behaviour: an update article. Neurosci Res. 2012;74:184–194. doi: 10.1016/j.neures.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, Hager J, Rousseau F, Curatolo P, Manzi B, Militerni R, Bravaccio C, Trillo S, Schneider C, Melmed R, Elia M, Lenti C, Saccani M, Pascucci T, Puglisi-Allegra S, Reichelt KL, Persico AM. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2010;15:38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci U S A. 2007;104:13501–13506. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadamatsu M, Kanai H, Xu X, Liu Y, Kato N. Review of animal models for autism: implication of thyroid hormone. Congenit Anom (Kyoto) 2006;46:1–9. doi: 10.1111/j.1741-4520.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio-Ruiz SL, Allan AM, Sheema S, Caldwell KK. Hippocampal N-methyl-D-aspartate receptor subunit expression profiles in a mouse model of prenatal alcohol exposure. Alcohol Clin Exp Res. 2010;34:342–353. doi: 10.1111/j.1530-0277.2009.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HC, Sun GY, Zoeller RT. Prenatal ethanol exposure selectively reduces the mRNA encoding alpha-1 thyroid hormone receptor in fetal rat brain. Alcohol Clin Exp Res. 1998;22:2111–2117. [PubMed] [Google Scholar]
- Sepulveda FJ, Bustos FJ, Inostroza E, Zuniga FA, Neve RL, Montecino M, van Zundert B. Differential roles of NMDA Receptor Subtypes NR2A and NR2B in dendritic branch development and requirement of RasGRF1. J Neurophysiol. 2010;103:1758–1770. doi: 10.1152/jn.00823.2009. [DOI] [PubMed] [Google Scholar]
- Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Minabe Y, Sugiyama T, Kawai M, Iyo M, Takei N, Mori N. Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Sittig LJ, Andrus BM, Schaffer DJ, Batra KK, Redei EE. Prenatal thyroxine treatment disparately affects peripheral and amygdala thyroid hormone levels. Psychoneuroendocrinology. 2010;35:791–797. doi: 10.1016/j.psyneuen.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PK, Sittig LJ, Ullmann TM, Redei EE. Candidate placental biomarkers for intrauterine alcohol exposure. Alcohol Clin Exp Res. 2011;35:559–565. doi: 10.1111/j.1530-0277.2010.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Herzing LB, Shukla PK, Redei EE. Parent-of-origin allelic contributions to deiodinase-3 expression elicit localized hyperthyroid milieu in the hippocampus. Mol Psychiatry. 2011a;16:786–787. doi: 10.1038/mp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Redei EE. Paternal genetic contribution influences fetal vulnerability to maternal alcohol consumption in a rat model of fetal alcohol spectrum disorder. PLoS One. 2010;5:e10058. doi: 10.1371/journal.pone.0010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Redei EE. Brain regional thyroid hormone status and Dio3: where genetics, epigenetics and psychiatric vulnerability meet. Expert Review of Endocrinology & Metabolism. 2011;6:649–652. doi: 10.1586/eem.11.64. [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Shukla PK, Herzing LB, Redei EE. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB J. 2011b;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timler GR, Olswang LB, Coggins TE. “Do I know what I need to do?” A social communication intervention for children with complex clinical profiles. Lang Speech Hear Serv Sch. 2005;36:73–85. doi: 10.1044/0161-1461(2005/007). [DOI] [PubMed] [Google Scholar]
- Tu HM, Legradi G, Bartha T, Salvatore D, Lechan RM, Larsen PR. Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology. 1999;140:784–790. doi: 10.1210/endo.140.2.6486. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci. 2012;35:284–292. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol Psychiatry. 2005;10:961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287:E318–326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Joh K, Ohta T, Masuzaki H, Ishimaru T, Mukai T, Niikawa N, Ogawa M, Wagstaff J, Kishino T. Neurons but not glial cells show reciprocal imprinting of sense and antisense transcripts of Ube3a. Hum Mol Genet. 2003;12:837–847. doi: 10.1093/hmg/ddg106. [DOI] [PubMed] [Google Scholar]
- Zoeller RT. Interspecies differences in susceptibility to perturbation of thyroid hormone homeostasis requires a definition of “sensitivity” that is informative for risk analysis. Regul Toxicol Pharmacol. 2004;40:380. doi: 10.1016/j.yrtph.2004.08.008. author reply 381–382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.