Abstract
Islet autotransplant (IAT) may ameliorate post-surgical diabetes following total pancreatectomy (TP), but outcomes are dependent upon islet mass which is unknown prior to pancreatectomy. We evaluated whether pre-operative metabolic testing could predict islet isolation outcomes, and thus improve assessment of TPIAT candidates. We examined the relationship between measures from frequent sample IV glucose tolerance tests (FSIVGTT) and mixed meal tolerance tests (MMTT) and islet mass in 60 adult patients, with multivariate logistic regression modeling to identify predictors of islet mass ≥2500 IEQ/kg. The acute C-peptide response to glucose (ACRglu) and disposition index from FSIVGTT correlated modestly with the islet equivalents per kilogram body weight (IEQ/kg). Fasting and MMTT glucose levels and HbA1c correlated inversely with IEQ/kg (r values −0.33 to −0.40, p≤0.05). In multivariate logistic regression modeling, normal fasting glucose (<100 mg/dL) and stimulated C-peptide on MMTT ≥4 ng/mL were associated with greater odds of receiving an islet mass ≥2,500 IEQ/kg (OR 0.93 for fasting glucose, CI 0.87–1.0; OR 7.9 for C-peptide, CI 1.75–35.6). In conclusion, parameters obtained from FSIVGTT correlate modestly with islet isolation outcomes. Stimulated C-peptide ≥4 ng/mL on MMTT conveyed 8 times the odds of receiving ≥2,500 IEQ/kg, a threshold associated with reasonable metabolic control post-operatively.
Keywords: Diabetes mellitus, islet transplant, pancreatitis, chronic pancreatitis, autoislet, total pancreatectomy
Introduction
Chronic pancreatitis is an irreversible inflammatory condition of the pancreas associated with severe pain, malabsorption, and endocrine dysfunction (1). Total pancreatectomy and islet autotransplantation (TPIAT) may be performed to relieve intractable pain and improve quality of life in patients with severe chronic pancreatitis (2–4). While the goal of islet autotransplantation is to preserve endocrine function to reduce or prevent postsurgical diabetes mellitus (1, 3), much uncertainty surrounds the diabetes outcome for an individual patient, and optimal timing for TPIAT—balancing preserved islet mass while not proceeding to surgery too early in the disease course—can be difficult to determine. Thus, better tools are needed to determine likelihood of a successful islet isolation for patients considering this surgery.
Overall about one-third of adult IAT recipients are insulin independent, with another one-third using minimal insulin (often once daily basal insulin). However the remaining third of patients require a standard basal-bolus insulin regimen and approximately 10% are C-peptide negative and may have a more labile post-pancreatectomy diabetes. The likelihood of success depends largely on the number of islets that are isolated for transplant from the diseased pancreas. In a recently published cohort of 409 TPIAT patients at the University of Minnesota, islet yields of <2500 islet equivalents (IEQ)/kg resulted in insulin independence in only 12% of cases at 3 years, compared to 22% with 2,501–5,000 IEQ/kg and 72% if >5,000 IEQ/kg. Perhaps more importantly, regardless of insulin use, the vast majority of patients with at least a moderate islet yield--≥2500 IEQ/kg—have a favorable metabolic outcome. Nearly all of these patients demonstrate islet graft function (C-peptide positive) and the majority maintain hemoglobin A1c levels in the American Diabetes Association goal range of <7% (3).
Unfortunately, potential islet yield is currently unknown until pancreatectomy is performed. Prior pancreatic surgery (particularly surgical drainage by lateral pancreaticojejunostomy) and atrophy on MRI have been shown to negatively correlate with islet yield but a wide range of islet yields has been found in patients with these risk factors (3–6). Preoperative predictors of islet yield could provide more patient-specific information to aid in counseling patients about the risk of diabetes after TPIAT.
Published literature from human cadaveric donor pancreas isolations and from porcine isolations suggest a significant correlation between tests of insulin secretion—particularly the acute insulin response to arginine or glucose—and the islet yield at isolation (7, 8). The acute insulin response (AIR) to arginine, glucose, and the glucose-potentiated arginine-induced insulin secretion post—transplant correlate well to transplanted islet mass (9). However, the relationship between pre-operative islet functional testing and isolation outcomes in chronic pancreatitis undergoing total pancreatectomy and islet autotransplantation remains largely unexplored. Preliminary data from 10 pediatric patients suggested a relationship between C-peptide on mixed meal tolerance testing and islet yield (10). TPIAT is performed more frequently in adult patients, yet presently there is no published literature examining the relationship between preoperative metabolic testing and islet yield in this population.
At our institution, TPIAT recipients enrolled in clinical trials undergo intravenous glucose tolerance testing and mixed meal tolerance testing immediately prior to surgery. Herein, we examined the potential of preoperative metabolic testing to predict islet isolation outcomes in a large cohort of adult patients undergoing TPIAT for chronic pancreatitis. While such tests are clearly useful for establishing preoperative baseline for islet function, we hypothesized that these results may also aid in individualized counseling regarding likelihood of a successfully isolation.
Materials and Methods
Subjects
Preoperative data was prospectively collected on 60 non-diabetic adult patients (≥18 yr) with chronic pancreatitis undergoing TPIAT at the University of Minnesota Medical Center from 2010–2012. Chronic pancreatitis was diagnosed on clinical and radiographic criteria and confirmed by histopathology at surgery. All participants were enrolled in a clinical trial which included metabolic testing performed immediately prior to TPIAT. Only patients without prior diabetes mellitus were enrolled in the study; patients were excluded if they were on oral anti-diabetic agents, insulin, or had a fasting blood glucose level ≥126 mg/dL. The University of Minnesota Institutional Review Board reviewed and approved the protocol (IRB# 1006M83756). Informed consent was obtained from all participants.
Basic demographic characteristics and pancreatitis history, including history of prior pancreatic surgeries, were collected for all participants. Prior pancreatic surgeries in this group included pancreaticoduodenectomy (Whipple procedure) or lateral pancreaticojejunostomy (Puestow or variants). History of lateral pancreaticojejunostomy is of particular relevance, as this procedure disrupts the pancreatic duct and thus impairs the later islet isolation because collagenase enzyme cannot be as efficiently diffused through the pancreas for digestion (a process that relies on an intact pancreatic duct).
Metabolic testing
Within 2 weeks prior to TPIAT, patients underwent frequent sample IV glucose tolerance testing (FSIVGTT) on day 1 and mixed meal tolerance testing (MMTT) on day 2. All testing was performed in the morning in the Clinical Research Unit following an overnight fast.
With the FSIVGTT, glucose, insulin, and C-peptide levels were measured at baseline and multiple times over the course of 180 minutes. At time 0, dextrose 0.3 grams/kg was given via intravenous push. Insulin, 0.025 units/kg, was injected at 20 minutes to augment glucose disposal. Insulin and C-peptide levels were measured at 1, 2, 3, 4, 5, 7, and 10 minutes after dextrose injection. The acute C-peptide response to glucose (ACRglu) was calculated by the trapezoidal area under the curve for the first 10 minutes after dextrose. The acute insulin response to glucose (AIRglu), insulin sensitivity index (SI), disposition index (AIRglu x SI), and glucose effectiveness (Sg) were calculated using MINMOD software (11, 12).
In the mixed meal tolerance test, the patient consumed 6 mL/kg Boost HP to a maximum of 360 mL over 5 minutes at time 0, and glucose and C-peptide were measured at -1 min, +30, +60, +90 and +120 minutes. Area under the curve for C-peptide (AUC C-peptide) and glucose (AUC glucose) were calculated using the trapezoidal method. In addition, hemoglobin A1c (HbA1C) was obtained and analyzed by high-performance liquid chromatography. Plasma glucose levels were measured by glucose oxidase assay. Plasma C-peptide levels were measured by Siemen’s immunolite 2000 chemiluminescent immunoassay and insulin levels were measured by two site immuno-enzymometric assay on a TOSOH 2000 autoanalyzer (13, 14).
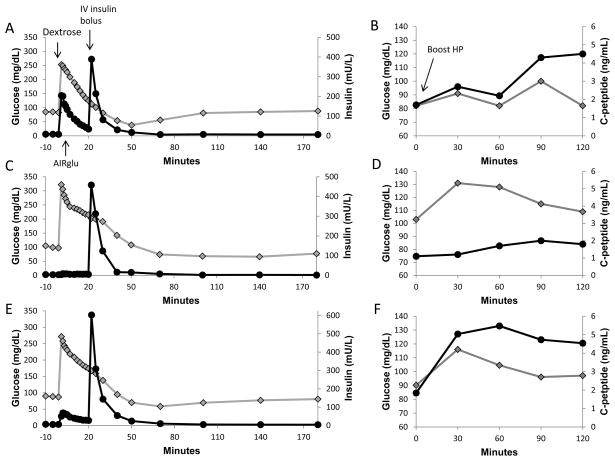
Representative examples of FSIVGTT and MMTT are displayed in figure 1.
Figure 1.
Illustrations of the frequent sample intravenous glucose tolerance test (1a) and mixed meal tolerance testing (1b) in a subject with idiopathic pancreatitis and higher islet yield of 4,219 IEQ/kg, and the same studies in a patient with genetic pancreatitis and a low islet yield of 807 IEQ/kg (1c and 1d). The subject with low islet yield has minimal acute insulin response to glucose (AIRglu) and lower C-peptide secretion with higher glucoses on mixed meal tolerance testing. The solid black lines with circles indicate insulin levels (a and c) and C-peptide levels (b and d), and the grey line with diamonds indicates glucose levels (all panels). In the FSIVGTT, a bolus of dextrose (0.3 g/kg) is administered intravenously at time 0, to elicit an acute insulin response, and a bolus of insulin (0.025 units/kg) is administered at time 20 minutes. In the MMTT, the patient ingests Boost HP (6mL/kg to max 360 mL) at time 0. Panels 1e and 1f illustrate the mean response for the entire group.
Pancreatectomy, islet isolation, and autologous transplantation
The surgical procedure of TPIAT has been described in the literature (1, 15). The islet isolations were performed in the University of Minnesota Molecular and Cellular Therapeutics GMP Facility using previously reported methods in compliance with federal regulations (16, 17). The pancreas was briefly distended with cold enzyme solution through the pancreatic duct using a pressure-controlled pump system (18). Enzymatic digestion was performed with Vitacyte Collagenase (Vitacyte LLC, Indianapolis, IN) and SERVA/Nordmark premium-grade neutral protease (SERVA Electrophoresis GmbH, Heidelberg, Germany) using 22–30 Wunsch units per gram pancreas of collagenase and 1.5–3.0 DMC units of neutral protease per gram pancreas, adjusted based on the donor age or severity of fibrosis of the pancreas (16, 19).
The pancreas was placed in a closed circuit Ricordi digestion chamber containing enzyme solution and shaken to separate the exocrine and endocrine tissues (20). The number of islets separated by enzymatic digestion was counted from an aliquot stained with diphenylthiocarbazone (Sigma, St. Louis, MO, USA) (21). Islet yield was quantified in terms of IEQ, which is islet mass standardized to an islet size of 150 μm diameter. If the total tissue volume of the digest was small (<0.25 mL/kg or <20 mL), the islet preparation was generally not further purified. If the digest volume was >0.25 mL/kg, the islets were purified by continuous iodixanol (OptiPrep, Axis-Shield, Oslo, Norway) density gradient on a COBE 2991 cell separator (22) unless, in the opinion of the isolation team, a large number of islets would be lost due to embedded tissue. The liberated islets were suspended in Connaught Medical Research Laboratories-1066 medium (Mediatech, Inc., Manassas, VA, USA) supplemented with 25 mM 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid (HEPES), antibiotics, and 2.5% human serum albumin.
Islets were infused into the portal vein after systemic heparinization in patients during the index operative procedure, and patients were managed in a protocolized fashion postoperatively as previously described (23).
Data analysis
Linear regression was used to determine univariate correlation coefficients for metabolic parameters and baseline parameters with islet isolation outcomes. Differences in means between groups were compared with Wilcoxon rank sum. Multivariate logistic regression was used to identify factors that predict islet mass ≥2,500 IEQ/kg. All analyses were performed using SAS (Cary, NC, USA). P-values ≤0.05 were considered statistically significant.
Results
Patient Characteristics
The 60 adult subjects had a mean age of 38.1 ± 11.5 years, were predominately female (n=50) and most often identified as non-Hispanic ethnicity (n=59) and Caucasian/white race (n=55). Mean body mass index was 25.5 ± 6.0 kg/m2. Etiologies of chronic pancreatitis were diverse, and included idiopathic disease (n=23), sphincter of Odi dysfunction (n=9), pancreas divisum (n=13), predisposing genetic mutation (PRSS1, SPINK1, or CFTR, n=12), hereditary disease without identified genetic etiology (n=1), annular pancreas (n=1), or biliary disease (n=1). Mean duration of diagnosed pancreatitis was 6.2 ±6.1 years (median 4 years). Baseline patient characteristics are outline in Table 1.
Table 1.
Patient characteristics, pancreatitis history, and preoperative labs
| Mean ± SD or n (%) | |
|---|---|
| Age (years) | 38.1 ± 11.5 |
| Gender (F/M) | 50/10 |
| BMI (kg/m2) | 25.5 ± 6.0 |
| Duration Pancreatitis (years) | 6.2 ± 6.1 |
| Narcotics | |
| Any use | 60 (100%) |
| Daily use | 47 (78%) |
| History of: | |
| ERCP (≥1 procedure) | 57 (95%) |
| Lateral pancreaticojejunostomy | 4 (7%) |
| Whipple procedure | 2 (3%) |
| # of hospitalizations (prior 1 year) | 6.3 ± 8.8 |
| Hemoglobin A1c (%) | 5.3 ± 0.3 |
| Fasting plasma glucose (mg/dL) | 90 ± 10 |
| Impaired fasting glucose | 11 (18%) |
Mean digest islet count (performed prior to purification) was 309,055 ±191,458 islet equivalents (IEQ). A COBE purification was performed to reduce tissue volume in 20 cases (33%). This was done only when the tissue volume was felt to be too high to safely permit intraportal infusion of the islets without purification. The mean islet count in the final (transplanted) product was 290,431 ± 156,619 IEQ and 4,307 ± 2,310 IEQ/kg body weight. Those patients undergoing purification had a mean loss of islet mass of 9.6± 16% in the final product. Mean final tissue volume was 11.7 ±5.7 mL.
Impact of disease etiology, prior surgical history, disease duration and BMI on islet isolation outcomes
A history of prior lateral pancreaticojejunostomy and genetic etiologies of disease (PRSS1, SPINK1, or cystic fibrosis) were associated with significantly lower islet yields at transplant (Table 2). PRSS1 mutations causing autosomal dominant hereditary pancreatitis were particularly detrimental—3 of 4 adults with this mutation had <1,000 IEQ/kg isolated for transplant. Only the youngest patient (age 19 years) without any prior surgical history received a moderate islet mass in the setting of a PRSS1 mutation at 4,239 IEQ/kg. A longer duration of disease was weakly but negatively correlated with islet mass (r= −0.25, p=0.05). The islet isolation outcomes did not differ by patient age, gender, narcotic use (daily vs not), number of ERCP procedures, or number of hospitalizations.
Table 2.
Prior surgery and genetic disease etiologies and impact on islet yield.
| Final Product IEQ | IEQ/kg | p-value* | |
|---|---|---|---|
| Surgical history | |||
| Prior LPJ (n=4) | 95,620 ± 59,702 | 1,638 ± 1,118 | ≤0.02 |
| No surgery or Whipple only (n=56) | 304,347 ± 152,190 | 4498 ± 2,258 | |
| Disease etiology | |||
| Any genetic cause (n=12) | 193,808 ± 159,325 | 2,842 ± 2,547 | ≤0.05 |
| All non-genetic etiologies (n=48) | 314,588 ± 147,891 | 4,674 ± 2,119 |
p-values are the comparison of prior LPJ vs no history of LPJ and genetic vs non-genetic etiology for IEQ and IEQ/kg.
Correlation of measures of insulin secretion and glycemic control and the isolation outcomes
We determined the regression coefficients for insulin/C-peptide secretory and glycemic measures with the islet mass. We measured islet mass in three ways: 1) The total islet equivalents in the digest (“digest IEQ”) which is the number of islets obtained from pancreatic digestion prior to any purification; this measure best reflects the number of islets that were available in the native pancreas; 2) The final islet mass expressed as total number of islets (“final product IEQ”) which reflects the end result of the isolation process; thus, the final product reflects what was infused into the patient for transplant; and 3) The relative islet mass for the recipient’s body weight in the final product (“final product IEQ/kg”) which is the measure most strongly associated in the medical literature with the glycemic control and metabolic outcomes in TPIAT recipients. Results for the univariate regression models are summarized in Table 3.
Table 3.
Correlation coefficients for key metabolic testing parameters and patient characteristics with the isolation outcomes, measured by digest IEQ, final product IEQ, and IEQ/kg, in a univariate analysis.
| Digest IEQ | Final Product IEQ | Final Product IEQ/kg | ||||
|---|---|---|---|---|---|---|
| Metabolic Parameter | r | p | r | p | r | p |
| Basic Labs | ||||||
| Hemoglobin A1c | −0.28* | 0.03 | −0.29* | 0.03 | −0.33* | 0.01 |
| Fasting Glucose | −0.24* | 0.07 | −0.24 | 0.06 | −0.41* | 0.001 |
| Fasting C-peptide | 0.20 | 0.13 | 0.21 | 0.11 | 0.03 | 0.85 |
| Mixed Meal Tolerance Test | ||||||
| AUC C-peptide | 0.18 | 0.19 | 0.26 | 0.09 | 0.06 | 0.66 |
| AUC insulin | 0.17 | 0.20 | 0.20 | 0.18 | 0.00 | 0.99 |
| AUC glucose | −0.36* | 0.007 | −0.34* | 0.001 | −0.40* | 0.002 |
| IV Glucose Tolerance | ||||||
| Peak C-peptide | 0.34* | 0.01 | 0.39* | 0.003 | 0.29* | 0.03 |
| ACR glucose | 0.30* | 0.02 | 0.36* | 0.007 | 0.26* | 0.05 |
| AIR glucose | 0.29* | 0.03 | 0.32* | 0.01 | 0.20* | 0.13 |
| Disposition Index (AIRg * SI) | 0.29* | 0.04 | 0.31* | 0.02 | 0.36* | 0.008 |
| Patient Characteristics | ||||||
| BMI | 0.37* | 0.004 | 0.33* | 0.01 | −0.11 | 0.40 |
| Duration of Disease | −0.21 | 0.11 | −0.22 | 0.09 | −0.25* | 0.05 |
indicates values that are statistically significant (p-value ≤0.05)
The acute insulin response (AIRglu) and the acute C-peptide response (ACRglu) were measured by frequent sample intravenous glucose tolerance testing. Both measures correlated positively with the final product IEQ, and ACRglu correlated with IEQ/kg. Insulin sensitivity (SI) was calculated from the 180 minute FSIVGTT and did not correlate with islet mass. Disposition index, which relates insulin secretion to insulin sensitivity (AIRglu * SI) was more strongly correlated with final product IEQ/kg than the AIRglu alone.
The AUC glucose from the MMTT, fasting glucose, and HbA1c correlated inversely with the final product IEQ/kg. These measures were more strongly correlated with the IEQ/kg than the final product total IEQ or digest IEQ. The 11 patients with impaired fasting glucose (IFG) had fewer islets isolated for transplant compared to those without IFG—2,509 ± 1,496 IEQ/kg vs 4,711 ± 2,276 IEQ/kg (p=0.003).
At the time of pancreatectomy, the islet lab assessed trimmed pancreas weight and severity of fibrosis (based on gross morphology and scored on a scale of 0–10, with 10 as most severe). The pancreas weight correlated positively with digest IEQ (r=0.44, p=0.007) and final product IEQ (r=0.38, p=0.002), while gross fibrosis correlated negatively with all measures of islet mass (r=0.4–0.5, p<0.001) similar to previous reports.
A simple model for clinical use in preoperative evaluation of TPIAT candidates
We next employed multivariate logistic regression analysis to identify a simple model that may help predict candidates likely to receive ≥2,500 IEQ/kg. This is a clinically relevant measure as we have previously demonstrated that patients with this islet mass are likely to do metabolically well (3). Using multivariate logistic regression modeling, we identified fasting glucose and peak stimulated C-peptide on MMTT as significant predictors, with an odds ratio (OR) of 0.93 (95% CI 0.87–1.0, p=0.041) for fasting glucose and OR of 7.90 (95% CI 1.75–35.6, p=0.007) for peak stimulated MMTT C-peptide ≥4 ng/mL. That is for each 1 mg/dL increase in fasting blood glucose, the odds of isolating >2,500 IEQ/kg are reduced, with an odds ratio of 0.93, while those patient with a stimulated C-peptide ≥4 ng/mL had 7.9 times the odds of receiving ≥2,500 IEQ/kg compared to those with peak C-peptide <4 ng/mL on mixed meal tolerance testing (figure 2).
Figure 2.
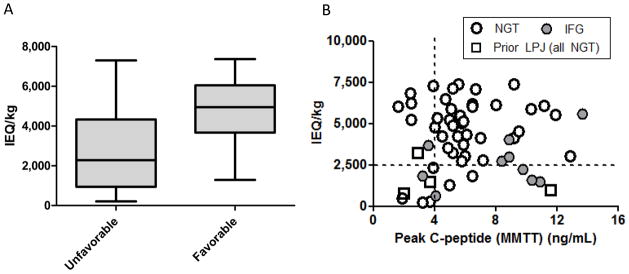
Figure 2a and 2b. (2a) Islet mass isolated in patients with all three of the following characteristics: 1) normal plasma fasting glucose (<100 mg/dL), 2) stimulated C-peptide ≥4 ng/mL on MMTT, and 3) no prior history of surgical drainage procedures (lateral pancreaticojejunostomy), (“favorable” group) compared to islet mass isolated in patients with any one or more of the following: 1) impaired fasting glucose, 2) peak stimulated C-peptide <4 ng/mL, or 3) history of lateral pancreaticojejunostomy (“unfavorable” group). Boxes indicate the 25th–75th percentile with line at the median value; whiskers indicate the minimum and maximum in each group. On average the favorable group had a higher islet mass isolated, ≥2500 IEQ/kg in 94% of cases. (*p<0.0001). (2b) Displays data for each participant for stimulated C-peptide on mixed meal test versus islet mass expressed as IEQ/kg. Open circles indicate patients with normal glucose tolerance, grey circles impaired glucose tolerance, and open squares history of lateral pancreaticojejunostomy (all 4 had normal fasting glucose).
In this cohort, there were 38 patients without a prior history of lateral pancreaticojejunostomy (surgical pancreatic drainage procedure), whom also had both a normal fasting glucose (<100 mg/dL) and a stimulated C-peptide ≥4 ng/mL on mixed meal tolerance testing. Notably, these patients had a successful isolation outcome in nearly all cases, with a final islet mass transplanted of ≥2500 IEQ/kg in 94% of cases and ≥5000 IEQ/kg in half. Only 2 patients (6%) had low yields (1290 IEQ/kg and 1847 IEQ/kg), with none <1000 IEQ/kg (a range where insulin independence is not possible). In contrast, patients with any one or more of impaired fasting glucose, peak stimulated C-peptide <4 ng/mL, or a history of lateral pancreaticojejunostomy had low yield (<2500 IEQ/kg) in 54% of cases (p <0.0001) (Figure 2).
Discussion
Total pancreatectomy and islet autotransplant may be considered for patients with severe chronic pancreatitis. The goal of the IAT is to ameliorate or reduce the severity of postoperative diabetes, and optimistically, we can achieve this goal in about two-thirds of patients. While 30–40% of patients are completely insulin-free, another 30% maintain normoglycemia on minimal insulin, typically once daily basal insulin. The remaining one-third of patients have a more severe form of post-pancreatectomy diabetes mellitus (23, 24), which may be labile and difficult to control. The risk of diabetes and severity of diabetes is determined in large part by the viable islet mass transplanted (3). Historically, those with <2500 IEQ/kg transplanted remain insulin dependent in the vast majority of cases and may be unable to safely achieve the American Diabetes Association goal of hemoglobin A1c <7%.
Because one of the major patient and physician concerns going into TPIAT is the risk for labile post-pancreatectomy diabetes mellitus, it is essential that we develop meaningful protocols for pre-operative assessment of islet mass and diabetes risk in these patients. We found a relationship between stimulated insulin and C-peptide secretion and the isolated islet mass in adult patients with chronic pancreatitis undergoing TPIAT. The acute insulin and C-peptide responses to an intravenous glucose tolerance test correlated modestly with islet mass. While these tests did not precisely predict islet mass for an individual patient, in those without a prior surgical drainage procedures (lateral pancreaticojejunostomy), a normal fasting glucose along with a mixed meal stimulated C-peptide >4 ng/mL accurately identified recipients highly likely to have a successful isolation outcome.
For the TPIAT recipient, metabolic testing is an important component of the pre-surgical evaluation. Such tests could serve two purposes: 1) to document baseline function prior to complete (and irreversible) removal of the pancreas; and 2) potentially to improve our preoperative counseling of potential TPIAT recipients. The latter is not yet established.
Previously published studies suggest a correlation between: 1) post-operative intravenous stimulation tests and islet mass transplanted in IAT recipients (9), 2) arginine or intravenous glucose stimulation tests and islet isolation results in cadaveric pancreas donors and porcine pancreas isolations (7, 8), and 3) a general relationship between metabolic testing and morphologic assessment of beta cell mass in animal models (25–27). In a prior analysis, glucose potentiated arginine stimulation and intravenous glucose tolerance testing performed after IAT in a small group of recipients correlated well with transplanted islet mass (9). However, pre-operative metabolic tests have never previously been studied in relation to TPIAT isolation outcomes in chronic pancreatitis patients undergoing this procedure.
We found a modest correlation (r value 0.3–0.4) between the acute insulin response (AIRglu) and C-peptide response (ACRglu) on IV glucose tolerance testing and the islet mass isolated. While insulin and C-peptide measures correlated best with total islet mass (IEQ), glycemic measures (HbA1c and glucose on MMTT) were better predictors of relative islet mass (IEQ/kg body weight), with lower glucose levels and lower HbA1c associated with better outcomes. While IVGTT measures provided a reasonable correlation with islet mass at a population level, these measured could not predict islet isolation outcomes for an individual patient.
Notably, we were able to identify the patients likely to receive a moderate or high islet mass of ≥2500 IEQ/kg based on prior surgical history, fasting glucose, and stimulated C-peptide after a mixed meal. Those patients with a stimulated C-peptide >4 ng/mL on MMTT had nearly 8 times the odds of receiving at least 2,500 IEQ/kg compared to the patients with low C-peptide. In patients without a prior surgical drainage procedure (lateral pancreaticojejunostomy, or Puestow), those patients with both normal fasting glucose and a stimulated C-peptide ≥4 ng/mL prior to surgery received ≥2,500 IEQ/kg in 94% of cases, with a notably high yield of ≥5,000 IEQ/kg in 50%. In contrast, a patient with a prior lateral pancreaticojejunostomy, or with impaired fasting glucose and/or stimulated C-peptide <4 ng/mL, fewer than half received ≥2500 IEQ/kg.
The majority of TPIAT recipients who receive a moderate islet mass of ≥2500 IEQ/kg are C-peptide positive and maintain goal glycemic control, with hemoglobin A1c <7% (3). Thus, this is a clinically relevant threshold for identifying those likely to perform well metabolically after islet transplant. The mixed meal test is relatively easy to perform in the clinical setting.
Our results showed a weaker correlation between insulin secretory measures and the islet mass than one might expect based on the published literature (7, 8, 25). Previous studies in animals and a limited number of studies in human partial pancreatectomy and islet transplant recipients reported correlation between AIRglu and beta cell mass, with correlation coefficients of 0.6–0.8. This difference may relate to: 1) variability inherent in our particular model—particularly the variations in the effectiveness of the islet isolation or islet count; or 2) a need for more sensitive measures of beta cell mass in this population. In particular, patients with chronic pancreatitis may not mimic the other populations in which these tests have been performed. It is possible that the chronic inflammatory environment present in chronic pancreatitis may impair beta cell function in response to oral and intravenous stimuli, thus reducing the reliability of such tests to adequately assess beta cell mass in this population (28).
Because there are inherent variabilities in the islet isolation process, there is not likely to be any test that correlates perfectly with islet yield using currently available technologies and enzymes solutions. The recovery of beta cells from the native pancreas, while presumed high, is less than 100% of the pancreatic islet mass, and varies depending on the condition of the pancreas and digestion of the pancreatic parenchyme. Pancreas digestion may be more successful in some cases than others. Second, islet counts are subjective, relying on a manual assessment of islet size and number in a small aliquot, thus, inherent errors in estimation of islet number may account for the imprecision around the regression line. Third, when comparing beta cell functional tests to isolated islet mass, we are making the assumption that all islets contain a similar proportion of functional beta cells, and that in fact, islet mass accurately reflects beta cell mass. This may or may not be true in patients with chronic pancreatitis. Stimulated insulin secretion is a measure of beta cell function, and it is has been proposed that some patients with chronic pancreatitis have a deficit in beta cell function which precedes the loss of islet mass (28). Ultimately, the clinical challenge is to: 1) estimate the true availability of islet mass in the pancreas; 2) efficiently liberate those islets into free suspension; and 3) engraft the islets with high efficiency into the patient.
There are a number of factors that are proposed to impact outcomes via islet mass, islet viability, or islet engraftment, and which were not the focus of this particular analysis. We noted that a smaller pancreas or more fibrotic pancreas is associated with a lower islet mass, although these variables were assessed post-pancreatectomy. The approach to pancreatectomy (ischemic time) may affect islet viability, while intraportal inflammation, the instant blood mediate inflammatory reaction, and efficacy of revascularization may all affect post-transplant islet survival and function. While recognizing this complexity of the islet transplant situation, we attempted to identify preoperative predictors for one important piece of the puzzle—islet mass available for transplant. It will additionally be important to follow these patients for long-term islet graft function, and correlate preoperative testing directly with long-term outcome. At this point, recipients remain engaged in a randomized treatment trial and metabolic outcomes are collected as a component of the clinical trial.
Although we could separate those likely to do well from those at high risk for poor isolation outcomes, we lacked in this study the ability to precisely measure islet mass, which would be highly valuable for longitudinal follow up of recipients to determine timing of TPIAT. Glucose potentiated arginine stimulation (GPAIS) measures insulin secretory response to an intravenous arginine bolus during hyperglycemia; because this test elicits a maximal insulin secretory response, it may be more sensitive for measuring early deficits in beta cell mass. Notably, GPAIS has been suggested as a superior measure of beta cell mass compared to either AIRglu or AIRarg (9, 25). GPAIS can be incorporated into future studies and evaluated as a potential predictor of islet mass in this model.
In summary, we found that in adult patients with chronic pancreatitis, stimulated insulin and C-peptide levels and glycemic measures obtained prior to surgery were weakly correlated to the number of islets subsequently isolated from the diseased pancreas. While a stimulated C-peptide and normal fasting glucose could help identify those recipients at low versus high risk for low islet yield and thus more severe post-operative diabetes, such tests lacked utility for accurately predicting islet mass within individual recipients in this population. Future research should investigate additional metabolic tests to determine measures which may be more sensitive in precisely predicting islet mass prior to TPIAT, to aid in preoperative counseling of recipients and, potentially, to aid in determining timing of TPIAT.
Acknowledgments
This work is supported by National Institute of Diabetes, Digestive, and Kidney Diseases (1K23DK084315-01A1), the American Diabetes Association (#1-11-CT-06), Merck and Co, and the National Pancreas Foundation. The authors thank K. Louise Berry RN for her contributions to the care of the participants.
Abbreviations
- TP
total pancreatectomy
- IAT
islet autotransplant
- CP
chronic pancreatitis
- IEQ
islet equivalents
- IEQ/kg
islet equivalents per kilogram body weight
- FSIVGTT
frequent sample intravenous glucose tolerance test
- MMTT
mixed meal tolerance test
- ACRglu
acute C-peptide response to glucose
- AIRglu
acute insulin response to glucose
- DI
disposition index
- AUC
area under the curve
- SI
insulin sensitivity index
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Blondet JJ, Carlson AM, Kobayashi T, Jie T, Bellin M, Hering BJ, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. The Surgical clinics of North America. 2007;87(6):1477–501. x. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Morgan K, Owczarski SM, Borckardt J, Madan A, Nishimura M, Adams DB. Pain control and quality of life after pancreatectomy with islet autotransplantation for chronic pancreatitis. J Gastrointest Surg. 2012;16(1):129–33. doi: 10.1007/s11605-011-1744-y. discussion 33–4. Epub 2011/11/02. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409–24. doi: 10.1016/j.jamcollsurg.2011.12.040. Epub 2012/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellin MD, Freeman ML, Schwarzenberg SJ, Dunn TB, Beilman GJ, Vickers SM, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(9):793–9. doi: 10.1016/j.cgh.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan KA, Theruvath T, Owczarski S, Adams DB. Total pancreatectomy with islet autotransplantation for chronic pancreatitis: do patients with prior pancreatic surgery have different outcomes? Am Surg. 2012;78(8):893–6. Epub 2012/08/04. [PubMed] [Google Scholar]
- 6.Khan KM, Desai CS, Kalb B, Patel C, Grigsby BM, Jie T, et al. MRI Prediction of Islet Yield for Autologous Transplantation After Total Pancreatectomy for Chronic Pancreatitis. Dig Dis Sci. 2012 doi: 10.1007/s10620-012-2448-1. Epub 2012/10/23. [DOI] [PubMed] [Google Scholar]
- 7.Hubert T, Jany T, Marcelli-Tourvieille S, Nunes B, Gmyr V, Kerr-Conte J, et al. Acute insulin response of donors is correlated with pancreatic islet isolation outcome in the pig. Diabetologia. 2005;48(10):2069–73. doi: 10.1007/s00125-005-1904-2. [DOI] [PubMed] [Google Scholar]
- 8.Hubert T, Strecker G, Gmyr V, Arnalsteen L, Garrigue D, Ezzouaoui R, et al. Acute insulin response to arginine in deceased donors predicts the outcome of human islet isolation. Am J Transplant. 2008;8(4):872–6. doi: 10.1111/j.1600-6143.2007.02131.x. Epub 2008/02/12. [DOI] [PubMed] [Google Scholar]
- 9.Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47(3):324–30. doi: 10.2337/diabetes.47.3.324. [DOI] [PubMed] [Google Scholar]
- 10.Bellin MD, Blondet JJ, Beilman GJ, Dunn TB, Balamurugan AN, Thomas W, et al. Predicting islet yield in pediatric patients undergoing pancreatectomy and autoislet transplantation for chronic pancreatitis. Pediatric diabetes. 2010;11(4):227–34. doi: 10.1111/j.1399-5448.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Computer methods and programs in biomedicine. 1986;23(2):113–22. doi: 10.1016/0169-2607(86)90106-9. Epub 1986/10/01. [DOI] [PubMed] [Google Scholar]
- 12.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5(6):1003–15. doi: 10.1089/152091503322641060. Epub 2004/01/08. [DOI] [PubMed] [Google Scholar]
- 13.Marcovina S, Bowsher RR, Miller WG, Staten M, Myers G, Caudill SP, et al. Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clinical chemistry. 2007;53(4):711–6. doi: 10.1373/clinchem.2006.082214. Epub 2007/02/03. [DOI] [PubMed] [Google Scholar]
- 14.Mahon JL, Beam CA, Marcovina SM, Boulware DC, Palmer JP, Winter WE, et al. Comparison of two insulin assays for first-phase insulin release in type 1 diabetes prediction and prevention studies. Clinica chimica acta; international journal of clinical chemistry. 2011;412(23–24):2128–31. doi: 10.1016/j.cca.2011.07.019. Epub 2011/08/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farney AC, Najarian JS, Nakhleh RE, Lloveras G, Field MJ, Gores PF, et al. Autotransplantation of dispersed pancreatic islet tissue combined with total or near-total pancreatectomy for treatment of chronic pancreatitis. Surgery. 1991;110(2):427–37. discussion 37–9. [PubMed] [Google Scholar]
- 16.Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93(7):693–702. doi: 10.1097/TP.0b013e318247281b. Epub 2012/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4(3):390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 18.Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM, et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell transplantation. 1999;8(3):285–92. doi: 10.1177/096368979900800309. [DOI] [PubMed] [Google Scholar]
- 19.Anazawa T, Balamurugan AN, Bellin M, Zhang HJ, Matsumoto S, Yonekawa Y, et al. Human islet isolation for autologous transplantation: comparison of yield and function using SERVA/Nordmark versus Roche enzymes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(10):2383–91. doi: 10.1111/j.1600-6143.2009.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl 1 Journal Article):140–2. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- 21.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, et al. Islet isolation assessment in man and large animals. Acta Diabetologica Latina. 1990;27(3):185–95. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 22.Lake SP, Bassett PD, Larkins A, Revell J, Walczak K, Chamberlain J, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes. 1989;38(Suppl 1 Journal Article):143–5. doi: 10.2337/diab.38.1.s143. [DOI] [PubMed] [Google Scholar]
- 23.Bellin MD, Balamurugan AN, Pruett TL, Sutherland DE. No islets left behind: islet autotransplantation for surgery-induced diabetes. Curr Diab Rep. 2012;12(5):580–6. doi: 10.1007/s11892-012-0296-1. Epub 2012/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellin MD, DERS, Robertson RP. Pancreatectomy and autologous islet transplantation for painful chronic pancreatitis: indications and outcomes. Hospital practice (1995) 2012;40(3):80–7. doi: 10.3810/hp.2012.08.992. Epub 2012/10/23. [DOI] [PubMed] [Google Scholar]
- 25.Robertson RP. Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes. 2007;56(10):2420–4. doi: 10.2337/db07-0742. Epub 2007/07/04. [DOI] [PubMed] [Google Scholar]
- 26.Larsen MO, Rolin B, Wilken M, Carr RD, Gotfredsen CF. Measurements of insulin secretory capacity and glucose tolerance to predict pancreatic beta-cell mass in vivo in the nicotinamide/streptozotocin Gottingen minipig, a model of moderate insulin deficiency and diabetes. Diabetes. 2003;52(1):118–23. doi: 10.2337/diabetes.52.1.118. Epub 2002/12/28. [DOI] [PubMed] [Google Scholar]
- 27.Larsen MO, Rolin B, Sturis J, Wilken M, Carr RD, Porksen N, et al. Measurements of insulin responses as predictive markers of pancreatic beta-cell mass in normal and beta-cell-reduced lean and obese Gottingen minipigs in vivo. American journal of physiology Endocrinology and metabolism. 2006;290(4):E670–7. doi: 10.1152/ajpendo.00251.2005. Epub 2005/11/10. [DOI] [PubMed] [Google Scholar]
- 28.Sasikala M, Talukdar R, Pavan kumar P, Radhika G, Rao GV, Pradeep R, et al. beta-Cell dysfunction in chronic pancreatitis. Dig Dis Sci. 2012;57(7):1764–72. doi: 10.1007/s10620-012-2086-7. Epub 2012/03/03. [DOI] [PubMed] [Google Scholar]