Abstract
Background & Aims
RM-131, a synthetic ghrelin agonist, greatly accelerates gastric emptying of solids in patients with type 2 diabetes and delayed gastric emptying (DGE). We investigated the safety and effects of a single dose of RM-131 on gastric emptying and upper gastrointestinal (GI) symptoms in patients with type 1 diabetes and previously documented DGE.
Methods
In a double-blind cross-over study, 10 patients with type 1 diabetes (age, 45.7 ± 4.4 y; body mass index, 24.1 ± 1.1 kg/m2) and previously documented DGE were assigned in random order to receive a single dose of RM-131 (100 μg, subcutaneously) or placebo. Thirty minutes later, they ate a radiolabeled solid–liquid meal containing EggBeaters (ConAgra Foods, Omaha, NE), and then underwent 4 hours of gastric emptying and 6 hours of colonic filling analyses by scintigraphy. Upper GI symptoms were assessed using a daily diary, gastroparesis cardinal symptom index (total GCSI-DD) and a combination of nausea, vomiting, fullness, and pain (NVFP) scores (each rated on a 0–5 scale).
Results
At screening, participants' mean level of hemoglobin A1c was 9.1% ± 0.5%; their total GCSI-DD score was 1.66 ± 0.38 (median, 1.71), and their total NVFP score was 1.73 ± 0.39 (median, 1.9). The t1/2 of solid gastric emptying was 84.9 ± 31.6 minutes when subjects were given RM-131 and 118.7 ± 26.7 when they were given a placebo. The median difference (Δ) was 33.9 minutes (interquartile range [IQR] −12, −49), or -54.7% (IQR, −21%, 110%). RM-131 decreased gastric retention of solids at 1 hour (P = .005) and 2 hours (P = .019). Numeric differences in t1/2 for gastric emptying of liquids, solid gastric emptying lag time, and colonic filling at 6 hours were not significant. Total GCSI-DD scores were 0.79 on placebo (IQR, 0.75, 2.08) and 0.17 on RM-131 (IQR, 0.00, 0.67; P = .026); NVFP scores were lower on RM-131 (P = .041). There were no significant adverse effects.
Conclusions
RM-131 significantly accelerates of gastric emptying of solids and reduces upper GI symptoms in patients with type 1 diabetes and documented DGE.
Keywords: Gastroparesis, Prokinetic, Drug, Clinical Trial
Gastroparesis is a clinical syndrome defined as delayed gastric emptying (DGE) in the absence of mechanical obstruction and the presence of upper gastrointestinal (GI) symptoms including nausea and/vomiting, postprandial fullness (early satiety), bloating, and epigastric pain.1,2 Approximately one third of cases at referral centers are caused by diabetes (diabetic gastroparesis).3 Although the cumulative 10-year incidence of gastroparesis in the community is estimated at 5.2% in type 1 diabetes and at 1% in type 2 diabetes,4 the impact of this disease is significant. It is associated with higher morbidity and mortality relative to US matched controls,5 as well as with impaired quality-of-life scores. These adverse statistics are independent of factors such as age, sex, smoking, alcohol use, and type of diabetes.6 Once diagnosed, patients often require multiple medications (such as prokinetics and anti-emetics) for several years and have much higher rates of hospitalizations, days in the hospital, and emergency room and office visits.7 The evidence for use of currently available agents is summarized in the Supplementary Materials and Methods.
In recent years, several new prokinetic medications have come under investigation, including several motilin agonists (eg, GSK962040 and RQ-00-20194)8,9 and the ghrelin analogs, TZP-101 (ulimorelin, an intravenous preparation),10,11 which showed only a slight improvement of GE t1/2 of solids by approximately 20% and TZP-102,12 (an oral preparation) which showed a lack of effect on GE in patients with diabetic gastroparesis. Ghrelin is the endogenous ligand for the growth hormone secretagogue-1a receptor and is a potential target for treatment of impaired gastric motility and energy balance.13 Recombinant human ghrelin accelerated GE of liquids in diabetic patients14 and GE of solids when administered at pharmacologic doses (reviewed by Camilleri et al13).
RM-131 is a synthetic ghrelin agonist with similar characteristics as native ghrelin, but with a 100-fold greater potency in reversing gastricileus in animal models and longer plasma t1/2 (Rhythm Pharmaceuticals, data on file). Pharmacokinetic information is included in the Supplementary Materials and Methods section. In a recent randomized controlled study of RM-131, we showed that single-dose administration of RM-131 was effective in greatly accelerating GE in patients with type 2 diabetes (T2DM) and DGE.15
The primary aim of this study was to investigate the effects on GE rate of a single dose of RM-131 in type 1 diabetes mellitus (T1DM) patients with prior documentation of DGE. Secondary aims included investigation of effects of RM-131 on upper GI symptoms, safety, and tolerability. The dose of 100 μg RM-131 was chosen based on the safety, pharmacokinetic, and pharmacodynamic profiles established in the single ascending dose study of healthy volunteers.
Materials and Methods
Trial Design
We conducted a randomized, double-blind, placebo-controlled, single-dose, 2-period, cross-over study with a 7-day washout, during the period from June 1 to November 30, 2012, in 10 patients with T1DM and prior documentation of DGE. Eligibility and identification of patients is included in Supplementary Materials and Methods.
Patients were randomized by a computer-generated allocation schedule to receive either subcutaneous injection of RM-131 or placebo (5% mannitol) with an identical appearance during period 1. Allocation was communicated to the research pharmacist in the Mayo Clinic Clinical Research Unit (CRU), and allocation was concealed from all investigators until completion of the entire study, at which time the randomization code was revealed to the study statistician.
After a 7-day washout period, patients crossed over to receive the alternative therapy in period 2. Consistent with the ethical review that patients should not be withdrawn from medication that may have been contributing to their health, patients were allowed to continue on antiemetics and prokinetics (eg, metoclopramide, erythromycin) when indicated, except on the day of testing. Doses of these drugs had to be stable for at least 2 weeks before randomization and throughout the study of both periods. The study was conducted on an outpatient basis in the CRU at a single tertiary referral center: Mayo Clinic in Rochester, Minnesota. All participants and members of the research team were blinded to the interventions.
Experimental Protocol
Details of the protocol are provided in the Supplementary Materials and Methods section.
Measurement of Gastric Emptying of Solids and Liquids and Small-Bowel Transit
Validated scintigraphy was used to assess GE of solids and colonic filling at 6 hours (CF6) after a standardized meal. Patients underwent dual-phase (solid and liquid) GE scintigraphy on the days the study drug was administered (day 1 of periods 1 and 2). The method has been described in detail elsewhere.16,17
Symptoms
Patients were asked to recall symptoms over the preceding 24 hours and fill out the gastroparesis cardinal symptom index daily diary (GCSI-DD)18 at screening and on day 2 of each period, 24 hours after each study visit, to assess their upper GI symptoms19 and return the completed form to the CRU.
The GCSI-DD is a patient-reported symptom questionnaire that covers 9 symptom severity items rated on a numeric scale from 0 (none) to 5 (very severe), including nausea/vomiting (3 items), fullness/early satiety (4 items), and bloating (2 items). Also included are 2 symptom severity items for upper abdominal pain, 2 items for vomiting/retching frequency, and an overall rating for the severity of gastroparesis. Patients are asked to recall symptoms over the past 24 hours. The total GCSI-DD score was calculated as the mean of the 3 subscale scores for severity of nausea/vomiting, postprandial fullness/early satiety, and bloating. The GCSI-DD was completed by participants at screening for baseline assessment and also at 24 hours after each treatment period. Patients were required to return the GCSI-DD to the clinic upon completion. A secondary composite score based on nausea, vomiting, fullness, and pain (NVFP) was derived post hoc in consideration of communications from the Food and Drug Administration to representatives of the American Neurogastroenterology and Motility Society regarding the requirement for assessment of pain in patients with gastroparesis. We perceive that this information foreshadows what will be required in clinical trials in gastroparesis and, therefore, we added it to the current article. The scores of NVFP symptoms were obtained based on GCSI-DD responses. Both total GCSI-DD and the post hoc NVFP scores were analyzed to compare symptom scores between the 2 treatment groups for descriptive purposes only.
Safety and Tolerability
Safety and tolerability were assessed by evaluation of adverse events (AEs), vital signs, physical examination, clinical laboratory tests (including blood glucose level), and an electrocardiogram. Each patient was monitored for the development of any AEs during study visits and by telephone follow-up evaluation on day 2 of both periods. Vital signs were assessed before the study dose, then after the study dose every 30 minutes for 6 hours. Blood glucose level was measured before the study dose, 2 and 4 hours after the study dose, and when clinically indicated.
Statistical Considerations: Outcomes, Power, and Analysis
Data are summarized predominantly as median and interquartile range (IQR) because most end points were not normally distributed, with the exception of GE at 1 and 2 hours and GCSI-DD and NVFP scores.
The primary end point of the study was the GE t1/2 for solids. The percentage difference in GE t1/2 for solids between treatments was defined as follows:
Secondary end points included the following: (1) GE of solids at 1, 2, and 4 hours, GE solid lag time, GE t1/2 for liquids, and CF6 (solids); (2) total GCSI-DD score18 and average score based on a scale of 0 to 5 for NVFP as documented in the GCSI-DD; and (3) AEs.
The intra-individual coefficient of variation of GE t1/2 of solids was 24%, justifying the sample size of 10 patients.20
Details of the statistical analysis are included in the Supplementary Materials and Methods section, including the correction method21 used to account for 2 composite symptoms scores. All co-authors had access to the study data, and reviewed and approved the final manuscript.
Results
Patient Characteristics at Study Entry and Disposition
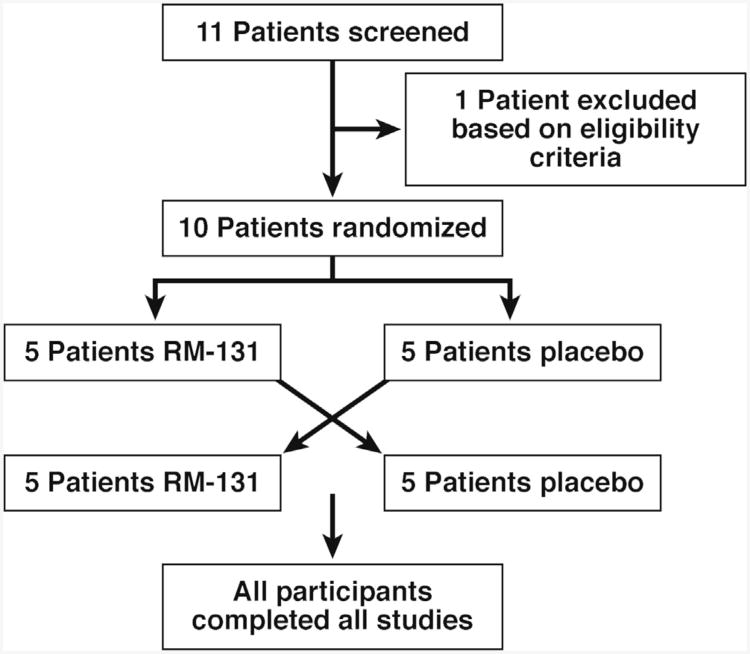
Table 1 shows the clinical features of the 10 patients. Patient disposition is shown in Figure 1. All participants completed all studies after randomization. One patient was receiving treatment for GI dysmotility during the cross-over study (pyridostigmine for chronic constipation that was stopped beginning 48 hours before day 1).
Table 1. Patient Demographics, Characteristics, and Features Associated With Diabetes.
| Patient number | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Average ± SEM | |
| Age, y | 33 | 46 | 24 | 42 | 44 | 65 | 61 | 51 | 31 | 60 | 45.7 ± 4.4 |
| Sex, male/female | F | F | F | F | M | M | F | F | F | F | N/A |
| BMI, kg/m2 | 24.1 | 19.8 | 22.8 | 24.4 | 23.8 | 30.8 | 19.7 | 21.6 | 27.4 | 27.1 | 24.1 ± 1.1 |
| Duration of diabetes, y | 23 | 33 | 22 | 38 | 17 | 35 | 49 | 42 | 17 | 45 | 32.1 ± 3.7 |
| Years since baseline gastric emptying testa | 2 | 3 | 3 | 2 | 10 | 3 | 2 | 2 | 10 | 12b | 4.9 ± 1.3 |
| Fasting glucose level, mg/dL | 118 | 119 | 233 | 151 | 163 | 210 | 278 | 154 | 119 | 314 | 185.9 ± 22.1 |
| HbA1c, % | 7.3 | 11.3 | 9.7 | 8.8 | 9.3 | 7.1 | 7.5 | 9.3 | 10.9 | 10.0 | 9.1 ± 0.5 |
| Peripheral neuropathy | - | + | + | - | + | + | + | + | + | + | N/A |
| Cardiovagal neuropathyc | - | + | - | + | + | + | - | - | + | + | N/A |
| Nephropathy | - | + | - | - | - | + | + | - | + | + | N/A |
| Retinopathy | - | + | + | + | + | - | + | + | + | + | N/A |
| Baseline total GCSI-DD (0–5)d | 0.5 | 2.5 | 2.6 | 1.1 | 0.3 | 2.3 | 2.9 | 3.4 | 0.8 | 0.3 | 1.66 ± 0.38 |
| Baseline composite NVFP score (0–5)d | 0.5 | 2.8 | 2.3 | 1.5 | 0.3 | 2.8 | 3.0 | 3.3 | 1.0 | 0 | 1.73 ± 0.39 |
| Prescription insulin | L, H | L, N | L, N | N | N | N | L, N | H | H | N | N/A |
BMI, body mass index; H, Humalog (Eli-Lilly; Indianapolis, IN) insulin; L, Lantus (Sanofi-Aventis, Bridgewater, NJ) insulin; N, Novolog (Novo Nordisk A/S, Bagsvaerd Denmark) insulin; N/A, not applicable; +, present; -, absent; baseline composite score averages of NVFP.
Time in years between baseline GE assessment and the current study.
Included as protocol deviation.
Absence of sinus arrhythmia on screening electrocardiogram used as an indicator of cardiovagal dysfunction.
Baseline scores obtained on initial screening evaluation.
Figure 1.
Patient disposition using the consolidated standards of reporting trials (CONSORT) approach. All participants completed all studies, and data were analyzed using intention-to-treat principles.
Effects of RM-131 on Transit
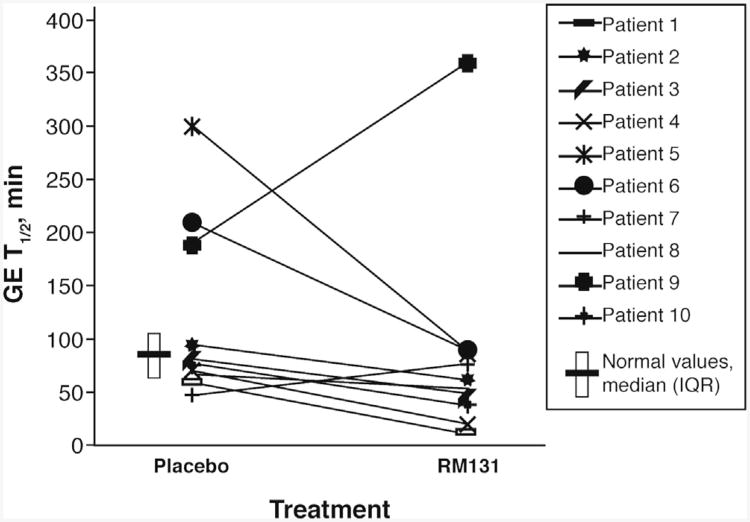
Most transit data are presented as median (IQR, range) values because of the presence of several outliers. All outliers were included in the data analysis. The 10 participants' individual data for GE t1/2 on RM-131 and placebo are shown in Figure 2. In general, 8 of 10 participants had faster GE on RM-131 relative to placebo treatment; 1 patient (patient 7) had slightly delayed GE on RM-131, and 1 patient (patient 9) was an outlier with greatly delayed GE with RM-131 relative to placebo. There were no significant order effects detected.
Figure 2.
Changes in GE t1/2 solids by treatment period for each patient (GE, minutes for solids for all 10 patients by treatment period: placebo and RM-131). Data are individual observations for each patient. The published median (83 min) and IQR (64–103 min) from 123 healthy volunteers using the identical radiolabeled meal are presented.16
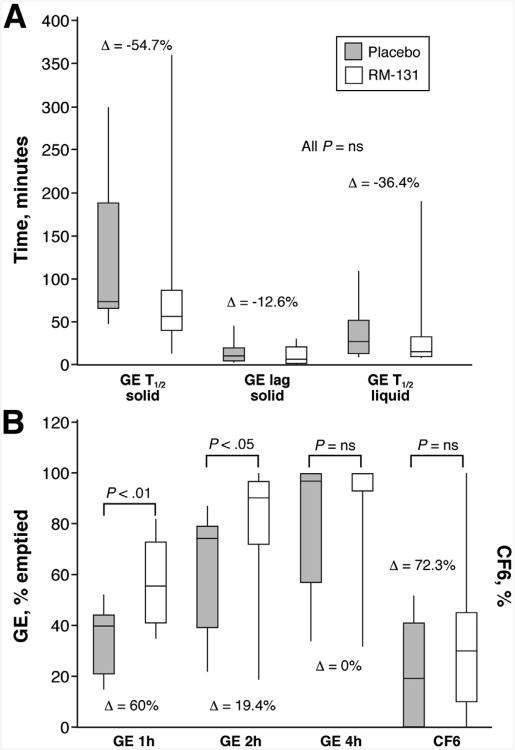
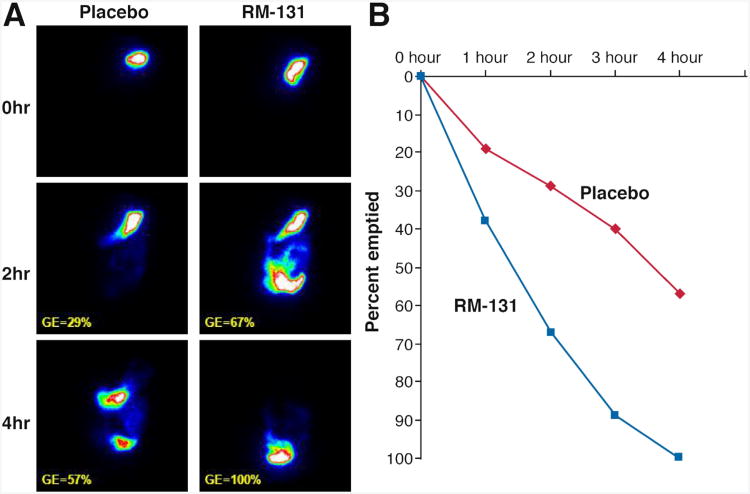
A summary of the effects of RM-131 on main transit parameters is shown in Figure 3. RM-131 was associated with an accelerated mean solid GE t1/2; the estimated treatment effect mean difference was −33.8 ±31.0 (SEM) minutes (P = NS). Similarly, the median solid GE t1/2 was faster with a median difference of −33.9 minutes (IQR, −12 to −49), equivalent to a −54.7% (IQR, −21 to −110) decrease relative to the overall mean (P = NS). RM-131 significantly increased GE of solids at 1 (P = .005) and 2 (P = .019) hours, with a mean difference of 24.7% ± 14.7% (SEM) relative to the overall mean at 2 hours. Figure 4 shows actual results of an abdominal scintigraphy in 1 patient with improvement in GE with RM-131 compared with placebo.
Figure 3.
Effect of RM-131 on main transit measurements in all 10 participants (GE, minutes for solids and liquids [upper panel]; percentage GE at 1, 2, and 4 hours, and CF6 at 6 hours [lower panel]). Gray, placebo; white, RM-131 100 μg. Data are median (IQR and range); P values by Wilcoxon signed rank test or paired t test (for GE 1 hour and 2 hour) comparing RM-131 versus placebo. Δ, median percentage difference for each end point. Data were not available for 1 participant for GE 1 hour.
Figure 4.
Assessment of GE of solids by scintigraphy in 1 patient showing delayed GE with placebo and normal GE with RM-131 (left panel). GE is shown as the percentage emptied over time for placebo and RM-131 in the same individual (right panel).
The presence of cardiovagal dysfunction did not modulate the effect of RM-131; thus, the drug-placebo differences were not different in those with or without cardiovagal dysfunction (data not shown).
There were numeric accelerations of liquid GE t1/2, GE solid lag time, and small-bowel transit (CF6), with mean percentage differences relative to the corresponding overall mean values of 30%, 41%, and 76%, respectively; however, these differences were not statistically significant.
Average Symptom Scores
Although patients participating in the study generally had lower symptom scores than at screening, clinically important treatment effects (Table 2) were observed with a significant reduction in total GCSI-DD (Hochberg adjusted P = .041, P =.026 by paired t test) and NVFP scores (Hochberg adjusted P =.041, P = .041 by paired t test) with RM-131 when compared with placebo.
Table 2. Summary of Main Transit, Symptoms, and Glycemic Indicators in All 10 Patients in the Randomized, Cross-Over Study.
| Placebo | RM-131 | P value | % Differencea | |
|---|---|---|---|---|
| GE t1/2 solid, min | 75.7 (66.4–188.6) | 58.2 (40.4–86.6) | NS | −54.7 |
| GE solid lag time (t10% GE), min | 9.0 (5.0–20.0) | 5.9 (2.8–21.4) | NS | −12.6 |
| GE at 1 h (%) | 40.3 (21.0–44.4) | 55.6 (40.5–73.1) | .005b | 60.0 |
| GE at 2 h (%) | 74.6 (38.5–79.4) | 88.7 (72.2–97.4) | .019b | 19.4 |
| GE at 4 h (%) | 97.1 (57.2–100) | 100 (92.9–100) | NS | 0 |
| GE t1/2 liquid, min | 26.4 (12.7–51.92) | 13.0 (10.4–32.6) | NS | −36.4 |
| CF6 solid, % | 19.0 (0.0–41.0) | 28.5 (10.0–45.0) | NS | 72.3 |
| Total GCSI-DD average score | 0.79 (0.75–2.08) | 0.17 (0.00–0.67) | .041c | −125.0 |
| Average score of combined NVFP | 1.00 (0.50–2.00) | 0.25 (0.00–0.50) | .041c | −141.8 |
| Blood glucose level at 120 min, mg/dL | 248 (182–273) | 231 (152–290) | NS | −11.4 |
NOTE. Data show median (IQR).
NS, not significant.
Median percentage difference among all participants for RM-131 minus placebo (within patient) relative to overall means (within patient); 100× ([within subject delta] / [within subject mean]). Data were compared using the Wilcoxon signed rank test unless otherwise noted.
Data were compared using the paired t test.
Data were compared using the paired t test with Hochberg21 step-up correction; 1 participant had missing 1-hour GE data at both study visits.
Glucose Levels
The median fasting blood glucose level before GE assessment was 165.0 mg/dL (IQR, 126–198) for placebo and 147.0 mg/dL (IQR, 133.0–167.0) for RM-131. Blood glucose levels at 120 minutes after the meal are shown in Table 2, and show no clinically important differences.
Safety and Tolerability of Single-Dose RM-131
RM-131 was generally well tolerated. A summary of all AEs is shown in Supplemental Table 1. No AEs were serious or significant. Only hunger was noted to be of borderline significance (P = .063) and was observed more frequently with RM-131. All AEs resolved spontaneously. No clinically important effects on physical examination, electrocardiogram, vital signs, or routine clinical laboratory tests were observed.
Discussion
The current study expands on our previous findings15 in which we showed that a single subcutaneous injection of RM-131 significantly accelerated GE t1/2 of solids by 66% compared with placebo in patients with T2DM and documented DGE. In the current study, we showed a 54.7% improvement in GE t1/2 solid in T1DM. RM-131 also significantly accelerated GE at 1 and 2 hours. A recent investigation of the ghrelin-receptor agonist, TZP-102,12 showed a lack of effect on GE in diabetic gastroparesis patients. TZP-101 showed improvement of GE T1/2 of solids by approximately 20%10 in diabetic patients with moderate to severe upper GI symptoms and confirmed DGE by baseline scintigraphy. Interestingly, 4 of 10 patients in the latter study had GE t1/2 values within the normal range10 with placebo treatment, similar to observations with placebo treatment in the current study. RM-131 was optimized based on the peptide characteristics of native ghrelin in contrast to TZP, which was optimized for binding of the growth hormone secretagogue-1a receptor.22
Assessment of symptoms with the use of a validated instrument showed significant improvement in total GCSI-DD and NVFP scores with RM-131. Most patients had mild to moderate symptoms at baseline and symptoms were generally fewer during study participation, potentially limiting our ability to assess effects of RM-131 on symptoms. Although it may be hypothesized that this improvement may reflect a time-dependent change in symptoms, symptom improvement may have resulted from treatment with either RM-131 or placebo. There was a 50% improvement in symptoms from baseline to placebo treatment observed in a study of TZP-101.23 Furthermore, the excellent test-retest reliability of GCSI-DD scores among gastroparesis patients has been shown previously,19 suggesting that the improvement in symptoms is a true treatment effect rather than a time-dependent change. The magnitude of effect on the main symptoms and the GCSI-DD observed in this study is consistent with the effect sizes observed in a prior study validating the response elements of the GCSI-DD and its individual components.19 This study was not specifically powered for assessment of symptoms scores, which was a secondary end point. Further investigation in patients with moderate to severe symptoms is needed; however, these data provide preliminary evidence in support of the clinical effectiveness of RM-131 in the treatment of diabetic gastroparesis.
We were unable to show significant change in our primary end point, GE t1/2, with RM-131. However, the ability to observe a statistically significant effect may have been attenuated by the relatively normal GE t1/2 observed in a number of the participants. We did not assess baseline GE by scintigraphy predominantly because of the radiation exposure that would have been necessitated by 3 procedures in a relatively short period. Only 3 patients had GE t1/2 solids of longer than 110 minutes during the placebo treatment after consuming the low-fat test meal, illustrating the potential impact of placebo in studies of gastric motor functions24 as well as the significant variability in GE rate on replicate studies.20 However, even with this wide range of GE t1/2, RM-131 accelerated GE at 1 and 2 hours. Acceleration of GE at 1 hour was significant even with correction for 5 primary and secondary end points (Hochberg21 adjusted P = .025). The fact that acceleration of GE was greatest in patients with the highest GE t1/2 on placebo is not surprising and likely reflects a floor effect in those with normal or fast GE t1/2. Such a floor effect with the relatively fast GE of solids of a low-fat meal likely contributed to a lack of detectable differences in GE at 4 hours because 4 patients emptied 100% of the test meal by 4 hours during both treatments. In retrospect, given the mode of action of pharmacologic doses of ghrelin, which induced a premature phase III of the migrating motor complex,25 and effects of ghrelin on decreasing gastric accommodation,13 we should have used the GE at 1 hour as the primary end point of our study.
Future investigations in patients with moderate to severe DGE will be needed to better characterize effects of RM-131 on all phases of GE. In this study, a fasting blood glucose value of less than 275 mg/dL before study drug administration and GE assessment was achieved in all participants except one; thus, effects of hyperglycemia were not studied in the present protocol. Significant hyperglycemia, reflecting poorly controlled diabetes, may attenuate effects of RM-131 on GE, as has been shown with erythromycin in healthy volunteers.26
In summary, RM-131 significantly accelerates early GE of solids and reduces upper GI symptoms in patients with T1DM and DGE. The magnitude of the effect in T1DM is in the range observed in T2DM and does not appear to be dependent on normal vagal function. A limitation of the study was the small sample size, and statistical inferences should be interpreted with caution. A larger sample size will be required to assess the generalizability of the data. These data suggest that RM-131 deserves further study of its medium- and longer-term efficacy in diabetic patients with upper GI symptoms and DGE of solids, given the shown efficacy, lack of significant AEs, or increase in post-prandial blood glucose levels, and unmet need in the treatment of diabetic gastroparesis.2
Supplementary Material
Suppementary Table 1. Summary of AEs in All 10 Patients in the Randomized, Cross-Over Study
NOTE. The total number of AEs are shown.
P = .0625; all others, P = not significant Comparisons were performed using the McNemar test.
Acknowledgments
The authors thank Cindy Stanislav for secretarial assistance; Jill Randolph, PharmD, RPh, for preparation of dosing solutions and the randomization scheme; and Keith Gottesdiener, MD, for discussion and comments.
Funding: Supported by a National Institutes of Health Clinical Translational Science Award grant (UL1 TR000135), and a research grant from Rhythm Pharmaceuticals, Boston, MA.
Abbreviations used in this paper
- AE
adverse event
- CF
colonic filling
- CRU
Clinical Research Unit
- DGE
delayed gastric emptying
- GCSI-DD
daily diary of gastroparesis cardinal symptom index
- GI
gastrointestinal
- IQR
interquartile range
- NVFP
nausea, vomiting fullness, pain
- t1/2
the calculated time point at which 50% of the radio-labeled meal has emptied from the stomach
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
Footnotes
Conflicts of interest: This author discloses the following: Michael Camilleri serves on an advisory board for Rhythm Pharmaceuticals, but does not receive any personal remuneration; Dr Camilleri is employed by the Mayo Clinic, which receives compensation on an hourly basis for his work in an advisory capacity. The remaining authors disclose no conflicts.
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2013.04.019.
References
- 1.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 4.Choung RS, Locke GR, 3rd, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82–88. doi: 10.1038/ajg.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talley NJ, Young L, Bytzer P, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71–76. doi: 10.1111/j.1572-0241.2001.03350.x. [DOI] [PubMed] [Google Scholar]
- 7.Hyett B, Martinez FJ, Gill BM, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology. 2009;137:445–452. doi: 10.1053/j.gastro.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 8.Sanger GJ, Westaway SM, Barnes AA, et al. GSK962040: a small molecule, selective motilin receptor agonist, effective as a stimulant of human and rabbit gastrointestinal motility. Neurogastroenterol Motil. 2009;21:657–664. e30–1. doi: 10.1111/j.1365-2982.2008.01270.x. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N, Koba N, Yamamoto T, et al. W1390 characterization of a novel, potent, and selective small molecule motilin receptor agonist, RQ-00201894. Gastroenterology. 2010;138:S–713. [Google Scholar]
- 10.Ejskjaer N, Vestergaard ET, Hellstrom PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 11.Wo JM, Ejskjaer N, Hellstrom PM, et al. Randomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting–randomised clinical study subset data. Aliment Pharmacol Ther. 2011;33:679–688. doi: 10.1111/j.1365-2036.2010.04567.x. [DOI] [PubMed] [Google Scholar]
- 12.Ejskjaer N, Wo JM, Esfandyari T, et al. Aphase 2a, randomized, double-blind 28-day study of TZP-102 a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25:e140–e150. doi: 10.1111/nmo.12064. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2009;6:343–352. doi: 10.1038/nrgastro.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin A, Camilleri M, Busciglio I, et al. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes Care. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology. 2006;131:1717–1724. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–680. doi: 10.1111/j.1365-2036.2009.04078.x. [DOI] [PubMed] [Google Scholar]
- 19.Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD) Neurogastroenterol Motil. 2012;24:456–463. e215–6. doi: 10.1111/j.1365-2982.2012.01879.x. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012;24:1076–e562. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochberg Y. A sharper Bonferroni procedure for multiple significance testing. Biometrika. 1988;75:800–802. [Google Scholar]
- 22.Bochicchio G, Charlton P, Pezzullo JC, et al. Ghrelin agonist TZP-101/ulimorelin accelerates gastrointestinal recovery independently of opioid use and surgery type: covariate analysis of phase 2 data. World J Surg. 2012;36:39–45. doi: 10.1007/s00268-011-1335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejskjaer N, Dimcevski G, Wo J, et al. Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2010;22:1069–e281. doi: 10.1111/j.1365-2982.2010.01519.x. [DOI] [PubMed] [Google Scholar]
- 24.Mearin F, Balboa A, Zárate N, et al. Placebo in functional dyspepsia: symptomatic, gastrointestinal motor, and gastric sensorial responses. Am J Gastroenterol. 1999;94:116–125. doi: 10.1111/j.1572-0241.1999.00781.x. [DOI] [PubMed] [Google Scholar]
- 25.Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayner CK, Su YC, Doran SM, et al. The stimulation of antral motility by erythromycin is attenuated by hyperglycemia. Am J Gastroenterol. 2000;95:2233–2241. doi: 10.1111/j.1572-0241.2000.02250.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppementary Table 1. Summary of AEs in All 10 Patients in the Randomized, Cross-Over Study
NOTE. The total number of AEs are shown.
P = .0625; all others, P = not significant Comparisons were performed using the McNemar test.