Abstract
Chronic kidney disease–mineral and bone disorder (CKD-MBD) is the term used to describe a constellation of biochemical abnormalities, bone disturbances that may lead to fractures, and extraskeletal calcification in soft tissues and arteries seen in CKD. This review focuses on the non-invasive diagnosis of renal osteodystrophy, the term used exclusively to define the bone pathology associated with CKD. Transiliac bone biopsy and histomorphometry with double-labeled tetracycline or its derivatives remains the gold standard for diagnosis of renal osteodystrophy. However, histomorphometry provides a “window” into bone only at a single point in time, is invasive, and not practical to study continuous changes in bone morphology. Further, CKD is a risk factor for fractures, and the etiology is multi-factorial and not fully explained by histomorphometry findings alone. The propensity of a bone to fracture is determined by bone strength, which is affected by bone mass and bone quality; the latter is a term used to describe the structure and composition of bone. Bone quantity is traditionally assessed by Dual X-ray Absorptiometry (DXA) and CT based methods. Bone quality is more difficult to assess non- invasively, but newer techniques are emerging and described in this review. Ultimately, the optimal diagnostic strategy for renal osteodystrophy may be a combination of multiple imaging techniques and biomarkers that are specific to each gender and race in CKD with a goal of predicting fracture risk and optimizing therapy.
Renal Osteodystrophy: Then and Now
Kidney Disease has been known to be associated with bone abnormalities for decades. As early as 1883, Lucas recommended the term “renal rickets” for the bone deformities associated with albuminuria(1). In 1943, Liu and Chu first used the term renal osteodystrophy as “the generic name to include cases of osseous disorder associated with renal insufficiency, while the exact nature of the pathological process in the skeleton is still undetermined”(2). The term “renal osteodystrophy” was then used to variably describe bone histology findings, skeletal abnormalities, and disordered biochemical and hormone levels (calcium, phosphorus, parathyroid hormone, vitamin D) associated with kidney disease through the rest of the 20th century.
In 2005 expert nephrologists in the field of bone and mineral disease felt the term renal osteodystrophy did not completely depict the full spectrum of systemic symptoms associated with mineral and bone disorders in CKD. The term Chronic kidney disease–mineral and bone disorder (CKD-MBD) was coined to encompass a constellation of abnormalities seen in progressive kidney disease that include 1) altered levels of calcium, phosphorus, parathyroid hormone (PTH), and vitamin D; 2) disturbances in bone modeling and remodeling, with the associated development of fractures or impaired linear bone growth (in children); and 3) extraskeletal calcification in soft tissues and arteries(3). It was recommended that the term renal osteodystrophy be used exclusively to define the bone pathology associated with CKD(3). Renal Osteodystrophy is one measure of the skeletal component of the systemic disorder of CKD-MBD. Transiliac bone biopsy and histomorphometry with double-labeled tetracycline or its derivatives remains the gold standard for diagnosis of renal osteodystrophy.
Bone in CKD
Bone remodeling is an ongoing lifelong process. Bone resorption occurs by osteoclasts and new bone or osteoid, is formed by osteoblasts. The new bone formed is mineralized to become mature bone. This process relies on complex cell signaling pathways to achieve coupling between these various processes. Together these processes control replacement of bone following micro-fractures that routinely occur with physical activity, thus maintaining bone architecture. At any given time, about 10–20% of the skeleton undergoes remodeling and a typical remodeling cycle can take up to 3–6 months(4). The mineral and endocrine functions disrupted in CKD are critically important in the regulation of bone remodeling. As a result, bone abnormalities are found almost universally in patients with CKD requiring dialysis and in the majority of patients with CKD Stages 3–5(5–7). The ultimate assessment of bone remodeling abnormalities is altered bone strength leading to fractures. Dialysis patients in their 40s have a relative risk of hip fracture 80-fold that of age and sex-matched controls(8). In patients with stage 4 CKD, the risk of hip fracture was nearly 4-fold that of the general population without CKD(9). Therefore identifying imaging techniques and biomarkers that can non-invasively identify those at risk for fractures is important both from a research perspective as well as for patient care.
Bone Strength and Fractures in CKD
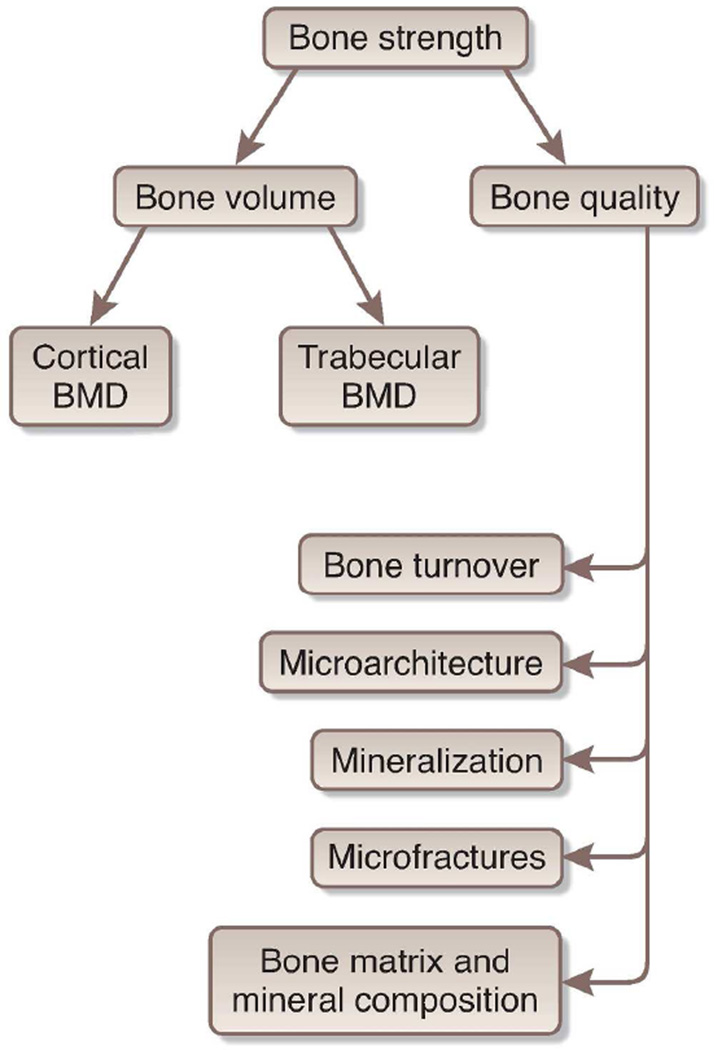
Fracture risk is determined by bone strength, or the ability of a bone to resist breakage. Both cortical and trabecular bone are important for bone strength - cortical bones resist bending or buckling, and trabeculae distribute force in cancellous bone. Bone strength is composed of both bone quantity and quality(10). Bone quantity is traditionally measured by bone mineral density (BMD) using Dual Energy X-Ray Absorptiometry (DXA), though newer CT based measures are now available. Bone quality is determined by bone turnover and mineralization (assesed by histomorphometry) as well as microarchitecture such as geometry, connectivity, and collagen cross-linking (see Figure 1). Microarchitecture of bone has been predominately evaluated in animal models but recent MRI techniques hold promise in humans. In CKD, metabolic abnormalities, altered bone cell differentiation pathways and disturbances in bone remodeling likely result in deterioration in bone quality. Thus, it is not surprising that there is increased fracture risk in CKD with abnormalities of both bone quantity and quality. Table 1 lists bone strength measurement techniques.
Figure 1. Determinants of Bone Strength.
Legend: Bone strength is comprised of both bone density and quality. Bone quality refers to bone turnover, microarchitecture, micro-fractures, mineralization as well as the composition of mineral matrix. Trabecular microarchitecture includes trabecular thickness, the ratio of plates and rods, their connectivity and spacing. Cortical microarchitecture includes cortical thickness, porosity and bone size. Composition of mineral matrix includes changes in the cross-linking of type 1 collagen and alterations in the size and structure of bone mineral. Bones accumulate microfractures over time even with normal physical activity. The ability to repair these affects bone quality.
Table 1.
Techniques to Measure Bone Parameters
| Bone measure | Technique |
|---|---|
| Total Bone Density | DXA |
| Cortical and Trabecular bone Density |
QCT, pQCT |
| Bone Turnover | Biomarkers (PTH, b-alp, Sclerostin) Histomorphometry |
| Microarchitecture | HRpQCT, HRMRI, histomorphometry, microCT, microMRI |
| Matrix composition | Infrared spectroscopy, Raman spectroscopy |
| Microfractures | Confocal microscopy, histology |
| Mineralization | Histomorphometry, spectroscopic techniques |
Bone histomorphometry
The clinical assessment of bone remodelling is best done with a bone biopsy, usually of trabecular bone at the iliac crest. The patient is given a tetracycline derivative approximately 3 to 4 weeks before the bone biopsy and a different tetracycline derivative 3 to 5 days before the biopsy. Tetracycline binds to hydroxyapatite and emits fluorescence, thereby serving as a label of the bone to allow assessment of bone change over time, termed dynamic assessment.
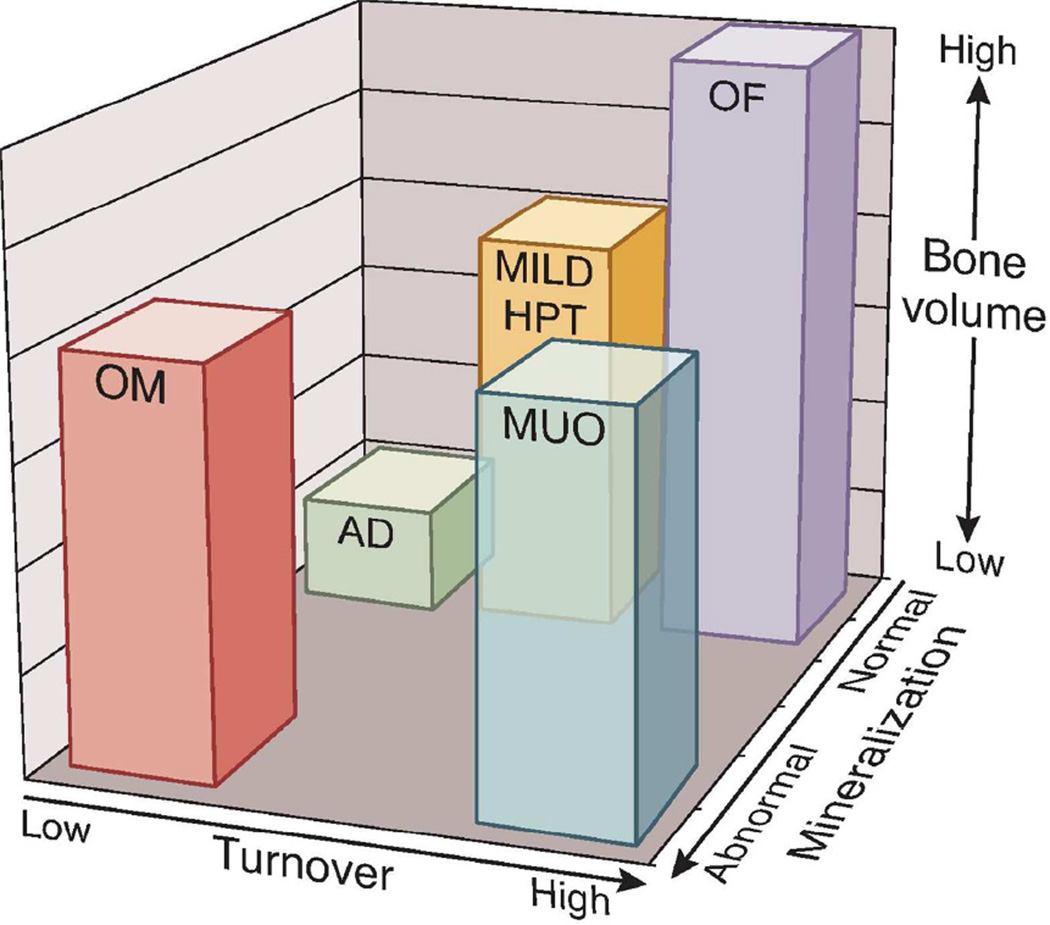
In 1983, Sherrard and others(11) proposed a classification system for the histomorphometric analysis of renal bone disease using parameters of bone turnover, percentage of unmineralized bone (osteoid) area as a percent of total bone area and fibrosis to distinguish the various forms of renal osteodystrophy. Three groups were named: high turnover disease (mild hyperparathyroidism or severe hyperparathyroidism with fibrosis called osteitis fibrosa cystica),low turnover bone disease (adynamic bone disease or osteomalacia) and mixed uremic osteodystrophy. Aluminum bone disease was diagnosed by special staining for aluminum deposits at the mineralization front. The focus on this classification scheme was on bone turnover, and at the time, it was felt that PTH was the main regulator of bone remodeling and therefore the primary noninvasive biomarker used. The prevalence of these various forms of renal osteodystrophy has changed over the years. There has been a decrease in osteomalacia and aluminum disease and an increased prevalence of adynamic bone disease (37 – 60% of dialysis patients), with a notably stable or slight reduction in the proportion of high bone turnover disease at 40 – 50%(12–14). These changes may be the result of a change in patient characteristics (more elderly patients and increased prevalence of diabetes) and/or treatment modalities. Recently the KDIGO initiative also standardized the nomenclature for reporting bone biopsy to reflect the role of turnover and also incorporate mineralization and volume (Figure 2)(15). The latter is a reflection of both the amount of bone at the onset of kidney disease and the severity and duration of the abnormalities with kidney disease. For example, if a patient starts dialysis after severe bone loss from post-menopausal or corticosteroid induced bone disease, the bone volume may be low even if remodeling is normal. If this same patient develops severe osteitis fibrosa, the resulting bone volume will be even lower than another patient with the same magnitude of osteitis fibrosa who may have had normal bones when starting dialysis.
Figure 2.
The figure is a graphical example of how the TMV system provides more information than the present, commonly used classification scheme. Each axis represents one of the descriptors in the TMV classification: turnover (from low to high), mineralization (from normal to abnormal), and bone volume (from low to high). Individual patient parameters could be plotted on the graph, or means and ranges of grouped data could be shown. For example, many patients with renal osteodystrophy cluster in areas shown by the bars. The red bar (OM, osteomalacia) is currently described as low-turnover bone with abnormal mineralization. The bone volume may be low to medium, depending on the severity and duration of the process and other factors that affect bone. The green bar (AD, adynamic bone disease) is currently described as low-turnover bone with normal mineralization, and the bone volume in this example is at the lower end of the spectrum, but other patients with normal mineralization and low turnover will have normal bone volume. The yellow bar (mild HPT, mild hyperparathyroid-related bone disease) and purple bar (OF, osteitis fibrosa or advanced hyperparathyroid-related bone disease) are currently used distinct categories, but in actuality represent a range of abnormalities along a continuum of medium to high turnover, and any bone volume depending on the duration of the disease process. Finally, the blue bar (MUO, mixed uremic osteodystrophy) is variably defined internationally. In the present graph, it is depicted as high-turnover, normal bone volume, with abnormal mineralization. In summary, the TMV classification system more precisely describes the range of pathologic abnormalities that can occur in patients with CKD.
From Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69(11):1945–53.
Bone histomorphometry findings provide tissue-level evidence of changes in turnover, mineralization and volume and thereby some aspects of bone quality. However, bone biopsies performed at the iliac crest may not reflect concurrent changes in bone in the vertebrae. Also histomorphometry depicts a single point in time and becomes impractical to perform repetitively to assess response to treatment. Historically, bone histomorphometry has evaluated only the changes in the trabecular bone, although cortical bone thickness and porosity are equally important in determining fracture risk(16, 17). This is especially important in CKD since hyperparathyroidism can cause thinning in the cortex(18). Simultaneously in the trabeculae, increased remodeling and increased bone volume may be observed, although new trabeculae may be irregular and lack connectivity and strength(18). There are no prospective studies of different histomorphometric patterns and the risk of fracture in CKD. Given the invasiveness of the procedure and limited availability of physicians trained to perform and interpret the results, long term prospective studies have not been feasible. Despite these problems, bone biopsy gives a true “picture” into the bone in CKD patients and remains the gold standard for the diagnosis of renal osteodystrophy, one component of CKD-MBD. However, it may not be the best test to study renal osteodystrophy progression and response to therapies due to the factors above. Therefore, there is an ongoing quest for biomarkers and imaging methods to study renal osteodystrophy.
Dual Energy X-Ray Absorptiometry (DXA)
Dual Energy X-Ray Absorptiometry (DXA) measures areal bone mineral density (aBMD) in gm/cm2 using minimal radiation and rapid scan times. BMD assessment by DXA has good reproducibility (<1–2% coefficient variation) and reliable reference ranges for age, gender, and race (19). The World Health Organization defines osteoporosis based on bone density 2.5 standard deviations below the mean for young white adult women (t-score) (20). In the general population, aBMD by DXA is an accepted surrogate end point after prospective studies demonstrated an age dependent predictive value of DXA for fractures(21). However, studies with some anti-osteoporosis agents such as sodium fluoride showed improved DXA yet worsened fractures. Other therapies have a much larger effect on fractures than on DXA(22).These findings led to increased appreciation of the importance of bone quality, which is not assessed by DXA, and forced the use of fractures as end points for approval of new therapeutics for the treatment of osteoporosis.
The KDIGO CKD-MBD guideline recommends DXA to assess fracture risk in patients with stage 1 through early stage 3 CKD, as long as biochemical testing does not suggest CKD-MBD(23). However, in CKD stages 3b–5, the guideline did not recommend DXA due to the lack of definitive data demonstrating that DXA predicts fracture in CKD-MBD. DXA assessed areal bone mineral density (aBMD) and therefore is only assesses bone mass not quality in both CKD and non-CKD patients. DXA does not correlate with bone histomorphometry or provide information on bone microarchitecture. It also does not provide compartmental assessment (cortical vs. trabecular bone), though using DXA at the ultradistal radius for fracture prediction is a valid assessment of cortical bone (the ultradistal radius is nearly all cortical bone).
However, multiple studies evaluating patients with CKD have utilized DXA and demonstrated use of this assessment technique. Secondary analyses of subjects with eGFR< 60 showed that BMD was predictive of fractures in intervention trials of bisphosphonates, teriparatide, denosumab, and raloxifene(24–26). In 2007, Jamal et al published a meta-analysis of six studies totaling 683 dialysis subjects that showed that those with fractures had significantly lower aBMD at all sites except for the femoral neck. The standardized mean difference between patients with fractures and those without fractures was largest for the ultradistal radius : −1.24 (−2 to –0.5)(27). Another cross-sectional study in 144 dialysis patients showed that those with fractures had significantly lower aBMD at the distal radius than those without fractures (28). More recently, an important study by Yenchek et al. analyzed the effect of CKD on fracture risk prediction by DXA in 587 older adults with CKD, enrolled in the Health, Aging and Body Composition Study. In this study with median follow-up of 11.3 years, prevalent osteoporosis and each SD decrease in BMD at the femoral neck was associated with greater than a 2-fold increased risk of fracture in patients with CKD compared to those without CKD(29). These data therefore support that DXA can predict fracture risk in patients with CKD. Importantly, DXA remains an inexpensive, widely available technique that can be easily standardized across sites. Given this reliability, DXA is likely a good tool in longitudinal CKD research studies for the serial assessment of bone mineral density in response to interventions. Unfortunately, to date, such studies are too limited in size and duration.
Quantitative Computerized tomography (QCT) and Peripheral Quantitative Computerized Tomography (pQCT)
QCT allows 3D imaging of cross-sections of the central and axial skeleton to provide spatial or volumetric bone mineral density (vBMD). It also allows distinction between cortical and trabecular compartments. The technique also allows the calculation of biomechanical parameters such as the ability of a bone to resist bending or torsion, which affect fracture risk by using special software. In CKD, QCT measures of trabecular bone density at the spine have been correlated with trabecular bone volume histomorphometry(30). A single study of 72 dialysis patients showed that prevalent vertebral fractures were best predicted by L1-L3 cortical BMD and that every 1 mg increase of bone mineral content in cortical bone; about 1 mg was associated with 4% decrease of fracture risk(31).
Peripheral QCTs (pQCTs) avoid the large dose of ionizing radiation exposure for patients by focusing on the tibia and distal radius. A cross-sectional study in 52 hemodialysis patients showed that decreases in distal radial cortical vBMD, cortical area, cortical thickness as well as decreases in torsional strength and bending strength were all significantly associated with odds of a fracture(32). Although this is a single study, these results are in line with expected associations between loss of bone quality and fracture risk in dialysis patients. Currently, pQCTs are not available for clinical purposes but show promise as predictors of fracture risk if similar results are shown in future larger prospective studies.
High Resolution Peripheral computerized Tomography (HRpQCT)
The resolution of QCTs (0.5 mm) is not sufficient for detection of trabecular architecture disruption. However, the resolution of HRpQCT is 100 µm which allows evaluation of trabecular microarchitecture (bone volume fraction, trabecular thickness, separation and number) in addition to the parameters measured by QCTs and pQCTs as detailed above. HR-pQCTof the radius and tibia was used to discriminate those with and without fractures in a cross-sectional study of 74 stage 5D CKD patients (with 30 prevalent fractures) and 40 controls without kidney disease or fractures(32, 33). All HR-pQCT parameters were found to differ significantly between patients with and without a history of fracture at the tibia except trabecular thickness. In contrast, at the radius, only trabecular number and trabecular thickness was significantly different in those with fractures and those without fractures(33, 34). This is surprising since trabecular vBMD was not associated with fractures in the pQCT study described above(32, 34).
In a HR-pQCT study in CKD stages 2–5, there was a difference in parameters in patients with and without a history of fractures(35). However, the power of the various HRpQCT parameters to individually discriminate between those with and without fractures by ROC (receiver operating curve) analyses was <0.75. When considering patients with the longest duration of CKD, the AUC improved to >0.8 for multiple parameters including radial cortical thickness, radial total vBMD and cortical vBMD. Interestingly, aBMD by DXA of the ultradistal radius performed similarly in its ability to discriminate prevalent fractures in this sub-population with the longest duration of kidney disease(35). In another study the discriminatory capacity of DXA was compared to HRpQCT in 211 patients with CKD 3–5, of whom 74 had fractures by X-ray or self-report. The greatest area under the ROC curve was obtained for aBMD by DXA at the ultradistal radius (AUC: 0.80; 95% confidence interval 0.74 to 0.87) even when compared to HRpQCT measures. The addition of vBMD and cortical thickness to aBMD at the ultradistal radius did not improve discrimination significantly(36). Therefore, high resolution computerized tomography techniques do provide assessment of bone architecture but their use at this time is limited to research centers and their additive value over available biomarkers and methods has not yet been proven.
Finite-element analysis (FEA) has been used to reconstruct HRpQCT images to study large regions of trabecular bone. This method allows for testing local stress and strain distributions in the trabeculae, as well as calculations of the percentage of the total load that is carried by trabecular bone(37). Recently, Trombetti et al. compared FEA for failure loading and stiffness in 33 hemodialysis patients and age and gender matched controls using HR-pQCTs. This population of women dialysis patients had lower radial cortical porosity and more disturbances of trabecular microarchitecture compared to controls. Consistent with this, both stiffness and predicted failure load in the distal radius and tibia were lower in women with ESRD, but not in men, compared with age-matched controls(38). The number of post-menopausal women was the same in both study groups, excluding menopausal state as the cause for this observed difference.
High-resolution MRI (HR-MRI) allows 3D imaging of the bone geometry and trabecular microarchitecture at peripheral sites. A critical benefit of this technique is its ability to generate information without ionizing radiation. HR-MRI imaging was used to analyze the trabecular bone structure of the calcaneus to compare this technique with bone mineral density (a BMD) by DXA in predicting therapy-induced bone loss and prevalent fractures. The study was done in 48 patients before and 12 patients after kidney transplantation respectively and 20 controls.. The strongest discriminators between patients with and without fractures were trabecular parameters, even after adjustment for age and BMD. Using receiver operating characteristic analysis the highest diagnostic performance was found for a combination of BMD and architecture measures AUC=0.85(39).
Micro-MRI is a technique that can be used to study bone architecture with excellent spatial resolution almost similar to an actual bone biopsy. Trabecular orientation and structure are clearly elucidated (40). A study of 17 hemodialysis patients with secondary hyperparathyroidism showed disruptions of the distal tibial trabecular network as compared to controls (40). There are no studies comparing this technique to other imaging methods in the ability to predict fractures in CKD.
Experimental technologies have been utilized in animal studies but are not yet utilized in humans. These include microCT which has very high spatial resolution and can assess many components of bone architecture(41). To measure the degree of mineralization, techniques such as quantitative back-scattered electron imaging and spectroscopy are used(42). Collagen morphology can be assessed by atomic force microscopy (43). Raman and Fourier transform infrared spectroscopy are used to study collagen cross linking and other features of the matrix(44). These techniques allow for more detailed examination of parameters of bone quality.
In summary, there are multiple newer imaging techniques that are available for non-invasive assessment of bone, especially in the research setting. DXA is the only imaging modality that is widely available for clinical purposes and the only modality standardized enough to be used as end points in clinical trials. Many of the other techniques have been studied only in cross-sectional studies in small populations of CKD patients. Prospective data to validate the ability of each method in predicting fracture risk in CKD is lacking, but will hopefully be a focus of future research and funding.
Blood Bone Biomarkers
An ideal biomarker for renal osteodystrophy should be able to detect the loss of bone quality and/or quantity early in CKD and/or predict future fractures. An ideal biomarker should be easily and reliably measured in a noninvasive or minimally invasive manner and be inexpensive to measure with low variability based on circadian rhythm. Further, an ideal biomarker should also be one that can be monitored serially in response to treatment and demonstrate predictive ability to discriminate between those with and without disease. Additionally in the CKD population, an ideal biomarker should not accumulate with GFR loss and should not be cleared with hemodialysis.
Bone is a “dynamic” organ with 10–20% constantly in a state of remodeling. The control of bone remodelling is highly complex but appears to occur in very distinct phases (1) osteoblast activation, (2) osteoclast recruitment and resorption, (3) preosteoblast migration and differentiation, (4) osteoblast deposition of matrix (osteoid or unmineralized bone), (5) mineralization, and (6) quiescent stage (4)(36) Each step of this chain is coupled to the subsequent one by signalling mechanisms. One of these is the osteoprotegerin (OPG) and receptor activator of nuclear-factor κB (RANK) system that explains the coupling of osteoblasts and osteoclasts. This control system is regulated by nearly every cytokine and hormone thought important in bone remodelling, including PTH, calcitriol, estrogen, glucocorticoids, interleukins, prostaglandins, and members of the TGF-β superfamily of cytokines (45). The role of uremia in regulation or dysregulation of this system is not completely understood. Given the bone milieu is so complex, it is unreasonable to expect that a single biomarker at a single point in time could portray all these changes, even in the absence of kidney disease. It is therefore not surprising that multiple biomarkers have been developed and can be broadly classified into markers of turnover, markers of bone formation and markers of bone resorption.
Biomarkers used in Clinical Practice for the care of Kidney Patients
Parathyroid hormone (PTH) and total bone specific alkaline phosphatase (b-alp) are biomarkers that may reflect bone turnover and bone formation and are currently used in clinical practice despite some assay limitations (Table 2). Extremes of PTH generally signify extremes of bone turnover; the KDIGO guideline suggests “maintaining intact PTH levels in the range of approximately two to nine times the upper normal limit for the assay in stage 5 CKD” in order to reflect these extremes where the predictive value of PTH is high(23). Unfortunately, the ability of PTH values to predict underlying bone histology is less discriminatory when within the 2–9 times normal level. B-alp or total alkaline phosphatase do not appear to have significantly higher positive predictive value as compared to PTH for underlying bone histology per a recent large analysis(46). Total alkaline phosphatase is not as specific as b-alp. Vitamin D (25(OH)vitamin D) is also routinely measured to detect vitamin D insufficiency/deficiency. Low levels are associated with osteomalacia and hip fractures, presumably due to mineralization defects. However, the data is less robust in CKD and the subject of many recent reviews and a recent Institute of Medicine report and thus will not be covered further(28, 47–49).
Table 2.
Bone Biomarkers used in Clinical Practice
| Biomarker | Sample collection and Assay | Predictor of histomorphometry |
Predictor of Fractures |
|---|---|---|---|
| PTH |
|
||
| Bone Specific Alkaline Phosphatase b-alp) |
|
|
PTH measured by the intact assay (Elecsys PTH 91–84) assay, Roche Diagnostics corporation, Indianapolis, IN, USA) was equally predictive to bone-specific alkaline phosphatase (BAP) of underlying bone turnover with a sensitivity of 0.58 vs 0.403, a positive predictive value of 0.373 vs 0.287, and a negative predictive value of 0.903 vs 0.877 (PTH vs BAP, respectively) for the detection of increased bone formation rates. The two together did not improve sensitivity or specificity (46).
Bone Turnover Markers (table 3)
Table 3.
Serum Bone Turnover Markers
| Serum Markers of Bone Resorption |
Levels dependent on eGFR (cleared renally)? |
|---|---|
| Serum amino-terminal cross- linking telopeptide of type 1 collagen (s-NTX) |
Yes |
| Serum carboxy-terminal cross-linking telopeptide of type 1 collagen (s-CTX) |
Yes |
| Carboxy-terminal cross linking telopeptide of type 1 collagen (s-ICTP or CTX-MMP) |
Yes |
| Serum tartarate-resistant acid phosphatase (TRAP5b) |
No |
|
Serum Markers of Bone Formation |
|
| Serum osteocalcin | Yes |
| Serum alkaline phosphatase | No |
| Bone specific alkaline phosphatase |
No |
| Procollagen type 1C propeptide (s-PICP) |
Yes |
| Procollagen type 1N propeptide (s-PINP) |
No |
Many bone turnover markers have been studied in the osteoporosis population for fracture prediction and to evaluate response to therapy. These bone turnover markers are released into circulation during skeletal metabolic activity and can be measured in the serum, if they are secreted into the extracellular space or in the urine when excreted in the urine.
Collagen based biomarkers
Osteoblasts secrete C- and N-terminal cleavage products of type 1 procollagen called Procollagen type 1N propeptide (s-P1NP) and Procollagen type 1C propeptide (s-P1CP), which are markers for bone formation. During bone formation pyridinoline cross-links bind collagen molecules together. Thus, serum carboxy-terminal cross-linking telopeptide of type 1 collagen (s-CTX) and serum amino-terminal cross-linking telopeptide of type 1 collagen (s-NTX) cross-linking telopeptide of bone collagen are measured as fragments of cross-links that are released when bone is resorbed. Among the serum biomarkers listed in table 3, many are also dependent on renal excretion. For example, osteocalcin is produced by osteoblasts during bone formation and is excreted by the kidney and its levels correlate with eGFR. The International Osteoporosis Foundation recommends that levels of s-PINP and s-CTX be used as reference standards for bone formation and bone resorption respectively in observational and interventional studies in the general population. However, each biomarker needs to be re-evaluated and studied prospectively in CKD and ESRD population, keeping its renal elimination and circulating fragments in consideration.
Procollagen type 1N propeptide (s-PINP) is an indicator of the synthesis of Type 1 collagen, which is a crucial step in bone formation. The most useful assay in patients with CKD appears to be one that measures only the intact s-PINP(50). Assays of total s-PINP, used in the general population recognize a smaller circulating procollagen 1 antigen in CKD, making them difficult to interpret in this population(50). S-PINP has little diurnal variation. S-PINP levels may be also useful in ESRD, where their concentrations show no significant changes during a dialysis session (as compared to other bone turnover markers such as s-CTX levels that can change as much as 30% during a single hemodialysis session)(51). Therefore, S-PINP is a potential biomarker that could be measured in future observational and interventional studies in CKD-MBD; such studies will help us classify its role in prediction of bone loss in CKD conclusively.
Tartrate Resistant Acid Phosphatase 5b (TRAP5b)
TRAP5b is released by osteoclasts during bone resorption. TRAP5b activity is measured by an immunocapture enzymatic assay with an inter-assay CV of 2·95%, an intra-assay CV of 2·15%, and is not influenced by TRAP5a activity(52). In a study of 98 CKD 3–5 patients, eGFR was not an independent predictor of TRAP5b(53). In contrast, in a study of 19 hemodialysis patients, TRAP5b insignificantly increased by 4.8 ± 2.4% as a result of a single hemodialysis session(54). There was a significant diurnal variation as well as differences with food intake in TRAP5b, however it was much less significant that other markers of resorption, such as s-CTX, at least in patients with normal kidney function(54). Overall, TRAP5b promises to be a valid marker of resorptive activity in CKD and ESRD, however studies correlating it with bone histomorphometry findings as well as prospective prediction of fractures are lacking.
Studies of biomarker prediction for bone histomorphometry or fractures in CKD
In 2009, The KDIGO guideline summarized the role of these collagen based biomarkers in predicting histomorphometry, fractures and bone mineral density based on studies published through 2007. Based on those studies the guideline had a weak (2C level) recommendation to not routinely measure bone-derived turnover markers of collagen synthesis (s-P1CP, s-P1NP) and breakdown (s-CTX, s-NTX). Since 2007, there are very few additional studies that examine correlations of these collagen based biomarkers with bone histomorphometry and no studies of their role in predicting fractures in CKD. However, there may be a potential role for combination of biomarkers with imaging for fracture prediction. A recent study illustrated this in 82 patients with CKD 3–5, 23 of whom had prevalent fractures, the highest tertile of formation (s-P1NP) and resorption (Trap-5b) markers were independently and positively associated with prevalent fracture(55). Further, when the highest tertile level of s-P1NP or Trap5b was combined with the T score at the femoral neck by DXA, it improved the discrimination of those with prior fracture over the T-score alone(55). This indicates an important future role for combining markers of bone resorption and formation and imaging in fracture prediction in clinical practice. Prospective studies in this field are needed. Most studies that attempt to correlate all these biomarkers to bone biopsy findings and fractures are of small sample size and it is important to remember that the correlations noted may be specific to only the race or ethnicity of patients studied. As we unravel the pathophysiology and regulatory mechanisms of bone remodeling additional biomarkers may prove valuable.
Biomarkers of Bone Currently used in Research Applications
Fibroblast Growth Factor 23 (FGF23) is produced by osteocytes and is elevated in patients on dialysis(56). Plasma FGF-23 levels are inversely correlated with both static and dynamic indices of osteoid mineralization in CKD5D(57). Thus, FGF23 may be a marker of bone mineralization, but more studies are needed to confirm this. Levels of FGF23 are 100 to 10000 fold higher in dialysis patients compared to healthy individuals(47). Given that FGF23 levels appear to be uniformly very high in dialysis patients, studies will need to demonstrate the clinical relevance of changes in FGF23 prior to widespread use as a biomarker in this last stage kidney population. Thus, FGF23 may be more useful in earlier stages of CKD or as a marker of cardiovascular disease or mortality(58, 59). Another biomarker of recent interest is sclerostin, the product of the SOST gene that is produced by osteocytes and is an inhibitor of the Wnt signaling pathway that leads to decreased bone formation(60). Its levels significantly decrease to increase with decreasing kidney function, even when adjusted for age(61). In a study of 60 dialysis patients, sclerostin was superior to PTH for positive prediction but PTH was superior for negative prediction of high turnover bone disease(62). In another study, sclerostin levels correlated with bone mineral density and abnormal microarchitecture assessed by pqCT(63). These promising data will need to be confirmed in additional prospective studies. There is no prospective data using serum FGF23 or sclerostin in fracture prediction published to date.
Conclusions
Fracture rates in CKD are high due to underlying abnormalities in bone quantity and quality. Bone histomorphometry evaluation is the gold standard for diagnosis of ROD. However, performing biopsies routinely is not practical; therefore the search for non-invasive methods to study bone and predict fractures is critical. The complex signaling mechanisms for bone remodeling are altered in CKD and a single biomarker may not be able to predict bone histology or fractures accurately. Imaging modalities have also become very important, but at this time the only clinically used imaging technique is the aBMD by DXA. Importantly, when performed at the ultra-distal radius DXA seems to correlate with fracture risk, though prospective studies are required. In patients with earlier stages of CKD, a recent study demonstrated other sites have at least an equivalent predictive role for fractures as in the general population.(29) DXA, however, does not inform us of bone quality. Newer imaging techniques to study bone quality are being developed and tested but are not yet standardized across sites. Ultimately, the approach may be a combination of multiple imaging techniques and biomarkers that are specific to each gender and race in CKD to predict fracture risk. More research is needed to evaluate these noninvasive approaches longitudinally in order to determine their use in predicting fractures – the bone abnormality of greatest concern.
Footnotes
Disclosures: Dr. Sharon Moe is a Consultant (receives honoraria) from Sanofi and Amgen and reports stock ownership in Eli Lily. Dr. Moorthi has no financial disclosures to report at this time.
References
- 1.Lucas RC. On a Form of Late Rickets Associated with Albuminuria. The Lancet. 1883;1(3119):993–994. [Google Scholar]
- 2.Liu SH, Chu HI. Treatment of Renal Osteodystrophy with Dihydrotachysterol (A.T.10) and Iron. Science. 1942;95(2467):388–389. doi: 10.1126/science.95.2467.388. Epub 1942/04/10. [DOI] [PubMed] [Google Scholar]
- 3.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney international. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 4.Parfitt AM. Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone. 2002;30(1):5–7. doi: 10.1016/s8756-3282(01)00642-1. [DOI] [PubMed] [Google Scholar]
- 5.Sprague SM. The role of the bone biopsy in the diagnosis of renal osteodystrophy. Semin Dial. 2000;13(3):152–155. doi: 10.1046/j.1525-139x.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 6.Malluche HH, Monier-Faugere MC. Renal osteodystrophy: what's in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol. 2006;65(4):235–242. doi: 10.5414/cnp65235. Epub 2006/04/25. [DOI] [PubMed] [Google Scholar]
- 7.Barreto FC, Barreto DV, Moyses RM, Neves CL, Jorgetti V, Draibe SA, et al. Osteoporosis in hemodialysis patients revisited by bone histomorphometry: a new insight into an old problem. Kidney international. 2006;69(10):1852–1857. doi: 10.1038/sj.ki.5000311. [DOI] [PubMed] [Google Scholar]
- 8.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney international. 2000;58(1):396–399. doi: 10.1046/j.1523-1755.2000.00178.x. Epub 2000/07/08. [DOI] [PubMed] [Google Scholar]
- 9.Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;51(1):38–44. doi: 10.1053/j.ajkd.2007.08.019. Epub 2007/12/25. [DOI] [PubMed] [Google Scholar]
- 10.Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocoritcoid-Induced Osteoporosis. Arthritis Rheum. 2001;44(7):1496–1503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, et al. The spectrum of bone disease in end-stage renal failure--an evolving disorder. Kidney international. 1993;43(2):436–442. doi: 10.1038/ki.1993.64. [DOI] [PubMed] [Google Scholar]
- 12.Jorgetti V. Review article: Bone biopsy in chronic kidney disease: patient level end-point or just another test? Nephrology (Carlton) 2009;14(4):404–407. doi: 10.1111/j.1440-1797.2009.01148.x. Epub 2009/07/01. [DOI] [PubMed] [Google Scholar]
- 13.Batista DG, Neves KR, Graciolli FG, dos Reis LM, Graciolli RG, Dominguez WV, et al. The bone histology spectrum in experimental renal failure: adverse effects of phosphate and parathyroid hormone disturbances. Calcif Tissue Int. 2010;87(1):60–67. doi: 10.1007/s00223-010-9367-y. Epub 2010/04/30. [DOI] [PubMed] [Google Scholar]
- 14.Monier-Faugere MC, Malluche HH. Trends in renal osteodystrophy: a survey from 1983 to 1995 in a total of 2248 patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(Suppl 3):111–120. doi: 10.1093/ndt/11.supp3.111. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney international. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 16.Parfitt AM. A structural approach to renal bone disease. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13(8):1213–1220. doi: 10.1359/jbmr.1998.13.8.1213. Epub 1998/08/26. [DOI] [PubMed] [Google Scholar]
- 17.Parfitt AM. Parathyroid hormone and periosteal bone expansion. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17(10):1741–1743. doi: 10.1359/jbmr.2002.17.10.1741. [DOI] [PubMed] [Google Scholar]
- 18.Leonard MB. A structural approach to skeletal fragility in chronic kidney disease. Semin Nephrol. 2009;29(2):133–143. doi: 10.1016/j.semnephrol.2009.01.006. Epub 2009/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hind K, Oldroyd B, Truscott JG. In vivo precision of the GE Lunar iDXA densitometer for the measurement of total-body, lumbar spine, and femoral bone mineral density in adults. J Clin Densitom. 2010;13(4):413–417. doi: 10.1016/j.jocd.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1999;10(4):259–264. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- 21.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. Journal of Clinical Investigation. 1988;81(6):1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesnut CH, 3rd, Rose CJ. Reconsidering the effects of antiresorptive therapies in reducing osteoporotic fracture. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(12):2163–2172. doi: 10.1359/jbmr.2001.16.12.2163. [DOI] [PubMed] [Google Scholar]
- 23.KDIGO. Clinical Practice Guidelines for the Management of CKD-MBD. Kidney international. 2009;76(S113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 24.Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20(12):2105–2115. doi: 10.1359/JBMR.050817. [DOI] [PubMed] [Google Scholar]
- 25.Miller PD, Schwartz EN, Chen P, Misurski DA, Krege JH. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18(1):59–68. doi: 10.1007/s00198-006-0189-8. Epub 2006/10/03. [DOI] [PubMed] [Google Scholar]
- 26.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca(2)(+) overload and cell death. The Journal of clinical investigation. 2012;122(4):1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamal SA, Hayden JA, Beyene J. Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;49(5):674–681. doi: 10.1053/j.ajkd.2007.02.264. Epub 2007/05/03. [DOI] [PubMed] [Google Scholar]
- 28.Ambrus C, Almasi C, Berta K, Deak G, Marton A, Molnar MZ, et al. Vitamin D insufficiency and bone fractures in patients on maintenance hemodialysis. Int Urol Nephrol. 2011;43(2):475–482. doi: 10.1007/s11255-010-9723-x. [DOI] [PubMed] [Google Scholar]
- 29.Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, et al. Bone Mineral Density and Fracture Risk in Older Individuals with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2012 doi: 10.2215/CJN.12871211. Epub 2012/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres A, Lorenzo V, Gonzalez-Posada JM. Comparison of histomorphometry and computerized tomography of the spine in quantitating trabecular bone in renal osteodystrophy. Nephron. 1986;44(4):282–287. doi: 10.1159/000184007. [DOI] [PubMed] [Google Scholar]
- 31.Mares J, Ohlidalova K, Opatrna S, Ferda J. Determinants of prevalent vertebral fractures and progressive bone loss in long-term hemodialysis patients. Journal of bone and mineral metabolism. 2009;27(2):217–223. doi: 10.1007/s00774-008-0030-x. [DOI] [PubMed] [Google Scholar]
- 32.Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical pQCT measures are associated with fractures in dialysis patients. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21(4):543–548. doi: 10.1359/jbmr.060105. Epub 2006/04/07. [DOI] [PubMed] [Google Scholar]
- 33.Cejka D, Patsch JM, Weber M, Diarra D, Riegersperger M, Kikic Z, et al. Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(9):2264–2271. doi: 10.2215/CJN.09711010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West SL, Jamal SA. Determination of bone architecture and strength in men and women with stage 5 chronic kidney disease. Semin Dial. 2012;25(4):397–402. doi: 10.1111/j.1525-139X.2012.01096.x. [DOI] [PubMed] [Google Scholar]
- 35.Nickolas TL, Stein E, Cohen A, Thomas V, Staron RB, McMahon DJ, et al. Bone mass and microarchitecture in CKD patients with fracture. Journal of the American Society of Nephrology : JASN. 2010;21(8):1371–1380. doi: 10.1681/ASN.2009121208. Epub 2010/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamal SA, Cheung AM, West SL, Lok CE. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012 doi: 10.1007/s00198-012-1908-y. [DOI] [PubMed] [Google Scholar]
- 37.van Rietbergen B, Weinans H, Huiskes R, Odgaard A. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech. 1995;28(1):69–81. doi: 10.1016/0021-9290(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 38.Trombetti A, Stoermann C, Chevalley T, Van Rietbergen B, Herrmann FR, Martin PY, et al. Alterations of bone microstructure and strength in end-stage renal failure. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012 doi: 10.1007/s00198-012-2133-4. [DOI] [PubMed] [Google Scholar]
- 39.Link TM, Saborowski, Kisters K, Kempkes M, Kosch M, Newitt D, et al. Changes in calcaneal trabecular bone structure assessed with high-resolution MR imaging in patients with kidney transplantation. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2002;13(2):119–129. doi: 10.1007/s001980200003. [DOI] [PubMed] [Google Scholar]
- 40.Wehrli FW, Leonard MB, Saha PK, Gomberg BR. Quantitative high-resolution magnetic resonance imaging reveals structural implications of renal osteodystrophy on trabecular and cortical bone. J Magn Reson Imaging. 2004;20(1):83–89. doi: 10.1002/jmri.20085. [DOI] [PubMed] [Google Scholar]
- 41.Allen MR, Chen NX, Gattone VH, 2nd, Chen X, Carr AJ, Leblanc P, et al. Skeletal effects of zoledronic acid in an animal model of chronic kidney disease. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012 doi: 10.1007/s00198-012-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchanque-Fossuo CN, Gong B, Poushanchi B, Donneys A, Sarhaddi D, Gallagher KK, et al. Raman spectroscopy demonstrates Amifostine induced preservation of bone mineralization patterns in the irradiated murine mandible. Bone. 2012 doi: 10.1016/j.bone.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris MD, Mandair GS. Raman assessment of bone quality. Clin Orthop Relat Res. 2011;469(8):2160–2169. doi: 10.1007/s11999-010-1692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace JM. Applications of atomic force microscopy for the assessment of nanoscale morphological and mechanical properties of bone. Bone. 2012;50(1):420–427. doi: 10.1016/j.bone.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. Journal of Bone & Mineral Research. 2000;15(1):2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 46.Malluche HHB-fE, Rojas E, Carvalho AB, D'Haese PC, Drueke TB, Ferreira MA, Jorgetti V, Moe SM, Sprague SM. Abstract presentation, Renal Week 2010. Denver, Colorado: American Society of Nephrology; Nov 16, 2010. Predictive Value of Biomarkers for Bone Turnover in ESKD. 2010. [Google Scholar]
- 47.institute of Medicine: Dietary References Intakes:Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; 1997. [PubMed] [Google Scholar]
- 48.Zidehsarai MP, Moe SM. Review article: Chronic kidney disease-mineral bone disorder: have we got the assays right? Nephrology (Carlton) 2009;14(4):374–382. doi: 10.1111/j.1440-1797.2009.01131.x. Epub 2009/07/01. [DOI] [PubMed] [Google Scholar]
- 49.Moorthi RN, Kandula P, Moe SM. Optimal vitamin D, calcitriol, and vitamin D analog replacement in chronic kidney disease: to D or not to D: that is the question. Current opinion in nephrology and hypertension. 2011;20(4):354–359. doi: 10.1097/MNH.0b013e3283470450. [DOI] [PubMed] [Google Scholar]
- 50.Koivula MK, Risteli L, Risteli J. Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clinical biochemistry. 2012;45(12):920–927. doi: 10.1016/j.clinbiochem.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez L, Torregrosa JV, Peris P, Monegal A, Bedini JL, Martinez De Osaba MJ, et al. Effect of hemodialysis and renal failure on serum biochemical markers of bone turnover. Journal of bone and mineral metabolism. 2004;22(3):254–259. doi: 10.1007/s00774-003-0476-9. [DOI] [PubMed] [Google Scholar]
- 52.Ohashi T, Igarashi Y, Mochizuki Y, Miura T, Inaba N, Katayama K, et al. Development of a novel fragments absorbed immunocapture enzyme assay system for tartrate-resistant acid phosphatase 5b. Clinica chimica acta; international journal of clinical chemistry. 2007;376(1–2):205–212. doi: 10.1016/j.cca.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Yamada S, Inaba M, Kurajoh M, Shidara K, Imanishi Y, Ishimura E, et al. Utility of serum tartrate-resistant acid phosphatase (TRACP5b) as a bone resorption marker in patients with chronic kidney disease: independence from renal dysfunction. Clinical endocrinology. 2008;69(2):189–196. doi: 10.1111/j.1365-2265.2008.03187.x. [DOI] [PubMed] [Google Scholar]
- 54.Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004;34(1):187–194. doi: 10.1016/j.bone.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, et al. Discriminants of prevalent fractures in chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2011;22(8):1560–1572. doi: 10.1681/ASN.2010121275. Epub 2011/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wesseling-Perry K. FGF-23 in bone biology. Pediatr Nephrol. 2010;25(4):603–608. doi: 10.1007/s00467-009-1384-6. Epub 2009/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, et al. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. The Journal of clinical endocrinology and metabolism. 2009;94(2):511–517. doi: 10.1210/jc.2008-0326. Epub 2008/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. Epub 2009/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. The New England journal of medicine. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. Epub 2008/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pelletier S, Dubourg L, Carlier MC, Hadj-Aissa A, Fouque D. The Relation between Renal Function and Serum Sclerostin in Adult Patients with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2013 doi: 10.2215/CJN.07670712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(4):877–882. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cejka D, Jager-Lansky A, Kieweg H, Weber M, Bieglmayer C, Haider DG, et al. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(1):226–230. doi: 10.1093/ndt/gfr270. [DOI] [PubMed] [Google Scholar]
- 64.Gardham C, Stevens PE, Delaney MP, LeRoux M, Coleman A, Lamb EJ. Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(7):1261–1267. doi: 10.2215/CJN.09471209. Epub 2010/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehmann G, Stein G, Huller M, Schemer R, Ramakrishnan K, Goodman WG. Specific measurement of PTH (1–84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney international. 2005;68(3):1206–1214. doi: 10.1111/j.1523-1755.2005.00513.x. Epub 2005/08/18. [DOI] [PubMed] [Google Scholar]
- 66.Herberth J, Branscum AJ, Mawad H, Cantor T, Monier-Faugere MC, Malluche HH. Intact PTH combined with the PTH ratio for diagnosis of bone turnover in dialysis patients: a diagnostic test study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55(5):897–906. doi: 10.1053/j.ajkd.2009.12.041. Epub 2010/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney international Supplement. 2009;(113):S1–S130. doi: 10.1038/ki.2009.188. Epub 2009/08/01. [DOI] [PubMed] [Google Scholar]
- 68.Sawaya BP, Butros R, Naqvi S, Geng Z, Mawad H, Friedler R, et al. Differences in bone turnover and intact PTH levels between African American and Caucasian patients with end-stage renal disease. Kidney international. 2003;64(2):737–742. doi: 10.1046/j.1523-1755.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 69.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;47(1):149–156. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 70.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000;36(6):1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 71.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. Journal of the American Society of Nephrology : JASN. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. Epub 2004/07/31. [DOI] [PubMed] [Google Scholar]
- 72.Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B. Fracture risk after parathyroidectomy among chronic hemodialysis patients. Journal of the American Society of Nephrology : JASN. 2007;18(8):2401–2407. doi: 10.1681/ASN.2007010022. Epub 2007/07/20. [DOI] [PubMed] [Google Scholar]
- 73.Sardiwal S, Gardham C, Coleman AE, Stevens PE, Delaney MP, Lamb EJ. Bone-specific alkaline phosphatase concentrations are less variable than those of parathyroid hormone in stable hemodialysis patients. Kidney international. 2012;82(1):100–105. doi: 10.1038/ki.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urena P, Hruby M, Ferreira A, Ang KS, de Vernejoul MC. Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. Journal of the American Society of Nephrology : JASN. 1996;7(3):506–512. doi: 10.1681/ASN.V73506. [DOI] [PubMed] [Google Scholar]
- 75.Coen G, Ballanti P, Bonucci E, Calabria S, Centorrino M, Fassino V, et al. Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998;13(9):2294–2302. doi: 10.1093/ndt/13.9.2294. [DOI] [PubMed] [Google Scholar]
- 76.Blayney MJ, Pisoni RL, Bragg-Gresham JL, Bommer J, Piera L, Saito A, et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney international. 2008;74(5):655–663. doi: 10.1038/ki.2008.248. [DOI] [PubMed] [Google Scholar]