Abstract
Transient Receptor Potential (TRP) channels were discovered while analyzing visual mutants in drosophila. The protein encoded by the transient receptor potential (trp) gene is a Ca2+ permeable cation channel activated downstream of the phospholipase C (PLC) pathway. While searching for homologues in other organisms, a surprisingly large number of mammalian TRP channels were cloned. The regulation of TRP channels is quite diverse, but many of them are either activated downstream of the PLC pathway, or modulated by it. This review will summarize the current knowledge on regulation of TRP channels by the PLC pathway, with special focus on TRPC-s, which can be considered as effectors of the PLC pathway, and the heat and capsaicin sensitive TRPV1, which is modulated by the PLC pathway in a complex manner.
Keywords: Phospholipase C, TRP channel, PIP2, phosphatidylinositol 4, 5-bispohosphate
Introduction
PLC catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to form the two classical second messengers inositol 1,4,5 trisphosphate (IP3) that releases Ca2+ from intracellular stores, and diacylglycerol (DAG) that activates protein kinase C (PKC). Since the storage capacity of the intracellular Ca2+ stores is finite, it has been appreciated long time ago that Ca2+ influx from the extracellular space is necessary for sustained Ca2+ signaling (Putney, 1986). The search for this enigmatic Ca2+ influx pathway was a very important driving force for the discovery of mammalian Transient Receptor Potential (TRP) channels. Putney proposed in 1990 that the major mechanism, by which Ca2+ influx is initiated upon PLC activation, is the emptying of the intracellular Ca2+ stores (Putney, 1990). He called this mechanism the “capacitative” Ca2+ influx, but later the term “store operated Ca2+ entry” became more widely used. Approximately at the same time, the channel responsible for generating the receptor potential in the drosophila photoreceptor cells was cloned (Montell and Rubin, 1989), and from the phenotype of the mutant it was named transient receptor potential (TRP). Since invertebrate vision is mediated by the PLC pathway, the search begun for the mammalian homologues of the drosophila TRP, as obvious candidates for store-operated or receptor-operated Ca2+ channels. It was soon found out that mammals have 7 relatively close homologues of the drosophila TRP channel; these were termed TRPC, for classical or canonical (Clapham et al., 2001). Essentially all mammalian TRPC-s are activated downstream of PLC, thus they can be considered as downstream effectors of PLC. The first part of this review will focus on the regulation of mammalian TRPC channels and the drosophila visual TRP channel complex. This topic has been reviewed recently in detail (Putney and Tomita, 2011), but the author felt that reviewing PLC regulation of TRP channels is not possible without discussing TRPC channels.
TRPC channels are however only one subfamily of TRP channels; based mainly on homology cloning, a large number of more distant mammalian homologues of the drosophila TRP were cloned. The superfamily is now divided into sub-families, named after their first discovered members. In mammals the two other major subfamilies TRPV (vanilloid) and TRPM (melastatin) have 6 and 8 members, respectively. More distant relatives of TRP channels are the 3 TRPP-s (polycystins), 3 TRPML-s (mucolipins) and the single TRPA (ankyrin) (Wu et al., 2010). The functions of these non-classical TRP channels are extremely diverse, and difficult to summarize. Many them channels are considered sensory ion channels, since they respond to physical cues such as heat (TRPV1, TRPV3, TRPM3), cold (TRPM8), or mechanical stimuli (TRPV4) and some are involved in taste perception (TRPM5) or vision (TRPM1). Their functions however include many non-sensory functions such as epithelial Ca2+ transport (TRPV5 and 6) and Mg2+ transport (TRPM6), and some of them such as TRPM7 and TRPV4 play important roles in development. Most TRP channels are non-selective, Ca2+ permeable, outwardly rectifying cation channels; their opening acts as an excitatory signal by both inducing depolarization and Ca2+ influx. The PLC pathway modulates many non-classical TRP channels, even though for most of them the PLC pathway is not the primary activator. The second part of the review will describe current knowledge on PLC modulation of non-classical TRP channels with special focus on the capsaicin- and heat-activated TRPV1, for which the PLC pathway plays important roles in both sensitization by pro-inflammatory agents and desensitization upon maximal pharmacological stimulation.
PLC modulation of TRP channels can not be discussed without considering the membrane phospholipid PI(4,5)P2, the substrate of PLC, which is also general regulator of many mammalian ion channels (Hilgemann et al., 2001; Suh and Hille, 2008; Gamper and Rohacs, 2012). Generally the activity of most phosphoinositide sensitive channels depends on PI(4,5)P2, in other words, this phospholipid is a necessary cofactor for channel activity (Suh and Hille, 2008). This is also true for most TRP channels (Rohacs, 2013). In many cases regulation of TRP channels by PLC happens through decreasing PI(4,5)P2 levels. We have discussed the literature on PI(4,5)P2 regulation of TRP channels in more detail in a concurrent review (Rohacs, 2013); this article will focus on the consequences of PLC activation.
PLC isoforms
PLCβ isoforms are probably the best-known PLC-s; these enzymes are activated by G-Protein Coupled Receptors (GPCR) through the Gq heterotrimeric G-proteins. Mammals have 4 PLCβ isoforms, numbered 1–4. The other classical pathway, Receptor Tyrosin Kinases activate one of the 2 PLCβ isoforms. PLCδ-s have no obvious activators, but they are the most sensitive to activation by increased cytoplasmic Ca2+. Mammals have 3 PLCδ isoforms, PLCδ1, 3, and 4. Besides the three major classical PLC groups, there are several more recently identified PLC isoforms, such as PLCε, two PLCη-s and a single PLCζ (Rhee, 2001; Fukami et al., 2010).
PLC and TRP channels in invertebrate vision
The invertebrate eye is different from its vertebrate counterpart both anatomically and in molecular signal transduction mechanisms. In both cases photons activate the G-protein coupled receptor rhodopsin, but the downstream mechanism is different (Raghu and Hardie, 2009). In mammals, the G-protein transducin activates a phosphodiesterase, which decreases cyclic nucleotide levels, and closes a cyclic nucleotide gated channel that will eventually lead to a hyperpolarizing receptor potential (Fain et al., 2010).
In drosophila and many other invertebrates the G-protein activated by rhodopsin is a homologue of Gq, and the downstream effector is PLC, which activates TRP cation channels leading to a depolarizing receptor potential. The channel complex mediating the receptor potential consists of a heterotetramer of TRP and TRPL channels. If you ever tried to catch a fly, you may have guessed already that insect vision is much faster than vertebrate vision, which is mainly due to the fact that the components of the signal transduction machinery are held in a complex by the scaffolding protein INAD. Even though the basic signal transduction pathway and its key players have been known for more than 15 years now, how exactly PLC activation leads to TRP channel opening in the insect eye, is still not clear (Montell, 2012). Ca2+ release from internal stores is clearly dispensable since knocking out the single drosophila isoform of the IP3 receptor did not cause blindness (Acharya et al., 1997).
Diacylglycerol (DAG), the downstream product of PI(4,5)P2 hydrolysis is an attractive candidate for being a messenger, since it activates several mammalian TRPC channels, see later. The effect of DAG analogues on the drosophila TRP and TRPL channels however is controversial. Stearyl-arachidonyl glycerol (SAG), but not oleoy-acetyl glycerol (OAG) activated heterologous TRPL channels (Estacion et al., 2001). Another article found that in native drosophila rhabdomeral patches OAG activated endogenous TRP channels (Delgado and Bacigalupo, 2009). A third article on the other hand found no effect of either OAG or SAG on heterologously expressed TRPL channels (Lev et al., 2012).
PI(4,5)P2 has been shown to inhibit recombinant TRPL channels in excised patches (Estacion et al., 2001), thus decrease in PI(4,5)P2 levels upon PLC activation is also a feasible candidate. In a contrary report however PI(4,5)P2 activated drosophila TRP channels in excised patches, while PI(4)P and PI inhibited them (Huang et al., 2010). Another report showed that depletion of PI(4,5)P2 using a rapamycin-inducible 5-phosphatase did not activate TRPL channels (Lev et al., 2012). The same study found that rapamycin-induced depletion of PI(4,5)P2 inhibited TRPL currents activated by carbachol. This result is consistent with PI(4,5)P2 serving as a co-factor for the TRPL channel, similar to other TRP(C) channels, see the TRPC channel section for further discussion.
The acyl chains in PI(4,5)P2 in vivo are a mixture, one of them is usually a poly-unsaturated fatty acid (PUFA), the other is usually saturated, in mammals arachidonyl-stearyl being the most common. Upon PLC activation, hydrolysis of PI(4,5)P2 results in the formation of IP3 and DAG, and the latter can be further hydrolyzed by DAG lipase enzymes to form PUFA. It was shown that in drosophila photoreceptors the native TRP channel complex, as well as recombinant TRPL channels are activated by PUFA (arachidonic acid and linoleic acid) (Chyb et al., 1999, Estacion et al., 2001). This finding was confirmed by (Lev et al., 2012), and the authors proposed that the most likely physiological activators of TRPL channels are PUFA. A DAG lipase enzyme required for normal phototransduction was also identified (Leung et al., 2008), and a DAG lipase inhibitor partially inhibited carbachol-induced activation of TRPL (Lev et al., 2012).
Upon hydrolysis of PI(4,5)P2 a H+ is also liberated, a fact overlooked for a long time. It was proposed recently, that local low pH also contributes to TRP channel activation in the highly localized phototransduction complex in the drosophila eye (Huang et al., 2010).
A recent paper proposed a radically new idea for the activation of drosophila photoreceptor TRP channels. Hardie et al found that light exposure evoked rapid PLC-mediated contractions in the photoreceptor cells. They also found that these contractions were sufficient to activate mechanosensitive channels introduced into the photoreceptor cells (Hardie and Franze, 2012). The authors suggested that the PLC-generated mechanical forces contribute to gating the light-sensitive channels. This idea is quite intriguing, since several mammalian TRPC channels have also been proposed to be mechanically regulated (Maroto et al., 2005; Spassova et al., 2006; Garrison et al., 2012; Quick et al., 2012). Mechanical activation of TRPC channels however is somewhat controversial (Gottlieb et al., 2008).
Mammalian TRPC channels – activation by PLC
After the cloning of the mammalian TRPC-s, the closest homologues of the drosophila light transduction channels, it quickly became accepted that these channels are activated by cell surface receptors that couple to PLC (Zhu et al., 1996; Vazquez et al., 2004). The opening of TRP channels leads both to increased Ca2+ influx and depolarization. Most cell types have some endogenous expression of several TRPC channels, but the currents are usually quite small. Quite a lot of the work on the activation mechanism of TRPC-s have been performed in expression systems. Most TRPC channels have been genetically deleted in mice, yielding important information on their biological functions. Interestingly, most knockout phenotypes are quite moderate, none of them are lethal, suggesting that the other isoforms can compensate the lack of a specific isoform (Birnbaumer, 2009). Because the activation mechanism of TRPC channels is still not elucidated, the various hypotheses will be briefly discussed below. It is quite possible that the activation mechanism of TRPC-s is multimodal, several of the mechanisms below contributing to different extents to the activation of various TRPC channels.
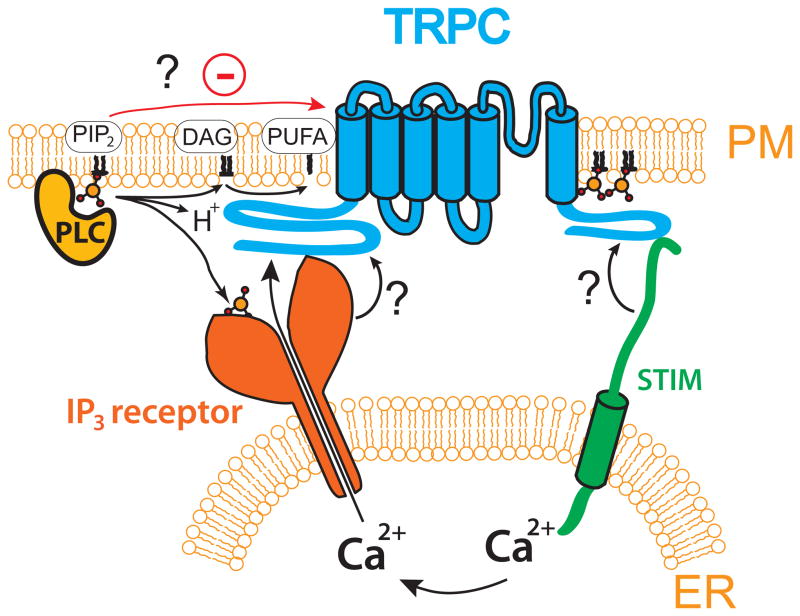
Activation by store depletion
As mentioned earlier TRPC-s were cloned in a quest for the holy grail of the “store operated Ca2+ channels”. Despite initial positive results (Zhu et al., 1996), the activation of these channels by store depletion quickly became controversial. Some laboratories could and some others could not activate these channels by pure store depletion. Some of the problems were caused by the lack of clear definition of what constitutes a pure store depletion, and the fact that many of the protocols used also had effects other than store depletion, such as increased cytoplasmic Ca2+ (Clapham et al., 2001; Parekh and Putney, 2005). Despite the controversies, TRP channels were considered store-operated by many for a long time, and even clearly non-store operated TRP channels were designated initially as such (Perez et al., 2002). As mentioned earlier, store depletion is clearly not the activation mechanism for the drosophila TRP channel complex, since phototransduction functions normally in the absence of IP3 receptor (Acharya et al., 1997), and also it is difficult to imagine store depletion to activate a channel with a speed characteristic of invertebrate vision.
An additional problem with the hypothesis that TRPC-s are responsible for store operated Ca2+ entry was the fact that the biophysical properties of the best-characterized store operated current ICRAC, (Hoth and Penner, 1992) were dramatically different from those of TRPC channels. Briefly, ICRAC is an inwardly rectifying, Ca2+ selective current, with unitary currents too small to measure, while TRPC-s are outwardly rectifying, non-selective cation channels with measureable single channel conductances. This led several laboratories for a renewed search for store-operated channels, using siRNA-based screens to knock down endogenous store operated Ca2+ entry pathways. In 2006 three groups reported the cloning of orai1, also know as CRACM that quickly became accepted as molecular counterpart of the store operated ICRAC current (Feske et al., 2006; Vig et al., 2006; Zhang et al., 2006).
It is noteworthy that, the cloning of orai1 resurrected efforts to study store operated activation of TRPC channels, mainly because of cloning of the endoplasmic reticulum Ca2+ sensor STIM1. Several laboratories showed that STIM1 interacts with TRPC channels, and it was proposed that in some cases it may contribute to opening of TRPC channels (Huang et al., 2006; Worley et al., 2007). It was also proposed that orai and TRPC channels interact to form store-operated channels (Liao et al., 2009). Nevertheless, the topic of store operated activation of TRPC channels remained controversial in most cases (Putney and Tomita, 2011). As opposed to TRPC channels orai mediated currents could be activated by store depletion by many different laboratories, with basically no contradictory results (Putney and Tomita, 2011).
Diacylglycerol
One of the products of PLC activation is diacylglycerol (DAG), which is best known as the activator of PKC. DAG activates TRPC3, TRPC6 and TRPC7, in a PKC independent manner (Hofmann et al., 1999). This effect has been reproduced by many different laboratories, and DAG analogues, especially OAG have been widely used as tools to specifically activate channels consisting of TRPC3,6 or 7. Even though the original paper claimed that DAG exerted its effect directly on the channels, this finding has been debated (Putney and Tomita, 2011). Nevertheless, whether or not this lipid acts directly on the channels, DAG activation still is the least controversial and best-accepted mechanism of TRPC activation. It cannot account however for the activation of other TRPC-s, such as TRPC4 and 5, which are not activated by DAG. While PUFA-s are prominent candidates for the activation of the drosophila visual TRP complex, they received less attention as potential activators of mammalian TRPC-s even though some TRPC-s, but not others, have been shown to be activated either by arachidonic acid or its downstream products (Beech, 2012).
Activation by the IP3 receptor
The IP3 receptor has a large enough cytoplasmic domain to traverse the gap between the endoplasmic reticulum and the plasma membrane (Fig 1) and it was proposed that upon binding to IP3, it interacts with TRPC3 channels and plays a role in their activation (Kiselyov et al., 1998). This activation was proposed to happen through displacing calmodulin form an inhibitory binding site (Zhang et al., 2001). Direct activation by the IP3 receptor is unlikely to be a general mechanism for TRPC activation, since most of these studies focused on TRPC3, and the drosophila visual TRP channels function well in the absence of the IP3 receptor (Acharya et al., 1997).
Figure 1.
Potential activation mechanisms of mammalian TRPC and drosophila visual TRP channels. The cartoon is a compilation of activation mechanisms proposed on a variety of channels in this family; note that there is not one specific channel where all of these mechanisms have been demonstrated.
Depletion of PI(4,5)P2
The first two publications on TRP channels and PI(4,5)P2 reported that this lipid inhibits two very distantly related TRP channels, the drosophila TRPL (Estacion et al., 2001) and the mammalian heat- and capsaicin-sensitive TRPV1 (Chuang et al., 2001). Thus it was an attractive hypothesis that PI(4,5)P2 is a general inhibitor of TRP channels, and that the activation mechanism by PLC is the depletion of PI(4,5)P2 and a relief from this inhibition. By now it is apparent that PI(4,5)P2 is not an inhibitor, but rather an activator of most TRP channels (Gamper and Rohacs, 2012; Rohacs, 2013). Nevertheless in many cases, including TRPC-s, the regulation by PI(4,5)P2 is complex, and concurrent inhibitory effects of the lipid may also exist. For example, TRPC5 channels can both negatively, and positively affected by decreasing PI(4,5)P2 levels, depending on the method used (Trebak et al., 2009).
Negative effects of phosphoinositide depletion and PLC
Despite the fact that the PLC pathway is the major activator of TRPC channels, and PI(4,5)P2 depletion has been one of the hypotheses explaining their activation, there are data arguing that decreasing membrane PI(4,5)P2 levels negatively regulate at least some TRPC channels. It was reported that despite its possible negative regulation by PI(4,5)P2, TRPC5 is activated by PI(4,5)P2 in excised patches (Trebak et al., 2009) similarly to TRPC3, 6 and 7 channels (Lemonnier et al., 2008). Similarly, a voltage dependent 5 phosphatases, that depletes PI(4,5)P5, was shown to inhibit TRPC3,6 and 7 channels (Imai et al., 2012). PI(4,5)P2 applied through the whole-cell patch pipette was shown to slow down desensitization of TRPC5 (Kim et al., 2008a). Overall, it appears that depletion of PI(4,5)P2 limits activation of these channels upon sustained PLC activation, either because PI(4,5)P2 is a co-factor, needed for channel activity, or because the loss of the substrate limits the generation of the second messenger, such as DAG. Overall, the regulation of TRPC channels by PI(4,5)P2 is quite complex, and we have discussed it in more detail in a recent review (Rohacs, 2013).
The role of TRP channels in mammalian vision
As mentioned earlier, the signal transduction mechanism of primary image forming photoreceptor cells (rods and cones) in the vertebrate eye is different from that in insects and most invertebrates. Intriguingly, other, non-image forming light responses of the mammalian eye, such as circadian rhythm, and pupil light responses are not mediated by rods and cones, but rather specialized light responsive ganglion cells. These cells express the light sensitive receptor melanopsin, which is more similar to insect rhodopsin than to mammalian opsins in rods and cones. Accordingly, melanopsin in the mouse eye couples to PLCβ4 and TRPC6 and TRPC7 channels and induces depolarization (Xue et al., 2011). This is in agreement with the idea that in early evolution common ancestors of the vertebrate and arthropod lineages had both types of photoreceptors. In each lineage one signal transduction pathway usually dominated, but vestigial functions of the other non-dominant phototransduction system remained. (Fain et al., 2010).
TRPV1 channels - involvement of the PLC pathway in sensitization and desensitization
TRPV1 is a multimodal nociceptive ion channel, activated by heat, low tissue pH, capsaicin, the pungent compound in chili peppers, and a plethora of other pain producing compounds (Caterina and Julius, 2001). This channel was the first non-classical mammalian TRP-s that was cloned, by expression cloning from sensory neuron mRNA using its chemical agonist capsaicin (Caterina et al., 1997). As opposed to most other TRP channels, this channel was extensively studied before it was cloned. Sensory dorsal root ganglion (DRG) and trigeminal ganglion (TG) neurons have been known to exhibit heat-activated (Cesare and McNaughton, 1996) and capsaicin-induced inward currents (Liu and Simon, 1996; Koplas et al., 1997). Capsaicin was also well known to stimulate sensory nerves (Szolcsanyi et al., 1988). The cloned receptor reproduced well the properties of endogenous channels when expressed heterologously (Tominaga et al., 1998). Genetic deletion of TRPV1 reinforced the role of this channel in certain forms of nociception, especially in thermal hyperalgesia, the increased sensitivity to heat upon inflammation (Caterina et al., 2000).
Sensitization and thermal hyperalgesia
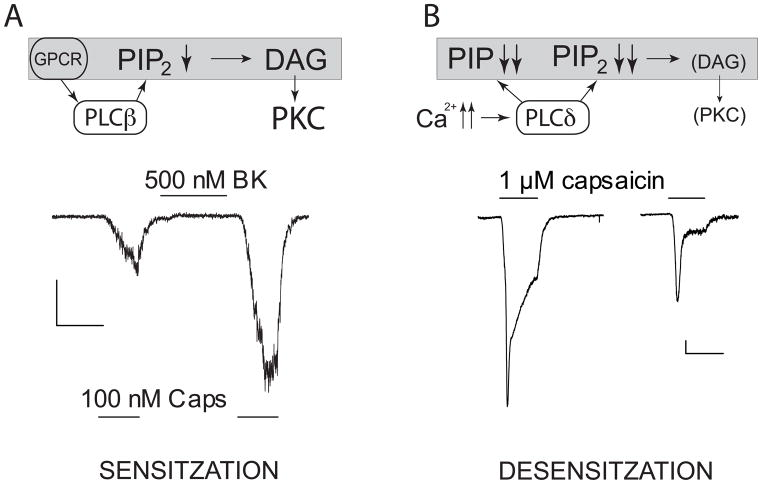
The threshold and sensitivity of TRPV1 to its activators is not static. Upon injury or inflammation, a plethora of pro-inflammatory mediators, such as bradykinin, ATP, prostaglandins and chemokines are released from immune cells and the damaged tissues. These agents sensitize TRPV1 to its activators: heat, low pH and capsaicin, at sub-maximal stimulation levels, see Fig. 2A for an example. These mediators activate GPCR-s that exert their effects mostly through the PLCβ pathway, and some through increasing intracellular cAMP. After sensitization of TRPV1 by the PLC pathway, lower concentrations of protons or capsaicin are required to activate the channel, but the maximal response does not change (Tominaga et al., 2001). Some other sensitizing agents, such as NGF act through Receptor Tyrosine Kinases and increase the maximal response, i.e. the number of available channels via the PI3K pathway (Zhang et al., 2005a; Stein et al., 2006).
Figure 2.
Sensitization and desensitization of TRPV1. A. Top, Graphical summary of changes in phosphoinositide levels during sensitization in DRG neurons, based on data from (Lukacs, 2013). Briefly, upon GPCR stimulation by Bradykinin, PLCβ activation leads to a moderate decrease in PI(4,5)P2, but no change in PI(4)P. The decrease in PI(4,5)P2 levels synergizes with PKC activation to potentiate TRPV1 activity. Bottom, Representative trace for sensitization of TRPV1 in a DRG neuron in the whole-cell configuration: the cell was stimulated with 100 nM capsaicin, then 500 nM bradykinin was applied, then the cell was stimulated by 100 nM capsaicin again. B. Top, Upon maximal TRPV1 activation, Ca2+-induced PLCδ activation leads to a robust decrease in PI(4,5)P2 and PI(4)P. Even though PKC is likely to be activated, its effect is over-ridden by the robust decrease in phosphoinostide levels and thus channel activity decreases. Bottom, Representative trace for desensitization in a DRG neuron in the perforated patch configuration: two subsequent stimulations by a saturating concentration of capsaicin (1 μM). Traces are modified from (Lukacs, 2013), scale bars are 100 pA and 1 min for both traces.
There are two alternative hypotheses for the mechanism of sensitization of TRPV1 upon GPCR-induced PLC activation. PKCε has been proposed to mediate sensitization of TRPV1 (Cesare et al., 1999) via direct phosphorylation of S502 and S800 (Numazaki et al., 2002). As mentioned earlier, an alternative hypothesis was that PI(4,5)P2 keeps TRPV1 under tonic inhibition, and upon PLC activation the decrease in PI(4,5)P2 levels relieves this inhibition, leading to potentiation (Chuang et al., 2001). An inhibitory binding site at the very distal C-terminus was proposed that mediates the inhibition by PI(4,5)P2 (Prescott and Julius, 2003).
Desensitization by high capsaicin concentrations
Prolonged application of maximal concentrations of capsaicin leads to a biphasic current response, an initial peak followed by a much smaller plateau both in native sensory neurons and in expression systems (Koplas et al., 1997; Liu et al., 2005; Lukacs, 2013) see Fig. 2B. This is a Ca2+ dependent phenomenon, since reducing extracellular Ca2+ to zero strongly reduced or eliminated desensitization (Koplas et al., 1997; Lukacs et al., 2007). It was shown that calmodulin (CaM) interacts with a C-terminal segment of TRPV1, removal of which inhibited desensitization (Numazaki et al., 2003). Neither calmodulin inhibitors nor a dominant negative mutant of calmodulin interfered however with desensitization, and the authors concluded that “CaM is not likely involved in Ca2+-dependent desensitization of TRPV1” (Numazaki et al., 2003). Nevertheless, CaM was shown to inhibit TRPV1 slowly and partially in excised patches (Rosenbaum et al., 2004) and another study found that a CaM inhibitor inhibited desensitization (Lishko et al., 2007).
The Ca2+ sensitive phosphatase calcineurin has also been implicated in desensitization of TRPV1 (Docherty et al., 1996; Mohapatra and Nau, 2005). In another study, however, a calcineurin presudosubstrate inhibitor did not inhibit acute desensitization (Piper et al., 1999).
Liu et al found that intracellular hydrolysable ATP was necessary for the recovery of TRPV1 from desensitization, and recovery was also inhibited by high concentrations of wortmannin, an inhibitor of type 3 phosphatidylinositol 4 Kinases, that inhibit resynthesis of PI(4,5)P2 (Liu et al., 2005). The same paper also showed that activation of TRPV1 by capsaicin inhibited the PI(4,5)P2 dependent Kir2.1 channels, suggesting that Ca2+ influx though TRPV1 depletes PI(4,5)P2. In subsequent articles, two laboratories found that PI(4,5)P2 in excised patches activates, rather than inhibits TRPV1 channels (Stein et al., 2006; Lukacs et al., 2007). Both the PLC inhibitor U73122 and inclusion of PI(4,5)P2 or PI(4)P in the whole-cell patch pipette inhibited desensitization (Lishko et al., 2007; Lukacs et al., 2007). Activation of TRPV1 with saturating capsaicin concentrations induced a translocation of a PI(4,5)P2 binding fluorescent probe form the plasma membrane to the cytoplasm, which was inhibited by U73122, signifying PLC dependent PI(4,5)P2 depletion (Lukacs et al., 2007). Furthermore, capsaicin induced the formation of IP3 and IP2, further supporting PLC activation (Lukacs et al., 2007). Overall, these data support a model for desensitization similar to that proposed for TRPM8 (Rohacs et al., 2005), and several other TRP channels, in which Ca2+ influx stimulates PLC, leading to depletion of PI(4,5)P2 and limiting channel activity, see Fig. 3.
Figure 3.
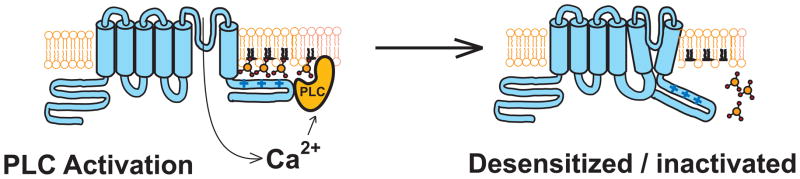
Model for Ca2+-dependent desensitization/inactivation of TRP channels. Calcium influx though various TRP channels activate a Ca2+ selective PLC, likely PLCδ isoform, that leads to depletion of PI(4,5)P2, and in some cases [PI(4)P], leading to diminished channel activity. This mechanism has been implicated so far in the regulation of TRPM8, TRPV1, TRPV2, and TRPV6, see text for further details.
On the other hand, these data were in direct contradiction with the PI(4,5)P2 depletion-based model of sensitization and raise two key questions. First, does PI(4,5)P2 activate or inhibit TRPV1 ? Second, how can the same PLC pathway play important roles in two phenomena, with opposing effects on channel activity?
Does PI(4,5)P2 activate or inhibit TRPV1?
In the original article proposing the inhibitory effect of phosphoinositides were not tested in excised inside-out patches (Chuang et al., 2001). All articles published later agree that in excised patches phosphoinositide chelating agents, such as poly-Lysine inhibit, while application of various phosphoinositides like PI(4,5)P2 and its precursor PI(4)P reactivate TRPV1 (Stein et al., 2006; Lukacs et al., 2007; Kim et al., 2008b; Klein et al., 2008). Dependence on phosphoinositides is further supported by the finding that TRPV1 is inhibited by the combined dephosphorylation of PI(4,5)P2 and PI(4)P by the dual specificity phosphatase pseudojanin (Hammond et al., 2012; Lukacs, 2013). On the other hand there are indications in recent articles that in intact cells, PI(4,5)P2 also exerts an inhibitory effect (Lukacs et al., 2007; Jeske et al., 2011; Lukacs, 2013). The detectability of PI(4,5)P2 inhibition in intact cells but not in excised patches suggested an indirect effect (Rohacs, 2009). A recent paper however showed that PI(4,5)P2 and other phosphoinositides inhibited the purified TRPV1 in lipid vesicles (Cao et al., 2013b). Currently it is difficult to bring all these observations into a coherent picture but it is safe to say that in the context of the cellular membrane there is a dependence of activity on PI(4,5)P2 and probably its precursor PI(4)P, but there also is a concurrent inhibitory effect by PI(4,5)P2. This seemingly complex and not completely resolved regulatory scheme is similar to that seen with TRPC channels. More discussion on phosphoinositide regulation of TRPV1 can be found in our concurrent review on this topic (Rohacs, 2013).
Opposing regulation by PLC in sensitization and desensitization
As we discussed earlier, activation of PLC by GPCR-s increases TRPV1 activity (sensitization), but activation of PLC by maximal Ca2+ influx through TRPV1 inhibits channel activity (desensitization). How is this possible? First of all, it is quite likely that the main PLC isoforms activated during these two processes are different, GPCR-s activate PLCβ isoforms, whereas Ca2+ influx though TRPV1 most likely activates PLCδ isoforms such as PLCδ4 (Lukacs, 2013). This does not explain the difference however, since the enzymatic reaction is the same in the two cases, most PLC isoforms hydrolyze PI(4,5)P2 and to a lesser extent its precursor PI(4)P (Rhee, 2001). One possible explanation can be that one of the pathways activates another signaling mechanism. PKC activation by GPCR-s is an obvious candidate, since it clearly participates in sensitization of TRPV1. PKC however is also activated by capsaicin, as expected upon PLC activation (Xu et al., 2012). The same argument can be made for all other candidates, such as increased cytoplasmic Ca2+, calmodulin, calcineurin, etc, since all of these will probably be activated by both pathways. Thus the difference is likely to be quantitative.
We have found that indeed, the phosphoinositide changes induced by GPCR activation and maximal stimulation of TRPV1 by capsaicin are quantitatively different. In DRG neurons, capsaicin induced a robust decrease in both PI(4)P and PI(4,5)P2, whereas bradykinin induced a moderate decrease in PI(4,5)P2 levels, but no change in PI(4)P (Lukacs, 2013), explaining lack of inhibition by bradykinin, despite the dependence of TRPV1 activity on these lipids. Consistent with the partial inhibitory effect of PI(4,5)P2, dialysis of PI(4,5)P2, but not PI(4)P through the patch pipette inhibited bradykinin-induced sensitization, and dephosphorylation of PI(4,5)P2 with a voltage dependent phosphatase potentiated the effect of sub-threshold stimulation of PKC on TRPV1 (Lukacs, 2013).
Overall, during sensitization by bradykinin, activation of PLCβ isoforms leads to a moderate reduction of PI(4,5)P2 levels that probably synergizes with PKC activation and leads to sensitization. The remaining PI(4,5)P2 and the unchanged levels of PI(4)P are sufficient to sustain channel activity (Lukacs, 2013). During desensitization maximal stimulation of TRPV1 induces activation of PLCδ isoforms, resulting in a robust depletion of PI(4,5)P2 and PI(4)P, limiting TRPV1 activity, which depends on these lipids (Lukacs, 2013). Figure 2 summarizes our model for the involvement of phosphoinositide changes and PKC in sensitization and desensitization based mainly on data in (Lukacs, 2013).
Other TRP channels
As described so far, activation of the PLC pathway may have both positive and negative effects on TRP channels. Many of the effects below are mediated by changes in PI(4,5)P2 levels. The details of TRP channel regulation by PI(4,5)P2 are beyond the scope of this article, but have been extensively discussed in a recent review (Rohacs, 2013).
Positive regulation by the PLC pathway
There are few other examples not discussed so far where positive regulation of TRP channels by the PLC pathway was described. In several cases, similar to TRPV1, this positive regulation coexists with a PLC dependent negative regulation.
TRPV3
TRPV3 is expressed in keratinocytes of the skin and activated by moderate heat (Dhaka et al., 2006). Deletion of this channel in mice impairs thermosensation (Moqrich et al., 2005) and results in wavy hair and thin stratum corneum (Cheng et al., 2010). Gain of function mutations in this channel cause Olmsted syndrome in humans, a rare congenital disorder characterized by palmoplantar and periorificial keratoderma, alopecia, and severe itching (Lin et al., 2012). This channels was shown to be inhibited by PI(4,5)P2 in excised patches, and activation GPCRs that activate PLC potentiated currents evoked by heat or its chemical agonist 2APB both in keratinocytes and in an expression system (Doerner et al., 2011).
TRPM4 and TRPM5
TRPM4 and TRPM5 are Ca2+-activated non-selective cation channels that do not permeate Ca2+. When PLC is activated, cytoplasmic Ca2+ increases, activating these channels, which induces depolarization. TRPM5 is selectively expressed in taste cells, and genetic deletion of either this channel or PLCβ2 abolished sweet, bitter and umami (amino acid) tastes (Zhang et al., 2003). Thus TRPM5 can be considered as a downstream effector of PLC in taste cells. TRPM4 is more widely expressed, and has been implicated in a variety of functions, including immune responses, insulin secretion, constriction of cerebral arteries, and cardiac dysfunction (Guinamard et al., 2011). Both TRPM4 and TRPM5 require PI(4,5)P2 for activity (Liu and Liman, 2003; Zhang et al., 2005b; Nilius et al., 2006), thus it is likely that the effect of PLC activation is biphasic, the channels are quickly activated by the increase in cytoplasmic Ca2+, but the following decrease in PI(4,5)P2 potentially limits their activity.
TRPA1
TRPA1 is a nociceptive ion channel, expressed in sensory neurons (Nilius et al., 2012). A wide range of noxious or painful chemicals activate this channel, including mustard oil, formaldehyde and compounds in tear gases; many of these agents stimulate the channels via covalent modification. TRPA1 is has been proposed to be both a cold sensor and a mechanosensor, but both of these hypotheses are controversial (Nilius et al., 2012). The role of TRPA1 in nociception in humans is demonstrated by the fact that its gain of function mutations cause Familial Episodic Pain Syndrome pain syndrome (Kremeyer et al., 2010).
Similar to TRPV1, TRPA1 is sensitized by pro-inflammatory agents such as bradykinin (Bandell et al., 2004) and protease-activated receptor PAR2 (Dai et al., 2007). The mechanism of this is far less studied than that of TRPV1, but the PLC pathway is quite likely to be involved either via increased cytoplasmic Ca2+ (Wang et al., 2008), reduced PI(4,5)P2 levels (Dai et al., 2007) or increased trafficking (Schmidt et al., 2009). TRPA also undergoes Ca2+-induced inactivation/desensitization, and PLC mediated PI(4,5)P2 depletion may also be involved in this phenomenon (Karashima et al., 2008). The regulation of this channel by PI(4,5)P2 is quite controversial, and have been discussed earlier (Rohacs, 2009).
Inhibition or desensitization by PLC activation
TRPM7 and TRPM6
These two channels are close homologues, and both have an atypical kinase domain at the C-terminus. Unlike any other TRP channels, genetic deletion of both TRPM6 (Walder et al., 2009) and TRPM7 (Jin et al., 2008) results in embryonic lethality in mice, due to various developmental defects. In humans, loss of function mutations in TRPM6 cause a more restricted disease phenotype, Hypomagnesemia with secondary hypocalcemia, presumably due to impaired Mg2+ absorption through this channel in the intestines and kidneys (Walder et al., 2002).
TRPM7 was the first TRP channel where dependence of activity on PI(4,5)P2 was demonstrated and it was proposed in the same study that activation of muscarinic receptors by carbachol inhibited these channels via depletion of PI(4,5)P2 (Runnels et al., 2002). Later this was challenged and it was proposed that PLC is not a major regulator of these channels, but rather the increase in cAMP exerts a robust inhibitory effect (Takezawa et al., 2004). In another paper (Langeslag et al., 2007) found that activation of PLC activates, rather than inhibits TRPM7 channels in the perforated patch configurations, but the inhibition observed earlier was reproduced in the whole-cell configuration. A recent paper demonstrated PI(4,5)P2 dependence of TRPM6, and showed that activation of PLC inhibited this channel (Xie et al., 2011).
TRPM8
TRPM8 is a sensory ion TRP channel activated by cold temperatures and menthol (McKemy et al., 2002; Peier et al., 2002). These channels are expressed mainly in sensory neurons of the dorsal root and trigeminal ganglia, and knockout studies confirmed their role in detecting moderately cold ambient temperatures (Bautista et al., 2007; Dhaka et al., 2007). The activation of TRPM8 both by cold and menthol requires the presence of PI(4,5)P2. The dependence of TRPM8 activity on PI(4,5)P2 has been demonstrated in excised patches (Liu and Qin, 2005; Rohacs et al., 2005; Yudin et al., 2011), planar lipid bilayers (Zakharian et al., 2009; Zakharian et al., 2010) and in intact cells, using various inducible lipid phosphatases (Varnai et al., 2006; Wang et al., 2008; Daniels et al., 2009; Yudin et al., 2011). Activation of the PLC pathway either by the inflammatory mediator bradykinin (Premkumar et al., 2005), or by Ca2+ influx through the channel (Rohacs et al., 2005; Daniels et al., 2009; Yudin et al., 2011) inhibits TRPM8. Ca2+ influx through the channel and the resulting PLCδ activation and PI(4,5)P2 depletion mediates desensitization/adaptation of responses to menthol and cold (Daniels et al., 2009; Yudin et al., 2011), (Figure 3). CaM has also been implicated in the acute phase of desensitization of TRPM8 (Sarria et al., 2011). The PKC pathway was implicated in the bradykinin-induced inhibition (Premkumar et al., 2005; Linte et al., 2007). As an alternative hypothesis for the bradykinin-induced inhibition it was proposed that Gq directly inhibits TRPM8 (Zhang et al., 2012). Overall, the PLC pathway is a negative regulator of TRPM8, and the relative roles of various downstream effectors have not fully been deciphered yet. We have recently reviewed the regulation of TRPM8 by various signaling pathways including PLC in more detail (Yudin and Rohacs, 2011).
TRPV2
TRPV2 was originally proposed to be a noxious heat sensor, but its role in thermosensation has been challenged, because TRPV2−/− mice have no defects in heat sensation (Park et al., 2011). On the other hand, TRPV2−/− mice had impaired phagocytosis in macropages (Link et al., 2010). Physiologically these channels are probably activated by various growth factors (Kanzaki et al., 1999). It was shown that TRPV2 undergoes desensitization in response to their chemical agonist 2-APB, with a mechanism similar to that of several TRP channels (Figure 3), i.e. Ca2+ influx mediated activation of PLC and the resulting depletion of PI(4,5)P2 (Mercado et al., 2010). The same article showed that these channels require PI(4,5)P2 for activity in excised patches.
TRPV4
TRPV4 is an ion channel activated both by moderate heat and cellular swelling induced by hyposmosis. Accordingly, the knockout mice have disturbances in thermosensation (Lee et al., 2005a) osmoregulation (Liedtke and Friedman, 2003), and some mutations in humans are associated with hyponatremia (Tian et al., 2009). Quite intriguingly, other mutations in TRPV4 cause a spectrum of developmental disorders (Nilius and Voets, 2013). A recent paper showed that the activation of this channel by heat and osmotic stimuli requires the presence of PI(4,5)P2, and activation of the PLC pathway inhibited channel activity (Garcia-Elias et al., 2013). Another article however reported that bradykinin and activation of PKC by phorbol esters potentiate TRPV4 channels (Fan et al., 2009).
TRPV5 and TRPV6
These two channels are close homologues of each other, but differ from other TRP channels considerably both biophysically and functionally. While most TRP channels are outwardly rectifying Ca2+ permeable non-selective cation channels, TRPV5 and 6 are inwardly rectifying Ca2+ selective channels. They are found in the apical membrane of various epithelial cells, including the distal tubule in the kidney, the duodenum and the epididymis. They participate in vectorial Ca2+ transport form the lumen to the interstitium (Hoenderop et al., 2005). Accordingly, TRPV5−/− mice have disturbances in Ca2+ reabsorption in the kidneys (Hoenderop et al., 2003), TRPV6−/− mice have moderately impaired Ca2+ absorption in the duodenum (Bianco et al., 2007) and the male TRPV6−/− animals are infertile (Weissgerber et al., 2011). The activity of both of these channels depend on PI(4,5)P2 (Lee et al., 2005b; Rohacs et al., 2005; Thyagarajan et al., 2008; Zakharian et al., 2011). Both channels are constitutively active, but undergo Ca2+-induced inactivation. We have shown earlier that Ca2+-influx through TRPV6 activates PLC, and inhibition of this pathway reduced Ca2+ induced inactivation (Thyagarajan et al., 2009) (Figure 3). The ubiquitous Ca2+ binding protein, calmodulin (CaM) is also implicated in Ca2+-induced inactivation of TRPV6 (Niemeyer et al., 2001; Derler et al., 2006) and TRPV5 (de Groot et al., 2011). We have found that CaM and PI(4,5)P2 reciprocally regulate TRPV6 in excised patches, i.e. high concentrations of PI(4,5)P2 alleviate Ca2+-CaM inhibition (Cao et al., 2013a). It is likely that CaM and PI(4,5)P2 depletion upon PLC activation cooperate to mediate Ca2+-induced inactivation and thus the channel serves as a coincidence detector of these two signals.
CONCLUSIONS
TRPC channels are downstream effectors of the PLC pathway, but their activation mechanism is not elucidated yet; it is possible that multiple signaling mechanisms cooperatively open these channels upon PLC activation. Figure 1 compiles potential mechanisms that have been implicated in activation of the drosophila visual TRP channel complex, or mammalian TRPC channels. The heat- and capsaicin-sensitive TRPV1 channels are regulated in a complex manner by two different PLC isoforms, during sensitization and desensitization, and signaling specificity may be mediated by differential changes in PI(4,5)P2 and PI(4)P levels (Figure 2). Many other TRP channels are also modulated by the PLC pathway, in several cases this is mediated by the reduction of PI(4,5)P2 levels, but other downstream effectors of the PLC pathway also contribute to their regulation.
Acknowledgments
This work was supported by grants R01NS055159 and R01GM093290 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18:881–887. doi: 10.1016/s0896-6273(00)80328-1. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Beech DJ. Integration of transient receptor potential canonical channels with lipids. Acta Physiol (Oxf) 2012;204:227–237. doi: 10.1111/j.1748-1716.2011.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, Luo H, Mauro T, Brown EM, Hediger MA. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res. 2007;22:274–285. doi: 10.1359/jbmr.061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- Cao C, Zakharian E, Borbiro I, Rohacs T. Interplay between calmodulin and phosphatidylinositol 4,5-bisphosphate in Ca2+-induced inactivation of transient receptor potential vanilloid 6 channels. J Biol Chem. 2013a;288:5278–5290. doi: 10.1074/jbc.M112.409482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013b;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, Knoff J, Eisinger B, Liu ML, Huang SM, Caterina MJ, Dempsey P, Michael LE, Dlugosz AA, Andrews NC, Clapham DE, Xu H. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141:331–343. doi: 10.1016/j.cell.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284:1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot T, Kovalevskaya NV, Verkaart S, Schilderink N, Felici M, van der Hagen EA, Bindels RJ, Vuister GW, Hoenderop JG. Molecular mechanisms of calmodulin action on TRPV5 and modulation by parathyroid hormone. Mol Cell Biol. 2011;31:2845–2853. doi: 10.1128/MCB.01319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R, Bacigalupo J. Unitary recordings of TRP and TRPL channels from isolated Drosophila retinal photoreceptor rhabdomeres: activation by light and lipids. J Neurophysiol. 2009;101:2372–2379. doi: 10.1152/jn.90578.2008. [DOI] [PubMed] [Google Scholar]
- Derler I, Hofbauer M, Kahr H, Fritsch R, Muik M, Kepplinger K, Hack ME, Moritz S, Schindl R, Groschner K, Romanin C. Dynamic but not constitutive association of calmodulin with rat TRPV6 channels enables fine tuning of Ca2+-dependent inactivation. J Physiol. 2006;577:31–44. doi: 10.1113/jphysiol.2006.118661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Hatt H, Ramsey IS. Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J Gen Physiol. 2011;137:271–288. doi: 10.1085/jgp.200910388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estacion M, Sinkins WG, Schilling WP. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J Physiol. 2001;530:1–19. doi: 10.1111/j.1469-7793.2001.0001m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Curr Biol. 2010;20:R114–124. doi: 10.1016/j.cub.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem. 2009;284:27884–27891. doi: 10.1074/jbc.M109.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Fukami K, Inanobe S, Kanemaru K, Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res. 2010;49:429–437. doi: 10.1016/j.plipres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Gamper N, Rohacs T. Phosphoinositide sensitivity of ion channels, a functional perspective. Subcell Biochem. 2012;59:289–333. doi: 10.1007/978-94-007-3015-1_10. [DOI] [PubMed] [Google Scholar]
- Garcia-Elias A, Mrkonjic S, Pardo-Pastor C, Inada H, Hellmich UA, Rubio-Moscardo F, Plata C, Gaudet R, Vicente R, Valverde MA. Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1220231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SR, Dietrich A, Stucky CL. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J Neurophysiol. 2012;107:913–922. doi: 10.1152/jn.00658.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Salle L, Simard C. The non-selective monovalent cationic channels TRPM4 and TRPM5. Adv Exp Med Biol. 2011;704:147–171. doi: 10.1007/978-94-007-0265-3_8. [DOI] [PubMed] [Google Scholar]
- Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 Are Essential But Independent Lipid Determinants of Membrane Identity. Science. 2012;337:727–30. doi: 10.1126/science.1222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science. 2012;338:260–263. doi: 10.1126/science.1222376. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:re19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest. 2003;112:1906–1914. doi: 10.1172/JCI19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 2010;20:189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Imai Y, Itsuki K, Okamura Y, Inoue R, Mori MX. A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P2-diacylglycerol signalling. J Physiol. 2012;590:1101–1119. doi: 10.1113/jphysiol.2011.221358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Por ED, Belugin S, Chaudhury S, Berg KA, Akopian AN, Henry MA, Gomez R. A-kinase anchoring protein 150 mediates transient receptor potential family V type 1 sensitivity to phosphatidylinositol-4,5-bisphosphate. J Neurosci. 2011;31:8681–8688. doi: 10.1523/JNEUROSCI.0020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch. 2008;457:77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim MT, Jeon JH, Kim SJ, So I. Involvement of phosphatidylinositol 4,5-bisphosphate in the desensitization of canonical transient receptor potential 5. Biol Pharm Bull. 2008a;31:1733–1738. doi: 10.1248/bpb.31.1733. [DOI] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Simkin D. Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol. 2008b;295:C92–99. doi: 10.1152/ajpcell.00023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Klein RM, Ufret-Vincenty CA, Hua L, Gordon SE. Determinants of molecular specificity in phosphoinositide regulation. Phosphatidylinositol (4,5)- bisphosphate (PI(4,5)P2) is the endogenous lipid regulating TRPV1. J Biol Chem. 2008;283:26208–26216. doi: 10.1074/jbc.M801912200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremeyer B, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K. Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J Biol Chem. 2007;282:232–239. doi: 10.1074/jbc.M605300200. [DOI] [PubMed] [Google Scholar]
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005a;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Cha SK, Sun TJ, Huang CL. PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+ J Gen Physiol. 2005b;126:439–451. doi: 10.1085/jgp.200509314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier L, Trebak M, Putney JW., Jr Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium. 2008;43:506–514. doi: 10.1016/j.ceca.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, An L, Doerge RW, Pak WL. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron. 2008;58:884–896. doi: 10.1016/j.neuron.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Katz B, Tzarfaty V, Minke B. Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: implications on gating of Drosophila TRPL (transient receptor potential-like) channel. J Biol Chem. 2012;287:1436–1447. doi: 10.1074/jbc.M111.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci U S A. 2009;106:3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90:558–564. doi: 10.1016/j.ajhg.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linte RM, Ciobanu C, Reid G, Babes A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp Brain Res. 2007;178:89–98. doi: 10.1007/s00221-006-0712-3. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs VYY, Hammond GR, Sharma E, Fukami K, Rohacs T. Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J Neurosci. 2013 doi: 10.1523/JNEUROSCI.5637-12.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Mercado J, Gordon-Shaag A, Zagotta WN, Gordon SE. Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2010;30:13338–13347. doi: 10.1523/JNEUROSCI.2108-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- Montell C. Drosophila visual transduction. Trends Neurosci. 2012;35:356–363. doi: 10.1016/j.tins.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C. Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc Natl Acad Sci U S A. 2001;98:3600–3605. doi: 10.1073/pnas.051511398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012;464:425–458. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. Embo J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013;14:152–163. doi: 10.1038/embor.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Piper AS, Yeats JC, Bevan S, Docherty RJ. A study of the voltage dependence of capsaicin-activated membrane currents in rat sensory neurones before and after acute desensitization. J Physiol. 1999;518(Pt 3):721–733. doi: 10.1111/j.1469-7793.1999.0721p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci. 2005;25:11322–11329. doi: 10.1523/JNEUROSCI.3006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Putney JW, Tomita T. Phospholipase C signaling and calcium influx. Adv Enzyme Regul. 2011 doi: 10.1016/j.advenzreg.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick K, et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2012;2:120068. doi: 10.1098/rsob.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P, Hardie RC. Regulation of Drosophila TRPC channels by lipid messengers. Cell Calcium. 2009;45:566–573. doi: 10.1016/j.ceca.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T. Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium. 2009;45:554–565. doi: 10.1016/j.ceca.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T. Phosphoinositide regulation of TRP channels. Handb Exp Pharmacol. 2013 doi: 10.1007/978-3-319-05161-1_18. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Sarria I, Ling J, Zhu MX, Gu JG. TRPM8 acute desensitization is mediated by calmodulin and requires PIP2: distinction from tachyphylaxis. J Neurophysiol. 2011;106:3056–3066. doi: 10.1152/jn.00544.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szolcsanyi J, Anton F, Reeh PW, Handwerker HO. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res. 1988;446:262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci U S A. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B, Benn BS, Christakos S, Rohacs T. Phospholipase C-mediated regulation of transient receptor potential vanilloid 6 channels: implications in active intestinal Ca2+ transport. Mol Pharmacol. 2009;75:608–616. doi: 10.1124/mol.108.052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B, Lukacs V, Rohacs T. Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J Biol Chem. 2008;283:14980–14987. doi: 10.1074/jbc.M704224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Fu Y, Garcia-Elias A, Fernandez-Fernandez JM, Vicente R, Kramer PL, Klein RF, Hitzemann R, Orwoll ES, Wilmot B, McWeeney S, Valverde MA, Cohen DM. A loss-of-function nonsynonymous polymorphism in the osmoregulatory TRPV4 gene is associated with human hyponatremia. Proc Natl Acad Sci U S A. 2009;106:14034–14039. doi: 10.1073/pnas.0904084106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Lemonnier L, DeHaven WI, Wedel BJ, Bird GS, Putney JW., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 2009;457:757–769. doi: 10.1007/s00424-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW., Jr The mammalian TRPC cation channels. Biochim Biophys Acta. 2004;1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- Walder RY, Yang B, Stokes JB, Kirby PA, Cao X, Shi P, Searby CC, Husted RF, Sheffield VC. Mice defective in Trpm6 show embryonic mortality and neural tube defects. Hum Mol Genet. 2009;18:4367–4375. doi: 10.1093/hmg/ddp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissgerber P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, Vennekens R, Wissenbach U, Middendorff R, Flockerzi V, Freichel M. Male fertility depends on Ca2+ absorption by TRPV6 in epididymal epithelia. Sci Signal. 2011;4:ra27. doi: 10.1126/scisignal.2001791. [DOI] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Sun B, Du J, Yang W, Chen HC, Overton JD, Runnels LW, Yue L. Phosphatidylinositol 4,5-bisphosphate (PIP2) controls magnesium gatekeeper TRPM6 activity. Sci Rep. 2011;1:146. doi: 10.1038/srep00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YP, Zhang JW, Li L, Ye ZY, Zhang Y, Gao X, Li F, Yan XS, Liu ZG, Liu LJ, Cao XH. Complex regulation of capsaicin on intracellular second messengers by calcium dependent and independent mechanisms in primary sensory neurons. Neurosci Lett. 2012;517:30–35. doi: 10.1016/j.neulet.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, Yau KW. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin Y, Lukacs V, Cao C, Rohacs T. Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J Physiol. 2011;589:6007–6027. doi: 10.1113/jphysiol.2011.220228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin Y, Rohacs T. Regulation of TRPM8 channel activity. Mol Cell Endocrinol. 2011;353:68–74. doi: 10.1016/j.mce.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E, Cao C, Rohacs T. Intracellular ATP supports TRPV6 activity via lipid kinases and the generation of PtdIns(4,5)P2. FASEB J. 2011;25:3915–3928. doi: 10.1096/fj.11-184630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E, Thyagarajan B, French RJ, Pavlov E, Rohacs T. Inorganic polyphosphate modulates TRPM8 channels. PLoS One. 2009;4:e5404. doi: 10.1371/journal.pone.0005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005a;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, McNaughton PA. Direct inhibition of the cold-activated TRPM8 ion channel by Galpha(q) Nat Cell Biol. 2012;14:851–858. doi: 10.1038/ncb2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem. 2005b;280:39185–39192. doi: 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci U S A. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]