Abstract
Eosinophil-produced cytokines have been shown to participate in the maintenance of antigen-specific plasma cells (PC) in bone marrow (BM), suggesting that eosinophils are required in the development and/or maintenance of alloantibody responses post-transplant.
To test this hypothesis, we sensitized eosinophil-deficient ΔdblGATA1 mice and WT control mice with allogeneic splenocytes or with allogeneic heart grafts and compared the kinetics and titers of serum donor-specific antibodies (DSA), as well as BM and spleen CD130+B220low PC populations between groups.
Spleen cells from naïve ΔdblGATA1 BALB/c mice contained higher percentages of PC than WT without detectable differences in BM PCs. After sensitization with allogeneic splenocytes, BALB/c ΔdblGATA1 mice contained fewer BM PCs but more splenic PCs compared to controls. These differences were associated with modestly lower titers of serum DSA 4 and 12 weeks after sensitization but secondary immunizations induced similar increases in both groups. Moreover, the kinetics and strength of DSA did not differ in WT and ΔdblGATA1 BALB/c mice transplanted with B6 cardiac allografts, nor did they differ in transplanted ΔdblGATA1 and WT mice on a B6 background.
Therefore, eosinophils are not required for alloantibody formation or maintenance in mice and are thus unlikely to be effective targets for antibody desensitization.
Keywords: eosinophil, donor-specific antibodies, GATA-1, transplant
INTRODUCTION
Preformed circulating anti-human leukocyte antigen (anti-HLA) antibodies at the time of kidney or heart transplantation expose patients to an increased risk of acute rejection and reduced 1-year graft survival [1–3]. De novo post-transplant anti-HLA antibodies develop in up to 60% [4] of kidney transplant patients and 80% [5] of heart transplant patients and increasing evidence indicates donor reactive alloantibodies contribute to late allograft loss [4, 6]. As sensitized transplant candidates comprise an increasing percentage of transplant waiting lists [7] and graft outcomes are worse in sensitized patients [1–3] there is an urgent need to better understand mechanisms of alloantibody formation and maintenance, so as to be able to better devise effective therapies.
B cell derived, antibody secreting plasma cells (PC) are crucial producers of alloantibodies. While long lived PC can be detected in the periphery, the majority of PC are maintained within specific niches in the BM. Increasing evidence indicates that the cytokines IL-6 and APRIL are essential for PC survival [8, 9]. A 2011 manuscript [10] provided intriguing evidence that the predominant cellular source of these survival factors is the eosinophil, as eosinophils were found in proximity to BM PCs, and mice deficient in eosinophils contained fewer antigen specific PCs following immunization with the hapten 2-phenyloxazolone (phOx).
These unexpected observations using a model antigen raised the intriguing hypothesis that BM eosinophils are essential for the production and maintenance of antibodies following exposure to alloantigens. If correct, the hypothesis would have important clinical implications for designing and testing novel, eosinophil-directed therapies to prevent and/or treat transplant-reactive alloantibodies. Herein we tested this hypothesis by comparing alloantibody formation and maintenance in eosinophil deficient mice.
METHODS
Mice
Wild type (WT) and double GATA1 mutant (ΔdblGATA1) BALB/c (H-2d) and C57BL/6 (H-2b, termed B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in the Mount Sinai School of Medicine Center for Comparative Medicine and Surgery in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. The study protocols described herein were reviewed and approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine, New York, NY, USA.
Reagents
Rat anti-murine B220, F4/80, and CD11b were from eBioscience (San Diego, CA, USA) and rat anti-murine CD138, Gr-1, and Siglec-F were purchased from BD Biosciences (Franklin Lakes, NJ USA). Appropriate Rat isotype controls were purchased from BD Biosciences.
Flow cytometry
Cell surface and intracellular staining was performed as described elsewhere [11], data were collected on a FACSCanto II (BD Biosciences) and analyzed using FloJo software (Tree Star, Ashland, OR USA). As nonspecific binding of serum to syngeneic thymocytes was never greater than 5% (not shown) we defined the DSA titer as the serum dilution that yielded >5% binding to donor thymocytes.
Eosinophil depletion
BALB/c and B6 mice were injected intraperitoneally (i.p.) three times (every other day) with 20 μg soluble rat anti-mouse Siglec-F (clone 238047, R&D Systems, Minneapolis, MN, USA or E50-2440, BD Biosciences). Rat IgG2a (R&D Systems) was used as the isotype-matched control antibody for both anti-Siglec-F clones. At day 4 after the final injection, eosinophils were analyzed in bone marrow, blood and spleen and the number of plasma cells in bone marrow and spleen determined.
Allosensitization and cardiac transplantation procedure
Animals were sensitized by allogeneic spleen cell i.p. injections (10×106 on day 0, 7, and 14). Heterotopic heart transplantation was performed as previously described. Heart graft function was monitored daily by palpation and rejection was defined as the day on which a palpable heartbeat was no longer detectable [11].
Alloantibody detection
Serum samples from recipient mice were diluted in PBS as indicated and incubated for 30 minutes at 4°C with syngeneic, donor or third-party thymocytes as target cells. Following a wash step with PBS 1% albumin, the bound antibody was detected by incubation with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse IgG (eBioscience) and quantified by flow cytometry.
Statistical analysis
To determine whether differences between groups were statistically significant, results were compared using Student’s t test. Log-rank test was employed to compare survival curves. A P value less than 0.05 was considered significant.
RESULTS
Eosinophil deficiency alters plasma cell distribution patterns in vivo
To study the impact of eosinophils on PCs and alloantibody formation, we used ΔdblGATA1 mice, which have a deletion in the high-affinity GATA-binding site of the promoter of the gene encoding the transcription factor GATA1. This mutation specifically blocks the development of mature eosinophils without other effects on hematopoiesis [12].
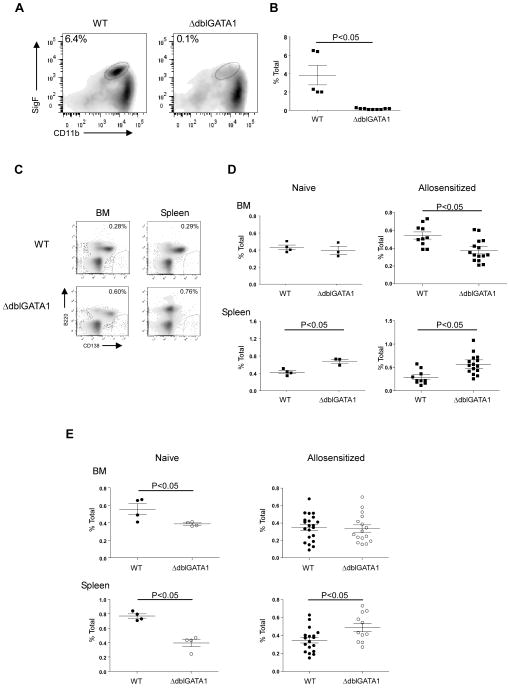
As previously reported by others [12] bone marrow and spleens of ΔdblGATA1 BALB/c mice contained no F4/80+Gr-1loCD11b+Sig-F+ eosinophils (Fig 1A–B). We quantified and compared frequencies of CD138+B220− PC [10] in naïve and alloantigen primed, WT and ΔdblGATA1 mice. Whereas the percentages of PCs were the same in the bone marrow of naïve WT and ΔdblGATA1 mice, we observed significantly higher frequencies of splenic PCs in the ΔdblGATA1 mice (Fig 1C–D). To test whether eosinophil deficiency affects PC populations following exposure to alloantigen we injected the mice (i.p.) with allogeneic B6 spleen cells and assessed PC frequencies 20 weeks later. We observed lower frequencies of BM PC in the ΔdblGATA1 recipients, but spleens of the allo-immunized GATA1Δdbl mice contained higher frequencies of PC than the WT controls. The results support the conclusion that eosinophil deficiency does not alter PC survival as previously reported [10], but instead alters homing patterns such that PC leave the BM and accumulate in the spleen.
Fig. 1. Eosinophil deficiency in ΔdblGATA-1 mice is associated with changes in plasma cell distribution.
(A) Representative flow plots and (B) quantification of CD11b+SigF+ eosinophils in the bone marrow of WT (black squares) and ΔdblGATA1 mice (white squares) gating on F4/80+Gr-1lo cells (n=5 in the WT and n=9 in the ΔdblGATA-1 group). (C) Representative flow plots of CD138+B220− plasma cells in the bone marrow (left) or spleen (right) of WT (top) and ΔdblGATA1 (bottom) mice. (D–E) Quantification of CD138+B220− plasma cells in the bone marrow and in the spleen of WT and ΔdblGATA1 BALB/c (D), and B6 (E) mice prior to i.p. injection (left, naïve; n= 3–4 per group) and at 20 weeks after sensitization with allogeneic splenocytes (right; BALB/c n= 10–15 and B6 n= 16–17 per group). Data are means ± SD.
We repeated the same experiments in WT and ΔdblGATA1 mice on a B6 background, first confirming that the ΔdblGATA1 B6 mice were eosinophil-deficient (3.16±0.06 vs. 0.73±0.03% of bone marrow cells; p<0.05). BM and spleens of naïve B6 ΔdblGATA1 mice contained fewer PCs than WT (Fig 1E). However, analysis of the same tissues at 20 weeks after i.p. immunization with allogeneic BALB/c spleen cells, revealed similar (BM) or higher (spleen) frequencies of PCs frequencies in the GATA1Δdbl mice (Fig 1E).
Absence of eosinophils lowers but does not eliminate DSA following i.p. injection of allogeneic cells
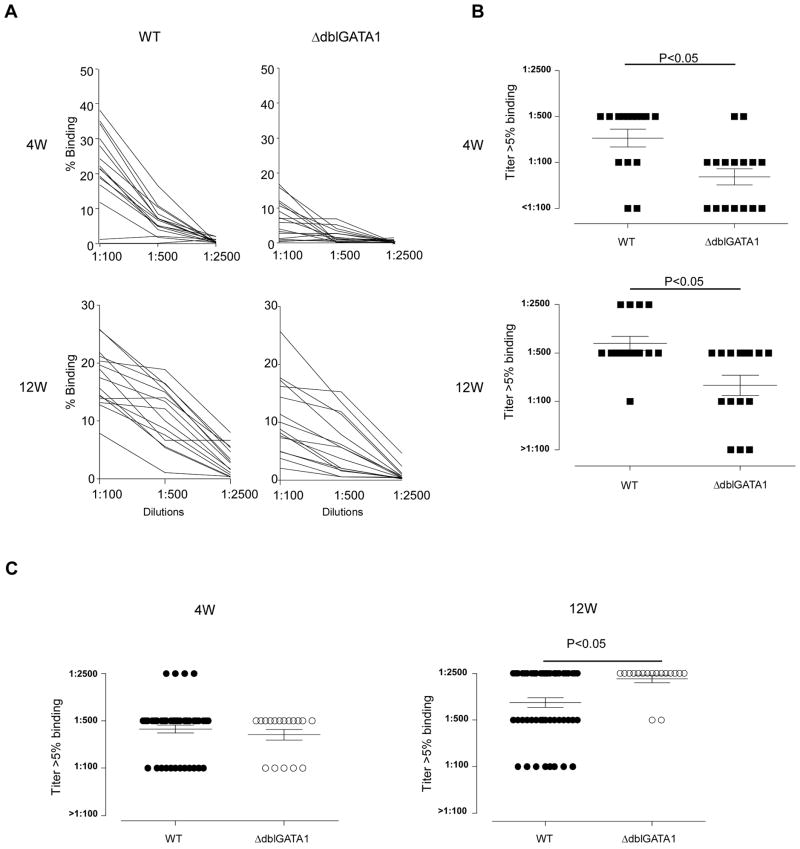
To test the impact of absent eosinophils on the kinetics and strength of alloantibody formation, we immunized WT and ΔdblGATA1 BALB/c mice with B6 splenocytes and quantified titers of serum DSA 4, 12, and 20 weeks later (Fig 2A–B). These analyses showed that all animals in both groups produced DSA, but the titers in the ΔdblGATA1 mice were significantly lower than the WT at all the time points. We repeated the same experiments in WT and ΔdblGATA1 mice on a B6 background by immunizing them with BALB/c spleen cells and testing for DSA 4–12 weeks later. These assays showed strong DSA in all animals and interestingly did not reveal differences in titers between the two groups (Fig 2C).
Fig. 2. Allosensitization by donor splenocytes elicits lower DSA titers in eosinophil deficient BALB/c, but not B6 mice.
A–B. Sera obtained from WT (black squares) and ΔdblGATA1 (white squares) BALB/c and B6 mice immunized with allogeneic B6 or BALB/c splenocytes (2 × 106 at d0 and d14), respectively, were tested by flow cytometry for binding to B6 thymocytes. A, % binding of each sera at various dilutions. (B) DSA titer (serum dilution at which binding to donor thymocytes is >5%) at 4w (top) and 12w (bottom) in WT and ΔdblGATA-1 BALB/c mice immunized with B6 splenocytes. (C) DSA titers at 4w (left) and 12w (right) from WT and ΔdblGATA-1 B6 mice immunized with BALB/c spleen cells. Similar trends in DSA titer were found at 20 weeks after B6 splenocyte injection or heart transplantation (not shown). Data are means ± SD. Binding to syngeneic BALB/c thymocytes was always <5% (not shown).
Absence of eosinophils does not impact DSA following heterotopic heart transplantation
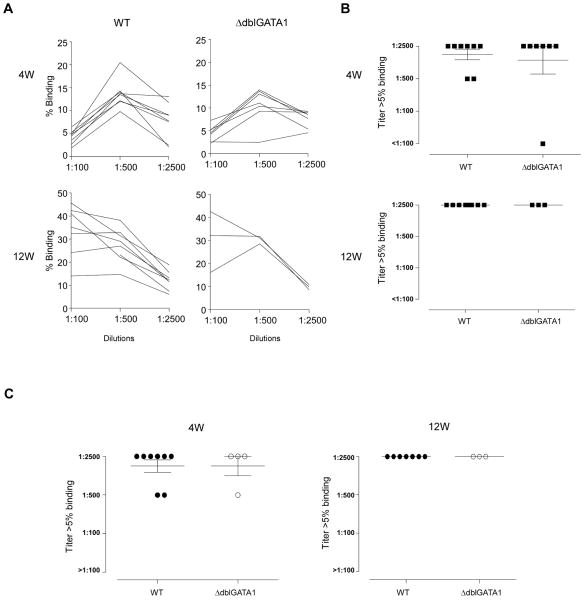
We expanded the experiments to a more clinically relevant model of solid organ transplantation in which allogeneic tissue persists long term in the host by serially analyzing the kinetics and strength of DSA in ΔdblGATA1 and WT BALB/c recipients of B6 heterotopic heart transplants. Allografts rejected 7–9 days post-transplantation in both groups (n=6–7 per group, p=ns). We tested serum from each animal for DSA 4 and 12 weeks later. In contrast to the above reported findings in mice allosensitized by i.p. injection of donor spleen cells, we did not observe differences in DSA titers between BALB/c ΔdblGATA1 and WT recipients of B6 hearts at either time point (Figure 3A–B). We performed analogous experiments by transplanting BALB/c hearts into WT or GATA1Δdbl B6 recipients and again observed no differences in DSA (Fig 3C).
Fig. 3. Eosinophil deficiency in both BALB/c and B6 mice is not associated with reduced DSA responses.
Sera obtained from WT (black squares) and ΔdblGATA1 (white squares) BALB/c and B6 mice following rejection of a heterotopic heart transplant were tested by flow cytometry for binding to B6 thymocytes. A, % binding of each sera at various dilutions. (B) DSA titer (serum dilution at which binding to donor thymocytes is >5%) at 4w (top) and 12w (bottom) in WT and ΔdblGATA-1 BALB/c mice immunized with B6 splenocytes. (C) DSA titers at 4w (left) and 12w (right) from WT and ΔdblGATA-1 B6 mice immunized with BALB/c spleen cells.. Data are means ± SD. None of the sera bound to syngeneic B6 thymocytes (not shown).
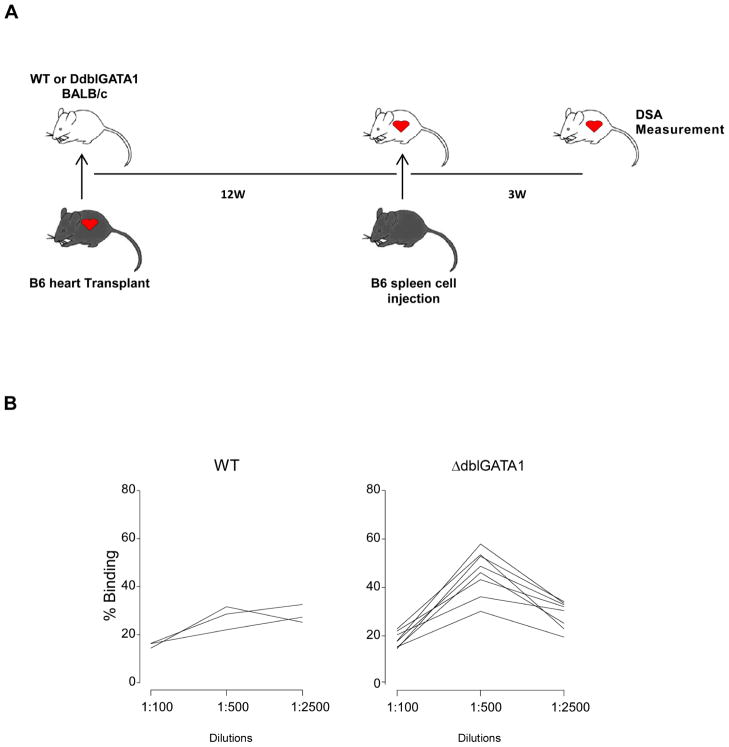
To test whether eosinophils are required for the development of secondary alloantibody responses, we immunized BALB/c WT and ΔdblGATA1 mice with allogeneic B6 spleen cells 12 weeks after they rejected B6 cardiac allografts and tested serum for DSA 3 weeks later (Fig 4A). These assays showed a significant increase in DSA binding to B6 thymocytes only in WT animals (max % binding: 27.4±0.9 vs. 46.7±1.1, p<0.05 and 30.5±0.6 vs. 27.5±1.6 in WT and ΔdblGATA1 mice, respectively), but the serum titer defined by >5% binding did not differ between the two groups (Fig 2B).
Fig. 4. Eosinophil deficiency does not affect secondary alloantibody response.
(A) Schematic of the experimental design to test secondary alloantibody response. (B) Donor specific antibody (DSA) in B6 heart transplant WT and ΔdblGATA-1 BALB/c mice challenged with B6 splenocytes at 12 weeks after grafting. DSA was measured at 3 weeks after reimmunization.
DISCUSSION
Whereas previous work by others implicated eosinophils as crucial for PC survival and function in the BM [10], our new data indicate that alloantibody production and maintenance are not eosinophil-dependent. We observed that eosinophil deficiency in the BALB/c background alters PC trafficking/homing with enhanced accumulation in the spleen compared to WT controls (Fig 1). The absence of eosinophils also resulted in modestly lowered DSA titers in animals immunized with allogeneic cells (Fig 2). However, the effects were only detectable in animals on a BALB/c background (Fig 2), the background strain studied in the previously published work using model antigens [10], and did not impact on primary or secondary alloantibody titers following solid organ transplantation (Fig 3). The strength and polyclonality of the allo-immune response induced by prolonged in vivo exposure to a solid organ transplant expressing a wide array of alloantigenic epitopes differs significantly from that induced by a single immunization to a hapten model antigen. Under such circumstances alternative sources of PC-promoting cytokines (beyond eosinophils) seem to be involved. It is also notable that the previously reported work that eosinophil deficiency reduced phOx-reactive BM PCs did not report effects on phOx-reactive antibody titers [10].
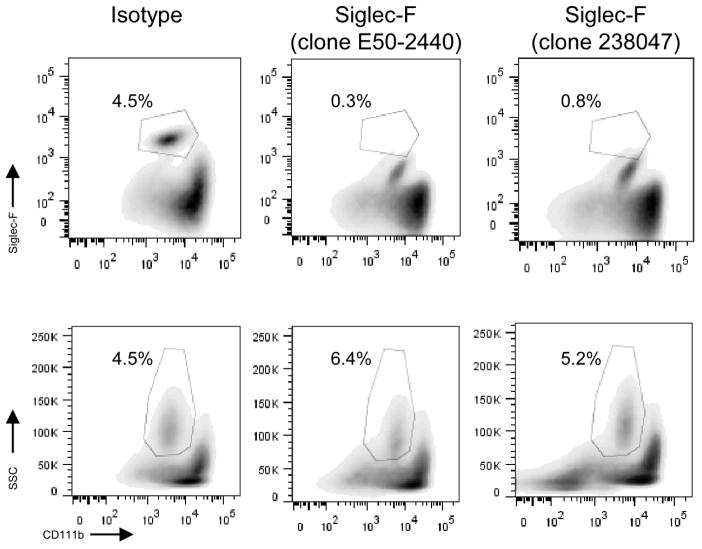
In an effort to test the role of eosinophils on allo-antibody production using an alternative experimental approach, we attempted to deplete them with an anti-Siglec-F mAb using published protocols. Importantly, the E50-2440 antibody clone (E50-2440) is the same as that used to identify eosinophils by flow cytometry (Fig 5). Pilot studies showed that in vivo treatment with anti-Sig-F mAb masks the relevant epitope, and that the eosinophils can only be detected by flow cytometry using an alternative gating strategy (Fig 5). Intriguingly, and in contrast to reports in which eosinophils were quantified by histology [10, 13], i.p. administration of anti-Sig-F had essentially no effect the frequency of eosinophils in BM or spleen (Fig 5) and did not alter antibody titers (not shown). We repeated the experiments using a different clone (238047) of the antibody and varied dosing schedules without discernible effects on the frequency of PCs in BM or spleen (Fig 5). Thus, our data do not support the use of anti-Siglec-F mAb as an effective eosinophil-depletion strategy in mice.
Fig. 5. Siglec-F antibody does not effectively deplete eosinophils.
Representative flow plots of CD11b+ eosinophils in the bone marrow of BALB/c mice at 4 days after last treatment with vehicle control or Siglec-F antibody (clones E50-2440 and 238047; three 20μg i.p. injections every other day). Cells were gated on F4/80+Gr-1lo cells and (top) Siglec-F+ CD11b+ or (bottom) SSC and CD11b+. We obtained similar results administering 5 i.p. Siglec-F antibody injections every day, both in BALB/c and in B6 mice and looking either in the bone marrow or in the spleen (not shown).
In sum, our results indicate subtle and strain dependent effects of eosinophils on alloantibody formation, and support the conclusion that eosinophils are not required for the development and/or maintenance of primary or secondary alloantibody responses to a transplant in mice.
Acknowledgments
The work was supported by NIH 3R01 AI43578 awarded to PSH. PC is a recipient of a fellowship award from the American Heart Association (12POST12050294).
ABBREVIATIONS
- BM
bone marrow
- PC
plasma cells
- DSA
donor-specific antibodies
- anti-HLA
anti-human leukocyte antigen
- phOx
2-phenyloxazolone
- WT
wild type
- ΔdblGATA1
double GATA1 mutant
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Patel AM, Pancoska C, Mulgaonkar S, Weng FL. Renal transplantation in patients with pre-transplant donor-specific antibodies and negative flow cytometry crossmatches. Am J Transplant. 2007;7:2371–2377. doi: 10.1111/j.1600-6143.2007.01944.x. [DOI] [PubMed] [Google Scholar]
- 2.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JD, Hamour IM, Banner NR, Rose ML. C4d fixing, luminex binding antibodies - a new tool for prediction of graft failure after heart transplantation. Am J Transplant. 2007;7:2809–2815. doi: 10.1111/j.1600-6143.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 4.Akalin E, Pascual M. Sensitization after kidney transplantation. Clin J Am Soc Nephrol. 2006;1:433–440. doi: 10.2215/CJN.01751105. [DOI] [PubMed] [Google Scholar]
- 5.Reinsmoen NL, Nelson K, Zeevi A. Anti-HLA antibody analysis and crossmatching in heart and lung transplantation. Transpl Immunol. 2004;13:63–71. doi: 10.1016/j.trim.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Smith JD, Banner NR, Hamour IM, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11:312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed on April 9, 2013];UNfOSh. Available at: http://www.unos.org.
- 8.Cassese G, Arce S, Hauser AE, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 9.Belnoue E, Pihlgren M, McGaha TL, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 10.Chu VT, Frohlich A, Steinhauser G, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 11.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257–268. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C, Cantor AB, Yang H, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann N, McBride ML, Yamada Y, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]