Abstract
Sex differences in autoimmune diseases are evolutionarily tied to the fact that the female immune system is confronted with intense alterations during menstrual cycles, pregnancy and childbirth. These events may be associated with breaches in the mucosal epithelial layers that are shielding us from environmental factors. Associations between environmental agents and autoimmune diseases have been described extensively in prior studies. Little evidence, however, exists for sex-specific environmental effects on autoimmune diseases. In this review, we summarize studies involving this often-neglected aspect. We give examples of environmental factors that may influence the sex bias in autoimmunity. We conclude that most studies do not give insight into sex-specific environmental effects due to the influence of gender-selective social, occupational or other exposures. Prospective studies are needed in order to determine true sex-biased environmental influences. Finally, humanized murine models might aid in better understanding the mechanisms involved in sex-specific environmental effects on autoimmune diseases.
Keywords: autoimmunity, chemicals, smoking, infectious agents, commensal bacteria, microbiota
1. INTRODUCTION
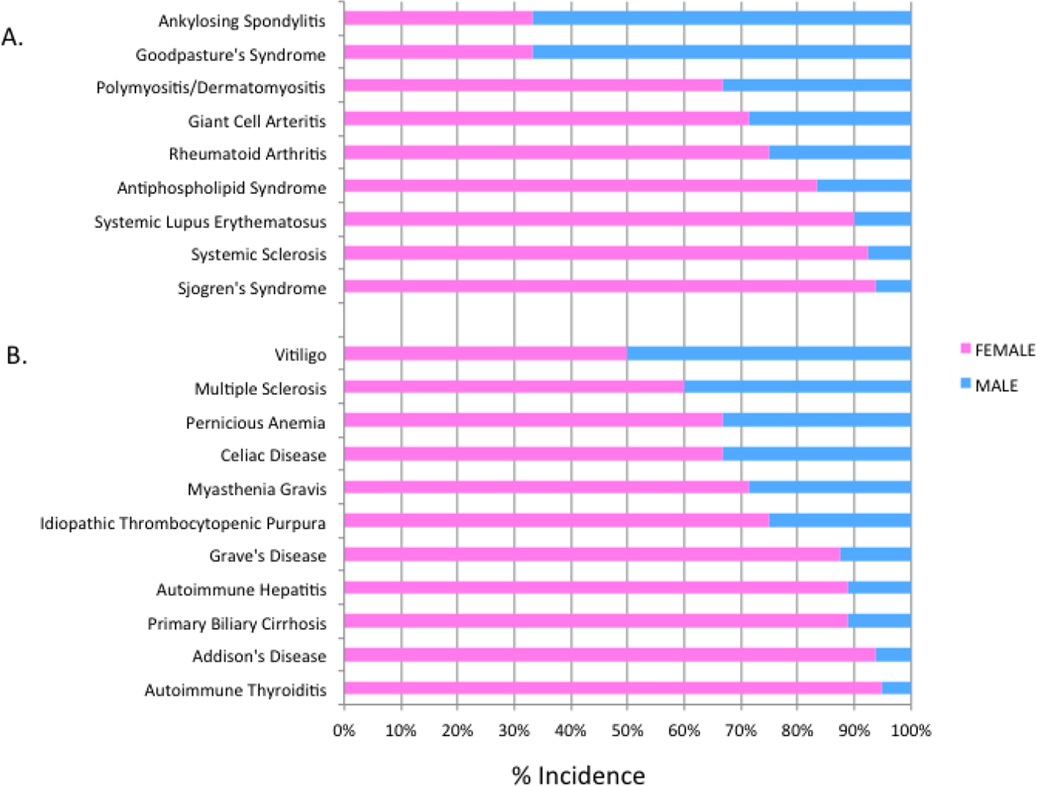
Autoimmune diseases include more than 70 different disorders affecting 5–8% of the general population [1–3]. Autoimmunity is thought to result from a combination of genetic factors and environmental triggers with a wide variability in terms of targeted tissues, age of onset, and response to immunosuppressive treatments. One feature that is shared by many autoimmune conditions, however, is the dramatically increased predisposition to autoimmunity among women. The predisposition is seen in organ-specific diseases such as multiple sclerosis, primary biliary sclerosis and myasthenia gravis. A striking female bias is especially evident in many systemic rheumatic diseases such as Sjogren's syndrome, systemic lupus erythematosus (SLE), mixed connective tissue disease and secondary antiphospholipid syndrome. These often co-existing diseases show a female predominance of approximately 70–90%. Rheumatoid arthritis (RA), polymyalgia rheumatica and primary antiphospholipid syndrome are about two-to-three times more common in females. The exceptions to the rule include ankylosing spondylitis and reactive arthritis where the sex ratio is reversed [2, 4]. Fig. 1 summarizes the spectrum of gender bias across various autoimmune diseases [2, 5].
Fig. 1.
Sex distribution of organ-specific and systemic autoimmune diseases [2, 5]. Panel A shows systemic rheumatic diseases. Panel B shows organ-specific autoimmune diseases.
Many theories have been developed trying to explain sex disparities in autoimmune disease prevalence. While the underlying mechanisms across the spectrum of autoimmune diseases are often related, they also have disease-specific and different underlying etiologies. Therefore, diseases should be examined separately in many cases, ideally with adequate numbers of subjects of both sexes.
Potential explanations for sex skewing of the autoimmune diseases include genetic factors, environmental exposures, gene-environment interactions and epigenetic effects [4, 6].
The influence of genetic factors includes effects of the genes encoded on sex chromosomes themselves, as well as indirect effects mediated by genes encoding sex hormones and related pathways [7]. Observations in humans show an elevated risk of developing SLE among XXY Klinefelter’s males and XX females, and a lower risk among XO females [8]. This phenomenon could be attributed to the presence of an extra X chromosome, although it has been difficult to separate this from the influence of sex hormones. To this end, Smith-Bouvier et al used an elegant model of sex chromosome complement without the confounding effects of differences in gonadal type [7]. They used this model system to study both multiple sclerosis and pristane-induced lupus, respectively, and demonstrated that the presence of two female sex chromosomes predisposes to autoimmunity independently of gonadal hormone production [7]. Several important SLE-associated X-linked genes, including FOXP3, CD40L (CD154), TLR7, SHP2, IL-13R α2, MECP2 and IRAK1 [5, 9], may be relatively over-expressed in women due to incomplete X inactivation. Increased dosage of one or more of these X-linked genes could theoretically promote development of systemic autoimmunity by various mechanisms. For example, follicular helper CD4+ T cells provide crucial costimulatory and survival signals to B cells via CD40L [10], and increased levels of TLR7 can augment RNA-autoantibody immune complex-driven IFNαproduction [11].
Besides the contribution of sex chromosomes to the female sex bias in autoimmune diseases, the female hormones themselves exert an effect on autoimmunity. There is ample evidence for that which is also reviewed elsewhere [8]. Androgens, for instance, tend to favor a Th1 response and activation of CD8+ cells, whereas estrogens exert the opposite effect, promoting Th2 dominance leading to antibody production. They promote extramedullary hematopoiesis, thereby increasing the likelihood of autoreactive B cells to escape negative selection. Estrogens are also known to support the survival of autoreactive T cell clones, which directly promotes autoimmunity [8]. Estrogen may also have immune-independent effects that may impact autoimmunity. Estrogen, for instance, induces DNA instability in breast and ovarian tissues through a mechanism that involves activation-induced deaminase transcription and function, which, in turn may play a role in autoimmunity as suggested by Pauklin et al [12]. These examples give some insights into the manifold hormonal influences on sex bias and autoimmunity development. This topic, however, goes beyond the focus of this review and is discussed elsewhere in this supplement of Clinical Immunology (pp..-.. “Sex Differences in Incidence and Prevalence of RA and SLE” by Tedeschi and Costenbader). In addition to endogenous (genetic or hormonal) factors, environmental factors may have effects on the sex skewing of autoimmune diseases. This additional variable will be discussed in the next section.
2. SEX DISPARITIES IN ENVIRONMENTAL EXPOSURES POTENTIALLY RELATED TO AUTOIMMUNE DISEASES
In a 1998 report, the Institute of Medicine addressed gender differences in susceptibility to environmental factors and defined the terms “sex”, as referring to biologic differences between males and females, and “gender” for societal or culturally-based observed differences [13]. The sex-biased development of autoimmunity could be associated with exposure to differential environmental agents or differential amounts of these agents. Patterns of these exposures may be due to mainly culturally constructed, gender-based reasons. Females are exposed to different factors than males for social and occupational reasons. For example, women in Western societies frequently wear lipstick whereas men are more likely to have occupational exposure to asbestos and silica. In order to be able to establish these as risk factors related to observed disparities, epidemiologic studies should include both men and women equally exposed to them. Environmental effects could be mediated through a variety of mechanisms including alterations in genetic or epigenetic regulation, i.e. changes in gene expression without affecting the DNA sequence itself [14, 15].
Quantification of exposure to environmental agents is another challenge for epidemiologic studies of sex-biased autoimmune diseases. For example, men generally tend to smoke more and heavier than do women. Many past investigations have been based on sampling of one sex, which precludes conclusions about sex disparities. When both sexes are included, it may be difficult to discern whether observed effects are due to chance, dose-effect or differential exposures. The data on sex-specific environmental influences in autoimmune diseases are therefore limited. We have included in this review primarily clinical studies with human subjects that have shown statistically significant differences (Table 1) but did not incorporate ex vivo or in vitro studies with human cells or tissues although these studies would provide additional insight in human disease. Finally, we allude to some studies in murine models where causal relationships are more easily addressed than in human epidemiological studies.
TABLE 1a.
| ENVIROMENTAL AGENT |
AUTO- IMMUNE DISEASE |
GENDER PREDOM- INANCE |
TYPE OF STUDY |
BIAS | STUDIES |
|---|---|---|---|---|---|
| SILICA | Systemic sclerosis | Male | Meta-analysis (9 case-controls, 3 cohort, 4 other) | Occupational exposure | 17–22 |
| SMOKING | RA | Male | Meta-analysis (observational studies) | None | 28–35 |
| HAIR DYE | SLE | Female (weak association) | 6 case-control | Occupational and social exposure | 24, 49–54 |
| HAIR DYE | RA | Female | 2 retrospective studies | Occupational exposure | 56, 57 |
| LIPSTICK | SLE | Female | 1 case-control | Social exposure | 55 |
| NAIL POLISH | SLE | Female | 1 case-control | Social exposure | 24 |
| ULTRAVIOLET RADIATION | DM | Female | 1 retrospective study | None | 64 |
| EXOGENOUS ESTROGENS | SLE | Female | 1 cohort study | Female participants | 69 |
Association of different environmental agents with systemic autoimmune diseases. Included are those studies that are discussed in the main text. Type of study, references, and any biasing factors that lead to gender- as opposed to sex-skewing are listed in the last three columns.
We applied the following methods that led to compilation of the studies listed in Table 1. We identified original, published, peer-reviewed studies and systematic reviews relevant to our subject that are published in English. The databases used were PubMed and Google Scholar. The search terms included ‘autoimmunity’, ‘sex’, ‘gender’ and ‘environment’. In addition, each factor was used separately with the above terms, i.e. silica, mercury, smoking, cosmetics, pristane, ultraviolet radiation, infections (EBV), commensals, microbiota, exogenous hormones, and oral contraceptive pills. Furthermore, distinction was made between murine and human studies with an emphasis on the latter. The initial screen of the literature using these parameters rendered 138 studies and reviews, that were subsequently supplemented with additional papers identified based on the references herein and targeted searches. We next focused only on those studies that appeared most relevant to the topic, and cited the ones that came closest to explaining the gender or sex predominance in autoimmune diseases (Table 1).
3. SEX- AND GENDER-BIASED ENVIRONMENTALLY INDUCED AUTOIMMUNITY
3.1. CHEMICAL EXPOSURES
3.1.1. SILICA
The major classes of synthetic amorphous silica are used in a variety of products, e.g. as fillers in the rubber industry, in tire compounds, as free-flow and anti-caking agents in powder materials, and as liquid carriers, particularly in the manufacture of animal feed and agrochemicals; other uses are found in toothpaste additives, paints, silicon rubber, insulation material, liquid systems in coatings, adhesives, printing inks, plastisol car undercoats, and cosmetics.
A meta-analysis published in 2002 included two case-control studies, two proportionate mortality studies and six cohort studies addressing the association between rheumatoid arthritis and silica exposure. Most of the included studies reported consistent elevation in the risk of RA with exposure to silica. The combined relative risk (RR) for silica exposure was 3.43 with 95% confidence interval (2.25–5.22) for all studies, and 4.45 (95% CI 2.24–8.86) for male cohorts, as most of them included only men [16]. Due to the insufficient number of female subjects, one cannot infer any firm conclusions about sex bias, but that the findings are most likely attributed to the fact that silica is largely a male occupational exposure.
A meta-analysis of sixteen studies (three cohort studies, nine case-control and four of other designs) examined the association between scleroderma and occupational exposure to silica. The authors detected a combined estimator of relative risk of 3.02 (95% CI, 1.24–7.35) for males, but no significant elevation in risk [1.03 (95% CI, 0.74–1.44)] among females [17–22].
Studies related to silica exposure and SLE and vasculitis have not shown sex predominance [23–25]. The Carolina Lupus Study and the Roxbury Lupus Study, both of which demonstrated associations of agricultural and occupational silica exposure with risk of SLE, included only women [26, 27]. Women may tend to work in professions with shorter or less intense silica exposures than typically encountered in the dusty trades; however, most SLE patients are women.
3.1.2. SMOKING
Many studies have demonstrated higher risk of RA associated with cigarette smoking, especially rheumatoid factor- or anti-citrullinated protein/peptide antibody-positive patients [28]. Epidemiologic studies have revealed that the risk higher in men (odds ratio [OR] summary for ever, current and past smokers were 1.89 (95% CI 1.56 to 2.28), 1.87 (1.49 to 2.34) and 1.76 (1.33 to 2.31), respectively [29] or in total 1.9–4.4[30–35]) compared to women (OR range 0.6– 2.5 [31, 32, 34, 36–46], for ever, current and past smokers were 1.27 (1.12 to 1.44), 1.31 (1.12 to 1.54) and 1.22 (1.06 to 1.40), respectively [29]). However, the risk for female smokers is now well established [34, 35].
An association between current cigarette smoking and risk of SLE has been reported in several past epidemiologic studies [44, 47, 48], but no direct comparison between the two sexes has been made.
3.1.3. COSMETICS
Cosmetic exposure is very common, especially among women. Five case-control studies and one cohort study examining permanent hair dye use have demonstrated no or weak association with SLE development [24, 49–54]. The interest in hair dyes and other hair products was based in part on the similarities of some of the constituents of these products (acrylamides) to medications involved in drug-induced lupus such as hydralazine and procainamide. One case-control study was conducted showing that ever using lipstick at least three days/week was significantly associated with an increased risk of SLE (OR = 1.71, 95%CI = 1.04–2.82) [55]. These studies are, of course, limited to the female population. Among women, hairdressers and beauticians (OR 2.7, 95% CI 0.8 – 8.6), as well as those exposed to hairdressing chemicals (OR 3.0, 95% CI 1.0 – 9.4) and meat products (OR 2.0, 95% CI 1.0–4.0), have been found to have an increased risk for rheumatoid arthritis [56, 57]. Another case-control study found associations between SLE and work that included applying nail polish or nail applications (OR 10.2; 95% CI 1.3, 81.5) [24]. Again, all the participants were females. Given the rarity of SLE among men, quite sensibly there have been no controlled randomized studies exposing male subjects to nail polish or lipsticks.
3.1.4. PRISTANE
The chemical tetramethylpentadecane (TMPD) is also called pristane. Little is known about the role of pristane or related substances in human SLE, but a well established model of pristane-induced lupus exists in mice [58]. Pristane (TMPD) is an isoprenoid alkane found in small quantities in many plants. It also occurs in crude oils and is a common constituent of mineral oil, a byproduct of the fractional distillation of petroleum. It has been shown that SJL/J female mice injected with pristane exhibit greater mortality, kidney disease, serum anti-nuclear and anti-dsDNA antibodies than their male siblings [58]. A female sex predominance exists in a murine, chemically-induced lupus model [58], which was also investigated for the relative contribution of sex chromosomes. The mechanisms of pristane-induced SLE involve chronic inflammation and formation of ectopic lymphoid tissue, and have been linked to the overproduction of the type 1 interferons (IFN) α and β [59–62].
3.2. PHYSICAL
3.2.1. ULTRAVIOLET RADIATION
Exposure to increased UV radiation intensity has been associated with increased risk of dermatomyositis (OR 2.3, 95% confidence interval [95% CI] 0.9–5.8) and with the expression of anti-Mi-2 autoantibodies (OR 6.0, 95% CI 1.1–34.1) in the U.S. A retrospective study demonstrated that these associations were found only among women (OR 3.8, 95% CI 1.3–11.0 for dermatomyositis and OR 17.3, 95% CI 1.8–162.4 for anti-Mi2 antibodies), suggesting that the threshold for UV radiation to trigger the development autoimmune disorders such as dermatomyositis may be lower among women [64].
3.3. BIOLOGICAL/MICROBIAL
3.3.1. HORMONES
Sex hormones have pleiotropic effects on the immune system. Progenitor and mature lymphocytes express receptors for both estrogen and androgens. This effect has been attributed to an enhanced Th2 response [8, 65]. Estrogens also support the survival of autoreactive T-cells, promoting autoimmunity, as it has been shown in patients with SLE [66]. Sex hormones have profound effects on sex-specific responses of the immune system as reviewed elsewhere [8, 67].
Estrogen, for instance, influences the cytokine profile of NKT cells [68], which are critical cell types at mucosal surfaces. Specifically in SLE, exposure to exogenous estrogens has been associated with the onset of disease among women. Oral contraceptive pills and postmenopausal hormone use have both been associated with an increased risk of developing SLE in large cohort studies of women [69]. Environmental estrogens are increasingly abundant in today’s societies and may also play a role in triggering autoimmunity in women and perhaps even men (reviewed in [70]). They can be found in a variety of foods and have immunomodulatory potential. Examples include daidzein in soybeans, genistein in vegetables, zearalenone in corn or 17β-estradiol in poultry meat. Furthermore, estrogen-mimicking chemicals are found in many household items like detergents, surfactants and plastics [70]. Finally, several estrogenic pesticides can exert immunologic effects, thereby contributing potentially to sex-biased autoimmunity, e.g. DDT, methoxychlor, chlordane, hexachlorbenzene, pentachlorphenol and aldicarb [70].
3.3.2. INFECTIOUS AGENTS
As any environmental or infectious agent needs to enter the host through mucosal or skin sites (so called “barrier organs” [71]; Fig. 2), it is not surprising that a sex-specific mucosal immune response leads to different responses to parasite infection or human vaccines based on sex [72, 73]. Given the manifold immunologic sex differences that exist in healthy subjects, it is likely that infectious agents contribute to a sex-skewed innate or adaptive immune response that may lead to inflammation or autoimmunity. This has been shown, for example, for coxsackieinduced myocarditis in mice [74]. Various pathogens have been associated with the onset or flare of human autoimmune diseases, most notably Epstein-Barr virus (EBV) in SLE [75]; a causal role, however, remains to be shown definitively. Molecular mimicry and concomitant adjuvant effects are thought to mediate these effects in murine models [76]. Antiphospholipid syndrome is particularly frequently associated with infectious triggers [77]. Interestingly, antiphospholipid antibodies can be induced by estrogen in mice [74, 78], but a link between sex, microbial agents and antiphospholipid syndrome has not yet been established.
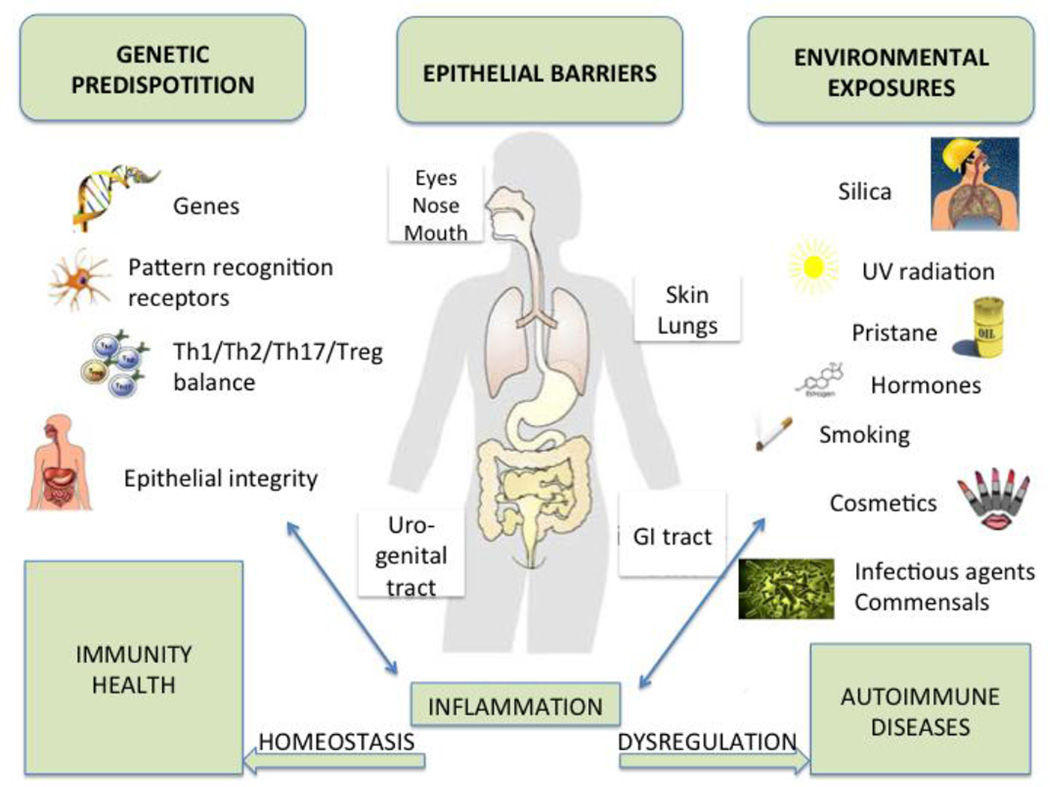
Fig. 2.
Inflammation and autoimmunity as a consequence of gene-environment interactions at mucosal sites (modified after [71]). The effects of environmental factors that penetrate human epithelial barriers, i.e. the skin, eyes, nasal/oral mucosa, lungs, urogenital or gastrointestinal (GI) tract, are influenced by the genetic predisposition of each individual. The combined effects of both genes and environment converge at the mucosal barrier organs where the majority of immunologically active cells reside physiologically. Any dysregulation at the epithelial barriers can lead to an inflammatory response to environmental agents that enter the body and may subsequently initiate or perpetuate autoimmune processes. Females are more frequently affected than males by autoimmune diseases due to different genetic, hormonal and immunologic factors (discussed in the main text). They may also be more susceptible to environmental (particularly microbial/commensal) agents due to physiologic changes in mucosal integrity during menses and childbirth, although this aspect is largely unexplored. Th, T helper cell (Th1/Th2/Th17, not shown is T follicular helper cell); Treg, T regulatory cell.
3.3.3. COMMENSALS
Even less is known about a potential contribution of commensal organisms in sex-specific effects on autoimmunity although intriguing examples exist in mice. Remarkable progress has been made in the last few years in our understanding of host/commensal interactions that are critical for the homeostasis of the immune system [79]. The collection of all bacteria that colonize a host, the microbiota, has now been extensively characterized in healthy subjects as part of the Human Microbiome Project [80]. Since the microbiota persists throughout life and is tolerated by the host’s immune system, it should be considered an “extended self”. The composition, however, changes over time and is easily disrupted by various interventions, which is why one could consider it also an external variable.
Some sex differences exist in the composition of the microbial communities in healthy humans [80, 81]. Similar to a sex-biased response to pathogens, the host immune system appears to be also skewed in response to commensals based on sex. A study of P. gingivalis-induced alveolar bone loss in mice revealed a sex-dependent effect of IL-17 signaling [82]. Female mice lacking the IL-17 receptor were significantly more susceptible to alveolar bone loss than males. Of note, the periodontal bacterium P. gingivalis has also been suggested to play a role in the generation of citrullinated antibodies in RA patients [83]. Interestingly, RA, an IL-17-driven autoimmune disease in murine models, is caused by a gut commensal in the K/BxN arthritis-prone strain [84] and associates with a less dynamic gastrointestinal microbiome in a female-biased, HLA-DR-transgenic model for collagen-induced arthritis [85]. Finally, it is notable that the K/BxN arthritis model is based on the autoimmune-prone non-obese diabetic (NOD) mouse strain. The NOD mouse, a model for female-biased polyglandular autoimmunity, exhibits a clear commensal-related sex bias based on segregation studies with naturally transmitted segmented filamentous bacteria [86]. Remarkably, it was more recently shown that sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity in the same model [87]. The microbiome of immature female NOD mice could be altered by transfer of gut microbiota from adult males, which subsequently led to protection from autoimmunity in the female recipients. These effects were accompanied by moderate elevations of testosterone levels that did not impair fertility of the female mice [87]. While the various animal studies cited here support that the gut microbiota is very likely another contributor to the sex bias of human autoimmune diseases, this notion remains to be tested in future studies in humans.
4. CONCLUSION
Sex differences in autoimmune diseases have long been known and are likely in part related evolutionarily to the fact that the female immune system must deal with major changes during menstrual cycles, pregnancy and childbirth – events that are associated with breaches in the mucosal barriers that are shielding us from environmental factors [71, 88] (Fig. 2). Numerous epidemiologic studies have shown an association between environmental factors and autoimmune diseases [89]. However, there is little evidence for sex-specific environmental influences on autoimmune diseases. We have summarized studies that relate to this important aspect of biology, and have included examples of potential chemical, physical and biological factors that may influence the sex bias in human systemic autoimmune diseases. Many studies are difficult to interpret due to the influence of gender-selective social, occupational or other exposures, and the sampling of exclusively, or predominantly, one sex versus the other. Studies that are largely missing in this field are those with sufficient and equal numbers of both male and female patients, as well as controlled for exposure and dosage of the environmental factor(s) prospectively prior to disease onset in order to avoid recall bias. Furthermore, observational studies typically can only establish an association, but cannot inform us about a potential causal relationship. Reverse causality is particularly difficult to exclude in human studies, which examine biomarkers among affected and unaffected individuals.
Ex vivo and in vitro studies using human tissues and/or peripheral blood cells exposed to environmental factors are potential ways to study causal relationships in humans and would nicely supplement prospective clinical trials. In addition, animal models can be very useful in leading to new to mechanistic insights, in particular in areas that have not yet been studied systematically in humans, such as the role of microbial pathogens or the benign microbiota. Animal models could point to further epidemiologic studies to investigate sex bias in autoimmune diseases. Since murine models are also limited, however, due to species differences in the immune system between mice and humans, generation of humanized mice might prove to be useful for this purpose [90]. Independently from clinical trials, more studies on human cells, cell lines and tissues would be useful. in order to delineate a non-causal association from causal influence of the environmental factors on the human immune system. Such ex vivo studies could, for instance, be carried out in sorted CD4+ cell subpopulations as done for the study of certain sex-biased endogenous factors (e.g. peroxisome proliferator activated receptor-αand -γ [91] The environmental factors derived from such studies would then need to be reproduced in animal models in order to further investigate cause versus effect in vivo. Going forward, we need more translational approaches of this nature combined with cohorts of well-defined patients and appropriate controls that are ideally tested at different stages of the disease [92]. While difficult to execute and not always feasible, such combined efforts should provide eventually important insight into the pathogenesis of sex-biased autoimmunity. In summary, much work is still to be done in order to advance our knowledge of sex-specific environmental influences on autoimmune diseases, but one can make incremental progress by considering both data obtained from murine models and those from human studies.
Highlights.
We review sex-specific environmental influences on systemic autoimmune diseases.
We address chemical, physical and biological (microbial) factors.
Most studies are biased due to gender-selective social, occupational or other exposures.
Murine autoimmune models could give insight into potential mechanisms of sex skewing
Acknowledgments
We apologize that not all papers related to the topic of sex differences in autoimmunity could be cited. We would like to thank Dr. R. R. Singh (UCLA) for helpful discussions on sex chromosomal effects on autoimmunity. This work was supported by a grant from the National Institutes of Health (K08 AI095318 to M.A.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
REFERENCES
- 1.Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. Journal of autoimmunity. 2007;28:1–6. doi: 10.1016/j.jaut.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Amur S, Parekh A, Mummaneni P. Sex differences and genomics in autoimmune diseases. Journal of autoimmunity. 2012;38:J254–J265. doi: 10.1016/j.jaut.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Pollard KM. Gender differences in autoimmunity associated with exposure to environmental factors. Journal of autoimmunity. 2012;38:J177–J186. doi: 10.1016/j.jaut.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitacre CC. Sex differences in autoimmune disease. Nature immunology. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 5.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nature reviews. Immunology. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 6.Lockshin MD. Invited review: sex ratio and rheumatic disease. J Appl Physiol. 2001;91:2366–2373. doi: 10.1152/jappl.2001.91.5.2366. [DOI] [PubMed] [Google Scholar]
- 7.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. The Journal of experimental medicine. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. Journal of autoimmunity. 2012;38:J282–J291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Jacob CO, Zhu J, Armstrong DL, Yan M, Han J, Zhou XJ, Thomas JA, Reiff A, Myones BL, Ojwang JO, Kaufman KM, Klein-Gitelman M, McCurdy D, Wagner-Weiner L, Silverman E, Ziegler J, Kelly JA, Merrill JT, Harley JB, Ramsey-Goldman R, Vila LM, Bae SC, Vyse TJ, Gilkeson GS, Gaffney PM, Moser KL, Langefeld CD, Zidovetzki R, Mohan C. Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2009;106:6256–6261. doi: 10.1073/pnas.0901181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman EI, Helfgott SM, Kriegel MA. Emerging therapies for systemic lupus erythematosus--focus on targeting interferon-alpha. Clin Immunol. 2012;143:210–221. doi: 10.1016/j.clim.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauklin S, Sernandez IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. Estrogen directly activates AID transcription and function. The Journal of experimental medicine. 2009;206:99–111. doi: 10.1084/jem.20080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setlow VP, Lawson CE, Woods NF, editors. Washington (DC): 1998. Gender Differences in Susceptibility to Environmental Factors: A Priority Assessment: Workshop Report. [PubMed] [Google Scholar]
- 14.Cooper GS, Gilbert KM, Greidinger EL, James JA, Pfau JC, Reinlib L, Richardson BC, Rose NR. Recent advances and opportunities in research on lupus, environmental influences and mechanisms of disease. Environmental health perspectives. 2008;116:695–702. doi: 10.1289/ehp.11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Molecular and cellular endocrinology. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Khuder SA, Peshimam AZ, Agraharam S. Environmental risk factors for rheumatoid arthritis. Reviews on environmental health. 2002;17:307–315. doi: 10.1515/reveh.2002.17.4.307. [DOI] [PubMed] [Google Scholar]
- 17.McCormic ZD, Khuder SS, Aryal BK, Ames AL, Khuder SA. Occupational silica exposure as a risk factor for scleroderma, a meta-analysis. International archives of occupational and environmental health. 2010;83:763–769. doi: 10.1007/s00420-009-0505-7. [DOI] [PubMed] [Google Scholar]
- 18.Makol A, Reilly MJ, Rosenman KD. Prevalence of connective tissue disease in silicosis (1985–2006)-a report from the state of Michigan surveillance system for silicosis. American journal of industrial medicine. 2011;54:255–262. doi: 10.1002/ajim.20917. [DOI] [PubMed] [Google Scholar]
- 19.Stolt P, Kallberg H, Lundberg I, Sjogren B, Klareskog L, Alfredsson L. Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Annals of the rheumatic diseases. 2005;64:582–586. doi: 10.1136/ard.2004.022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stolt P, Yahya A, Bengtsson C, Kallberg H, Ronnelid J, Lundberg I, Klareskog L, Alfredsson L. Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Annals of the rheumatic diseases. 2010;69:1072–1076. doi: 10.1136/ard.2009.114694. [DOI] [PubMed] [Google Scholar]
- 21.Barnes J, Mayes MD. Epidemiology of systemic sclerosis, incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Current opinion in rheumatology. 2012;24:165–170. doi: 10.1097/BOR.0b013e32834ff2e8. [DOI] [PubMed] [Google Scholar]
- 22.Burns CJ, Laing TJ, Gillespie BW, Heeringa SG, Alcser KH, Mayes MD, Wasko MC, Cooper BC, Garabrant DH, Schottenfeld D. The epidemiology of scleroderma among women: assessment of risk from exposure to silicone and silica. The Journal of rheumatology. 1996;23:1904–1911. [PubMed] [Google Scholar]
- 23.Cooper G, Gilbert K, Greidinger E, James J, Pfau J, Reinlib L, Richardson B, Rose N. Recent advances and opportunities in research on lupus: environmental influences and mechanisms of disease. Ciencia & saude coletiva. 2009;14:1865–1876. doi: 10.1590/s1413-81232009000500028. [DOI] [PubMed] [Google Scholar]
- 24.Cooper GS, Wither J, Bernatsky S, Claudio JO, Clarke A, Rioux JD, Fortin PR. Occupational and environmental exposures and risk of systemic lupus erythematosus: silica, sunlight, solvents. Rheumatology (Oxford) 2010;49:2172–2180. doi: 10.1093/rheumatology/keq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tervaert JW, Stegeman CA, Kallenberg CG. Silicon exposure and vasculitis. Current opinion in rheumatology. 1998;10:12–17. doi: 10.1097/00002281-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Parks CG, Walitt BT, Pettinger M, Chen JC, de Roos AJ, Hunt J, Sarto G, Howard BV. Insecticide use and risk of rheumatoid arthritis and systemic lupus erythematosus in the Women's Health Initiative Observational Study. Arthritis care & research. 2011;63:184–194. doi: 10.1002/acr.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finckh A, Cooper GS, Chibnik LB, Costenbader KH, Watts J, Pankey H, Fraser PA, Karlson EW. Occupational silica and solvent exposures and risk of systemic lupus erythematosus in urban women. Arthritis and rheumatism. 2006;54:3648–3654. doi: 10.1002/art.22210. [DOI] [PubMed] [Google Scholar]
- 28.Hoovestol RA, Mikuls TR. Environmental exposures and rheumatoid arthritis risk. Current rheumatology reports. 2011;13:431–439. doi: 10.1007/s11926-011-0203-9. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, Kumagai S. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Annals of the rheumatic diseases. 2010;69:70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 30.Uhlig T, Hagen KB, Kvien TK. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. The Journal of rheumatology. 1999;26:47–54. [PubMed] [Google Scholar]
- 31.Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L, Alfredsson L. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Annals of the rheumatic diseases. 2003;62:835–841. doi: 10.1136/ard.62.9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis and rheumatism. 2004;50:3085–3092. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 33.Heliovaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. The Journal of rheumatology. 1993;20:1830–1835. [PubMed] [Google Scholar]
- 34.Krishnan E, Sokka T, Hannonen P. Smoking-gender interaction and risk for rheumatoid arthritis. Arthritis research & therapy. 2003;5:R158–R162. doi: 10.1186/ar750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reckner Olsson A, Skogh T, Wingren G. Comorbidity and lifestyle, reproductive factors, and environmental exposures associated with rheumatoid arthritis. Annals of the rheumatic diseases. 2001;60:934–939. doi: 10.1136/ard.60.10.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vessey MP, Villard-Mackintosh L, Yeates D. Oral contraceptives, cigarette smoking and other factors in relation to arthritis. Contraception. 1987;35:457–464. doi: 10.1016/0010-7824(87)90082-5. [DOI] [PubMed] [Google Scholar]
- 37.Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5:525–532. [PubMed] [Google Scholar]
- 38.Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, Silman AJ. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis and rheumatism. 1997;40:1955–1961. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez Avila M, Liang MH, Willett WC, Stampfer MJ, Colditz GA, Rosner B, Roberts WN, Hennekens CH, Speizer FE. Reproductive factors, smoking, and the risk for rheumatoid arthritis. Epidemiology. 1990;1:285–291. doi: 10.1097/00001648-199007000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Hazes JM, Dijkmans BA, Vandenbroucke JP, de Vries RR, Cats A. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. Annals of the rheumatic diseases. 1990;49:980–982. doi: 10.1136/ard.49.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis and rheumatism. 1999;42:910–917. doi: 10.1002/1529-0131(199905)42:5<910::AID-ANR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 42.Criswell LA, Merlino LA, Cerhan JR, Mikuls TR, Mudano AS, Burma M, Folsom AR, Saag KG. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women's Health Study. The American journal of medicine. 2002;112:465–471. doi: 10.1016/s0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- 43.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. The American journal of medicine. 2006;119:503, e501–e509. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 44.Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15:737–745. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 45.Lahiri M, Morgan C, Symmons DP, Bruce IN. Modifiable risk factors for RA: prevention, better than cure? Rheumatology (Oxford) 2012;51:499–512. doi: 10.1093/rheumatology/ker299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergstrom U, Jacobsson LT, Nilsson JA, Berglund G, Turesson C. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford) 2011;50:2005–2013. doi: 10.1093/rheumatology/ker258. [DOI] [PubMed] [Google Scholar]
- 47.Costenbader KH, Karlson EW. Cigarette smoking and systemic lupus erythematosus: a smoking gun? Autoimmunity. 2005;38:541–547. doi: 10.1080/08916930500285758. [DOI] [PubMed] [Google Scholar]
- 48.Costenbader KH, Kim DJ, Peerzada J, Lockman S, Nobles-Knight D, Petri M, Karlson EW. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis and rheumatism. 2004;50:849–857. doi: 10.1002/art.20049. [DOI] [PubMed] [Google Scholar]
- 49.Freni-Titulaer LW, Kelley DB, Grow AG, McKinley TW, Arnett FC, Hochberg MC. Connective tissue disease in southeastern Georgia: a case-control study of etiologic factors. American journal of epidemiology. 1989;130:404–409. doi: 10.1093/oxfordjournals.aje.a115348. [DOI] [PubMed] [Google Scholar]
- 50.Petri M, Allbritton J. Hair product use in systemic lupus erythematosus. A case-control study. Arthritis and rheumatism. 1992;35:625–629. doi: 10.1002/art.1780350605. [DOI] [PubMed] [Google Scholar]
- 51.Reidenberg MM, Drayer DE, Lorenzo B, Strom BL, West SL, Snyder ES, Freundlich B, Stolley PD. Acetylation phenotypes and environmental chemical exposure of people with idiopathic systemic lupus erythematosus. Arthritis and rheumatism. 1993;36:971–973. doi: 10.1002/art.1780360714. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Guerrero J, Karlson EW, Colditz GA, Hunter DJ, Speizer FE, Liang MH. Hair dye use and the risk of developing systemic lupus erythematosus. Arthritis and rheumatism. 1996;39:657–662. doi: 10.1002/art.1780390418. [DOI] [PubMed] [Google Scholar]
- 53.Hardy CJ, Palmer BP, Muir KR, Powell RJ. Systemic lupus erythematosus (SLE) and hair treatment: a large community based case-control study. Lupus. 1999;8:541–544. doi: 10.1191/096120399678840800. [DOI] [PubMed] [Google Scholar]
- 54.Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Smoking and use of hair treatments in relation to risk of developing systemic lupus erythematosus. The Journal of rheumatology. 2001;28:2653–2656. [PubMed] [Google Scholar]
- 55.Wang J, Kay AB, Fletcher J, Formica MK, McAlindon TE. Is lipstick associated with the development of systemic lupus erythematosus (SLE)? Clinical rheumatology. 2008;27:1183–1187. doi: 10.1007/s10067-008-0937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olsson AR, Skogh T, Wingren G. Occupational determinants for rheumatoid arthritis. Scandinavian journal of work, environment & health. 2000;26:243–249. doi: 10.5271/sjweh.538. [DOI] [PubMed] [Google Scholar]
- 57.Olsson AR, Skogh T, Axelson O, Wingren G. Occupations and exposures in the work environment as determinants for rheumatoid arthritis. Occupational and environmental medicine. 2004;61:233–238. doi: 10.1136/oem.2003.007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith DL, Dong X, Du S, Oh M, Singh RR, Voskuhl RR. A female preponderance for chemically induced lupus in SJL/J mice. Clin Immunol. 2007;122:101–107. doi: 10.1016/j.clim.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends in immunology. 2009;30:455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Summers SA, Hoi A, Steinmetz OM, O'Sullivan KM, Ooi JD, Odobasic D, Akira S, Kitching AR, Holdsworth SR. TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. Journal of autoimmunity. 2010;35:291–298. doi: 10.1016/j.jaut.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Herman S, Kny A, Schorn C, Pfatschbacher J, Niederreiter B, Herrmann M, Holmdahl R, Steiner G, Hoffmann MH. Cell death and cytokine production induced by autoimmunogenic hydrocarbon oils. Autoimmunity. 2012;45:602–611. doi: 10.3109/08916934.2012.719948. [DOI] [PubMed] [Google Scholar]
- 62.Minhas U, Das P, Bhatnagar A. Role of reactive intermediates in the immunopathogenesis of the pristane-induced Balb/c model of lupus. Lupus. 2011;20:1421–1425. doi: 10.1177/0961203311418791. [DOI] [PubMed] [Google Scholar]
- 63.Dahlgren J, Takhar H, Anderson-Mahoney P, Kotlerman J, Tarr J, Warshaw R. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site a cross sectional study. Environmental health : a global access science source. 2007;6:8. doi: 10.1186/1476-069X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Love LA, Weinberg CR, McConnaughey DR, Oddis CV, Medsger TA, Jr, Reveille JD, Arnett FC, Targoff IN, Miller FW. Ultraviolet radiation intensity predicts the relative distribution of dermatomyositis and anti-Mi-2 autoantibodies in women. Arthritis and rheumatism. 2009;60:2499–2504. doi: 10.1002/art.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verthelyi D. Sex hormones as immunomodulators in health and disease. International immunopharmacology. 2001;1:983–993. doi: 10.1016/s1567-5769(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 66.Panchanathan R, Choubey D. Murine BAFF expression is up-regulated by estrogen and interferons: Implications for sex bias in the development of autoimmunity. Molecular immunology. 2013;53:15–23. doi: 10.1016/j.molimm.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. 2012;11:A502–A514. doi: 10.1016/j.autrev.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gourdy P, Araujo LM, Zhu R, Garmy-Susini B, Diem S, Laurell H, Leite-de-Moraes M, Dy M, Arnal JF, Bayard F, Herbelin A. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood. 2005;105:2415–2420. doi: 10.1182/blood-2004-07-2819. [DOI] [PubMed] [Google Scholar]
- 69.Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis and rheumatism. 2007;56:1251–1262. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- 70.Chighizola C, Meroni PL. The role of environmental estrogens and autoimmunity. Autoimmun Rev. 2012;11:A493–A501. doi: 10.1016/j.autrev.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 71.Ehlers S, Kaufmann SH. Infection, inflammation, and chronic diseases: consequences of a modern lifestyle. Trends in immunology. 2010;31:184–190. doi: 10.1016/j.it.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite immunology. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 73.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–3555. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 74.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, Rose NR, Fairweather D. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 75.James JA, Harley JB, Scofield RH. Epstein-Barr virus and systemic lupus erythematosus. Current opinion in rheumatology. 2006;18:462–467. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 76.Rose NR. Infection, mimics, and autoimmune disease. The Journal of clinical investigation. 2001;107:943–944. doi: 10.1172/JCI12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blank M, Shoenfeld Y. Beta-2-glycoprotein-I, infections, antiphospholipid syndrome and therapeutic considerations. Clin Immunol. 2004;112:190–199. doi: 10.1016/j.clim.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 78.Ahmed SA, Hissong BD, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E. Gender and risk of autoimmune diseases: possible role of estrogenic compounds. Environmental health perspectives. 1999;107(Suppl 5):681–686. doi: 10.1289/ehp.99107s5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 80.A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL. The interleukin-17 receptor plays a gender-dependent role in host protection against Porphyromonas gingivalis-induced periodontal bone loss. Infection and immunity. 2008;76:4206–4213. doi: 10.1128/IAI.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nature reviews. Rheumatology. 2011;7:569–578. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomez A, Luckey D, Yeoman CJ, Marietta EV, Berg Miller ME, Murray JA, White BA, Taneja V. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PloS one. 2012;7:e36095. doi: 10.1371/journal.pone.0036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science. 2013 doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 88.Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, Neph S, Sabo P, Kim JM, Liao W, Li MO, Leslie C, Stamatoyannopoulos JA, Rudensky AY. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, De Roos AJ. Epidemiology of environmental exposures and human autoimmune diseases: Findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. Journal of autoimmunity. 2012 doi: 10.1016/j.jaut.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willinger T, Rongvaux A, Strowig T, Manz MG, Flavell RA. Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends in immunology. 2011;32:321–327. doi: 10.1016/j.it.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 91.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, Dunn SE. Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFNgamma and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci U S A. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Selmi C, Leung PS, Sherr DH, Diaz M, Nyland JF, Monestier M, Rose NR, Gershwin ME. Mechanisms of environmental influence on human autoimmunity: a national institute of environmental health sciences expert panel workshop. Journal of autoimmunity. 2012;39:272–284. doi: 10.1016/j.jaut.2012.05.007. [DOI] [PubMed] [Google Scholar]