Abstract
Brown adipose tissue (BAT) is of great scientific interest as a potential target to treat obesity. The development of novel strategies to quantify brown fat thermogenesis in adult humans now enables minimally invasive assessment of novel pharmacotherapeutics. Input from the central nervous system (CNS) via sympathetic efferents is widely regarded as the key controller of BAT-mediated thermogenesis in response to changes in body temperature or nutrient availability. More recently, however, it has become clear that locally secreted signals and endocrine factors originating from multiple organs can control the recruitment of brown adipocytes and, more importantly, induce thermogenesis in brown fat. Thus, they provide an attractive strategy to fine tune brown fat thermogenesis independent of classical temperature sensing. Here, we summarize recent findings on bone morphogenetic protein (BMP) signaling as an example of secreted factors in the regulation of brown adipocyte formation and systemic control of energy metabolism. We further highlight endocrine communication routes between the different types of brown adipocytes and other organs that contribute to regulation of thermogenesis. Thus, emerging evidence suggests that the classical mechanisms of central temperature sensing and sympathetic nervous system–driven thermogenesis are complemented by local and endocrine signals to determine systemic energy homeostasis.
Keywords: brown adipose tissue, bone morphogenetic proteins, systemic thermogenic capacity
Introduction
The presence of brown adipose tissue (BAT) in adult humans is well established.1 The current renaissance of brown fat research has recognized this tissue as a metabolically active part of systemic energy homeostasis and thermoregulation. BAT is detectable in humans and, importantly, can be readily quantified by a combination of positron emission tomography (PET) and computed tomography (CT). In humans, this type of fat is found in different anatomical locations throughout the upper torso and is distinguishable from its energy-storing cousin, white adipose tissue (WAT), by several morphological and functional parameters. While white adipocytes contain a single large lipid droplet to store triglycerides, brown adipocytes are characterized by multiple small lipid droplets and abundant mitochondria that oxidize nutrients and generate heat. Central to its inherent thermogenic activity is uncoupling protein 1 (UCP1), which is uniquely expressed in BAT and therefore serves as a definite marker of brown adipocytes.
Brown fat formation: the role of bone morphogenetic proteins
It is now clear that two types of brown fat exist within the body: classical or constitutive BAT (cBAT), such as interscapular cBAT in mice, resides in preformed depots that are fully developed at birth and that are, at least in rodents, present throughout life. During embryogenesis, this population of brown adipocytes arises from a common progenitor that expresses myogenic transcription factors such as Myf5.2 These cells give rise to skeletal muscle in addition to cBAT, a process that involves lineage determination through the transcription factor PR domain–containing 16 (Prdm16), thus setting the developmental origin of cBAT apart from WAT.2 The second type of brown adipocytes resides predominantly within white fat, thus representing a mixture of white and a subset of brown-like adipocytes with a currently unresolved nomenclature, which includes beige, brite (brown-in-white), inducible, or recruitable BAT (rBAT).3,4
The formation of brown adipocytes is subject to regulation by developmental signals from the morphogens. Among the classical developmental pathways, hedgehog,5 Wnt6 fibroblast growth factor (FGF),7 and bone morphogenetic protein (BMP) signaling4,8,9 have been implicated in the development of brown adipocytes. Work from our laboratory, among others, has helped to establish the specific role of BMPs in brown adipocyte biology. These secreted proteins regulate a number of developmental processes among which the formation of brown adipocytes represents a previously unrecognized feature. Specifically, the ligand BMP7 is required for the embryonic formation of cBAT, and loss of BMP7 in mice leads to a marked decrease in cBAT mass and a significant reduction in the expression of UCP1 protein. Conversely, in vitro administration of BMP7 to brown pre-adipocytes promotes their differentiation into brown adipocytes. Importantly, in multipotent progenitors and primary white pre-adipocytes, BMP7 exposure determines BAT lineage commitment and differentiation, altogether providing compelling evidence for the role of BMP signaling in brown adipocyte formation and metabolism.8,9 Along the same lines, a recent study demonstrated that BMP4 may act as a brown adipogenic morphogen: overexpression of this factor in white adipose tissue of mice results in a conversion of white into brown-like adipocytes and is accompanied by a healthy metabolic phenotype that shows increased energy expenditure and insulin sensitivity.10 Likewise, BMP8b increases sensitivity to sympathetic activation of brown adipocytes by increasing lipolysis, suggesting that this BMP ligand acts as a modulator of sympathetic nervous system (SNS)-mediated thermogenesis. In support of this notion, expression of BMP8b in cBAT is regulated by thermogenic and nutritional stimuli that may require such local modulation.11 This finding is entirely consistent with the observation that systemic administration of BMP7 synergistically increases expression of brown fat markers after co-injection with the sympathetic agonist CL316,243.8
To more broadly address the role of BMP signaling in brown adipogenesis, we recently generated two mouse models with genetic ablation of the type 1A BMP receptor, BMPR1A, in (1) Myf5+ progenitors that give rise to skeletal muscle and cBAT (Myf5–BMPR1A knockout (KO)), and in (2) all adipocytes using the aP2 driver, thus targeting cBAT, rBAT, and WAT (aP2-BMPR1A KO).4 In Myf5–BMPR1A KO mice, a striking cBAT paucity was observed, whereas skeletal muscle development was mostly normal. Similarly, in aP2-BMPR1A KO mice, the formation of both types of brown, but not white, adipocytes was markedly inhibited, altogether suggesting that BMP signaling is essential for and plays a specific role in the development and differentiation of brown adipogenic progenitors. Mechanistically, the canonical downstream targets of BMPs, p38 mitogen–activated protein kinase (p38MAPK) and Smad signaling, have been implicated in these processes.4,9 Ablation of BMPR1A alone markedly inhibits BMP7-mediated Smad phosphorylation, and a complete blockage of differentiation could be achieved by double deletion of BMPR1A and a second type 1 BMPR, activin A receptor type 1 (ACVR1). Interestingly, the transcription factor Zfp423, which is known to determine an adipogenic fate in progenitors, increases sensitivity to BMP signals12 and could be further enhanced by feedback mechanisms, as BMP7 has been shown to increase expression levels of this transcription factor.8 Of note, the neuropeptide orexin, which is required for embryonic development and maturation of brown adipocytes, requires intact BMP signaling through BMPR1A for its brown adipogenic action, indicating that modulation of this signaling pathway may affect brown adipogenic processes at several stages.13
Systemic regulation of brown fat mass and thermogenic capacity
The general consensus is that BAT is the most important controller of non-shivering thermogenesis. However, the concept of BAT as a metabolic regulator beyond thermoregulation has recently emerged, suggesting a requirement for regulation of brown fat mass and function independent of temperature sensing in the central nervous system (CNS). Along these lines, several studies have shown that BAT may regulate nutrient levels, glucose homeostasis, and systemic insulin sensitivity.14–16 These effects are at least partially mediated by endocrine signals that originate from brown adipocytes and that may not necessarily require input from central temperature sensing.
It has recently been suggested that thermogenic activity of brown adipocytes may be regulated by mechanisms other than the classical central-to-peripheral axis of the SNS.17 Sympathetic tone, by far the best-characterized regulator of brown fat activity, induces thermogenesis in cBAT and lipolysis in WAT as a short-term measure to meet increased thermogenic requirements. A more long-term adjustment to a low-temperature challenge is hyperplasia of cBAT and/or browning of WAT (i.e., the recruitment of brown-like adipocytes within white fat depots).18 This is consistent with observations made in the Myf5–BMPR1A KO mouse model with severe paucity of cBAT, where compensatory recruitment of brown adipocytes occurs within WAT.4 As a result of this process, these animals display normal energy homeostasis and body weight, even under obesity-promoting conditions such as feeding a high-fat diet and thermoneutrality. Taken together, these findings emphasize the physiological potential of compensatory browning of WAT to maintain and fine tune the thermogenic capacity according to metabolic demands and environmental challenges. Our findings further indicate that the crosstalk between different depots of brown fat is mediated, at least in part, by the SNS, suggesting that direct sympathetic input to WAT serves as an important component of compensatory browning to maintain a normal thermogenic capacity (Fig. 1). However, our analysis does not rule out that secreted factors, such as some of the recently identified browning molecules, may also play a role in systemic regulation of browning and thermogenic capacity that could even act independently of the SNS. In fact, we believe that such signals originating from different organs may be integrated with the temperature sensing–related sympathetic tone to determine systemic capacity for thermogenesis by modulating total brown fat mass (Fig. 1). Therefore, we discuss here the modes of action of some exemplary secreted factors to highlight possible communication principles through which these and other factors may act to fine tune BAT-mediated control of systemic energy homeostasis alone or in conjunction with sympathetic input.
Figure 1.
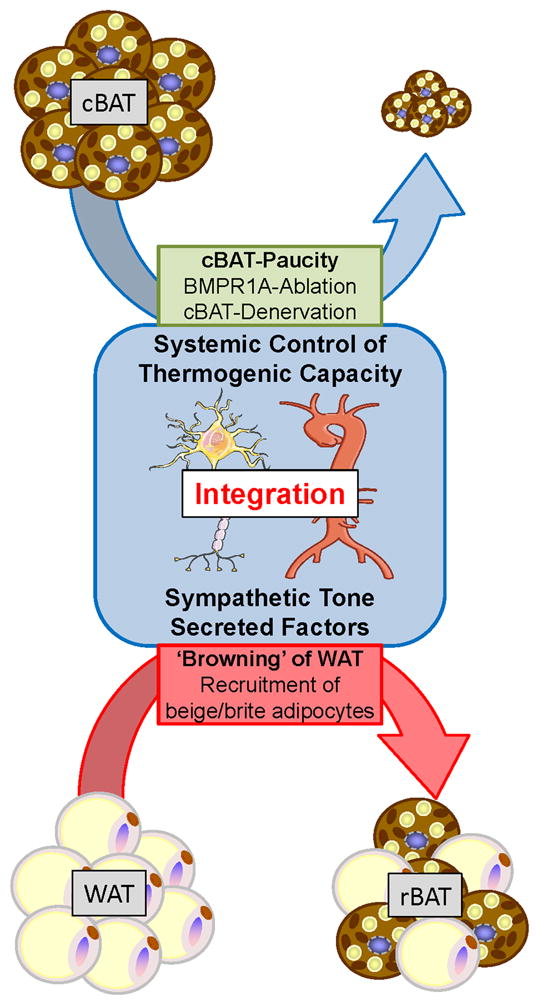
Systemic integration of brown adipogenic signals. Energy homeostasis is rigorously controlled by integration of central and peripheral mechanisms. The sympathetic nervous system (SNS) plays a key role in relaying brown adipogenic cues from constitutive BAT (cBAT) to white adipose tissue (WAT) to determine systemic capacity for thermogenesis and energy expenditure. For example, in situations where cBAT activity is reduced, for instance, due to ablation of bone morphogenetic protein receptor 1A (BMPR1A) or surgical denervation, this system is able to induce a compensatory recruitment of the beige/brite adipocytes within WAT to sustain normal body temperature and energy balance.3,4 Additionally, the recent identification of secreted factors that may affect browning of WAT and thermogenic activity of brown adipocytes adds an attractive communication mechanism as a target for therapeutic interventions.
BMP7, BMP8b, the myokine irisin, FGF21, prostaglandins (PGs), and cardiac natriuretic peptides (NPs), among numerous other secreted factors, have recently been identified to regulate brown fat physiology.8,11,19–22 Several recent articles have thoroughly reviewed the role of the browning factors.23–25 Here, we focus on some of the exemplary factors to discuss potential mechanisms of action and the possibility that these factors may act independently of the SNS to induce browning, increase the sensitivity to SNS input, or facilitate a crosstalk between the peripheral and central regulation of thermogenesis.
Recently, it has been discovered that BMP8b is expressed in the hypothalamus and induces energy expenditure through BAT in an AMP-activated protein kinase (AMPK)–dependent manner by increasing sympathetic input to cBAT.11 Similarly, systemic administration of BMP7 reduces body weight gain, at least in part due to induction of BAT-mediated energy expenditure. Intriguingly, BMP7 can also directly inhibit food intake following intracerebroventricular injection, which is independent of leptin activity, and through activation of the mTOR-p70S6K pathway, indicating that BMP7 can directly act in the CNS. This study also showed that BMP receptors are present in different hypothalamic regions that are involved in metabolic control.26 As discussed above, a close interaction of the brown adipogenic and pro-thermogenic signals of BMPs with SNS-driven activation of thermogenesis suggests that a crosstalk between these two pathways on peripheral and central levels may be critical to modulate overall thermogenic capacity. BMP8b appears to be made by mature brown adipocytes and its expression is regulated by temperature and other metabolic cues,11 suggesting that it is under control of central temperature sensing. By contrast, BMP7 is produced by the stromal vascular cells within the adipose tissue and thus may serve as a niche factor to drive the tissue resident progenitors to differentiate into brown fat lineage8 (and T.J. Schulz and Y. Tseng, unpublished observations). Whether BMP7 expression is regulated by neuronal/ sympathetic stimuli is still unknown. When administered to mice receiving β3-adrenergic stimulation, BMP7 synergistically increased expression of UCP1 in WAT, suggesting that BMP7, like BMP8b, may increase the sensitivity of adipocytes to sympathetic tone.8 While these synergistic effects on thermogenesis have been clearly documented, it remains to be determined whether BMPs can induce heat production in BAT without sympathetic stimulation.
Irisin was recently discovered as a protein secreted from skeletal muscle following physical activity that promoted the recruitment of brown adipocytes within WAT.19 Importantly, increased irisin levels are anti-obesogenic and associated with increased energy expenditure and glucose tolerance, suggesting that at least some of the beneficial effects of physical activity may be due to enhanced browning of WAT. Thus, irisin represents a prominent example of signals emerging from peripheral organs and non-sympathetic regulation of systemic thermogenic capacity. It remains to be determined whether neuronal input may also affect secretion of irisin from skeletal muscle. Thus far, this protein presents an intriguing opportunity to increase systemic thermogenic capacity through a secreted and circulating molecule.
Recently, a similar browning phenotype has been observed in animals that were raised in an enriched environment. Like physical exercise, increased browning was observed in WAT of these animals. Strikingly, this did not require changes in temperature or activity. The authors show that hypothalamic signaling initiated by brain-derived neurotrophic factor (BDNF) is responsible for the peripheral browning of WAT, altogether further emphasizing that a close interaction between circulating factors and neuronal control of thermogenesis is in place.27
Another example of BAT-to-WAT communication to promote browning comes from FGF21, a protein thought to be expressed and secreted from cBAT after sympathetic stimulation, for instance, in response to cold exposure.20 FGF21 can act as a potent messenger molecule secreted from BAT to induce browning of WAT as a more long-term adaptation to a cold environment. Unlike irisin, local expression of FGF21 is regulated by sympathetic input, suggesting that the SNS acts through several pathways to control systemic capacity for thermogenesis. Aside from autocrine and endocrine effects on WAT following its secretion from brown adipocytes, the liver is the predominant source of FGF21, where it serves to regulate hepatic nutrient metabolism and the fasting response.28 Given that FGF21 is secreted in response to different metabolic signals (e.g., fasting) it appears likely that some of these may impact thermogenic capacity without temperature-related sympathetic input. Interestingly, a recent study demonstrated that intracerebroventricular administration of FGF21 had insulin-sensitizing effects and increased energy expenditure, indicating that FGF21 may act in the CNS, possibly through a crosstalk mechanism that affects thermoregulation in the CNS.29
Like FGF21, synthesis of PGs is regulated by the SNS and could add another regulatory cue to browning of WAT. Expression of cyclooxygenase-2 (COX2), which catalyzes a rate-limiting step of PG biosynthesis, is upregulated by sympathetic stimuli such as cold exposure. Moreover, administration of PGs promotes the differentiation of brown adipocytes from mesenchymal progenitors, and overexpression of COX2 promotes browning of WAT in vivo. In this study, the authors propose a feed-forward mechanism that results in the formation of rBAT with increased sensitivity to the sympathetic neurotransmitter norepinephrine.21 Interestingly, norepinephrine is also secreted by alternatively activated macrophages, thus providing a non-SNS source of this hormone that may act through different molecular pathways to activate thermogenic gene expression in brown adipocytes and lipolysis within WAT.30 As demonstrated by Nguyen et al., cold exposure leads to enhanced alternative activation of WAT resident macrophages that is dependent on interleukin (IL)-4 and IL-13. These findings add another layer of complexity to the regulatory sensing mechanisms that determine local and systemic thermogenic capacity. With regard to a possible central mechanism of action, it is well established that circulating PGs are involved in the fever response by acting on central temperature sensing.31 Direct administration of PGs to the anterior hypothalamic preoptic area (POAH) similarly results in activation of brown adipose thermogenesis.32 Given that the POAH is considered to be the responsible component of temperature sensing in the brain, these findings provide interesting evidence that this circulating browning molecule also acts centrally to affect thermoregulation in response to metabolic cues.33,34
Cardiac NPs, both atrial (ANP) and ventricular forms (brain natriuretic peptide, BNP), are believed to interact with the SNS to mediate browning of WAT.22 Following cold exposure, circulating levels of these peptides increase along with expression of their receptors in WAT, ultimately leading to increased rBAT recruitment. Interestingly, this is accompanied by a decreased expression of the NP clearance receptor (NPRC), and genetic ablation of this receptor leads to browning of WAT, altogether indicating that the communication route from cardiac tissues may contribute to systemic energy metabolism by controlling brown fat physiology.22 While it is currently unclear whether NPs can also affect thermogenesis independently of sympathetic drive, these peptides are also secreted in response to numerous signals, for instance cytokines and hormones, which mostly act through NF B and p38MAPK signaling, and may regulate thermogenic activity of brown fat.35
Conclusion
A number of other signaling molecules have been implicated in the formation and activation of brown adipocytes.17 Here, we highlight only a small portion of them to exemplify the importance of systemically active signals originating from different anatomical sources. Total brown fat mass may be seen as a readout of maximum thermogenic capacity that could be utilized to regulate body temperature in response to changing ambient temperatures and possibly also adjustments following metabolic challenges and in response to variable nutrient availability. We propose that systemic integration of neuronal signals from the central and sympathetic nervous systems, as well as endocrine and locally secreted paracrine/autocrine factors originating from different organs in the periphery, determine total brown fat activity for thermoregulation and energy homeostasis. An important component of this system is the crosstalk between the two different types of BAT: cBAT and rBAT. Our recent findings suggest that sympathetic input induces a compensatory recruitment of beige/brite adipocytes within white fat under conditions of cBAT paucity.4 Accumulating evidence also indicates that secreted factors acting as endocrine or autocrine/paracrine signals are important regulators of brown adipocyte physiology in both types of BAT. It remains to be determined whether these secreted signals are modulators of the main driving signal, the SNS, or independent regulators that can directly promote BAT-mediated thermogenesis beyond the sympathetic tone. Additionally, it is currently unclear whether these factors exclusively act in the periphery or may have targets in the CNS. While we discuss existing published evidence that would allow such action in the CNS, it is currently unclear to what extent this also pertains to systemic regulation of thermogenic capacity. The hypothalamus could act as a central mediator that could integrate such signals with conventional thermo-sensing and regulation within the POAH. Identification of signals and their peripheral as well as central targets would provide valuable tools to improve therapeutic strategies that incorporate increased energy expenditure by brown adipocytes. Taken together, secreted factors originating from multiple locations of the periphery could help to fine tune systemic thermogenic capacity either by affecting sensitivity of cBAT to sympathetic input or by promoting adaptive recruitment of brown adipocytes within WAT. The notion that many of these factors also may act on targets within the CNS could help to integrate these thermogenic cues with the conventional thermoregulation in response to ambient temperature. A combination of individual, highly specific signals could therefore provide a veritable treatment for obesity and metabolic dysfunction.
Acknowledgments
This work was supported in part by NIH Grants R01 DK077097 (Y.-H.T.), and Joslin Diabetes Center’s Diabetes Research Center (DRC; P30 DK036836 from the NIDDK), a research Grant from the American Diabetes Association, and by funding from the Harvard Stem Cell Institute (to Y.-H.T.). T.J.S. was supported by the Mary K. Iacocca Foundation, the German Research Foundation (DFG; SCHU 2445/1-1 and SCHU 2445/2-1), and the European Research Council (ERC-StG-311082).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.NEDERGAARD J, BENGTSSON T, CANNON B. Three years with adult human brown adipose tissue. Ann N Y Acad Sci. 2010;1212:E20–36. doi: 10.1111/j.1749-6632.2010.05905.x.:E20-E36. [DOI] [PubMed] [Google Scholar]
- 2.SEALE P, BJORK B, YANG W, KAJIMURA S, CHIN S, KUANG S, SCIME A, DEVARAKONDA S, CONROE HM, ERDJUMENT-BROMAGE H, TEMPST P, RUDNICKI MA, BEIER DR, SPIEGELMAN BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WU J, BOSTROM P, SPARKS LM, YE L, CHOI JH, GIANG AH, KHANDEKAR M, VIRTANEN KA, NUUTILA P, SCHAART G, HUANG K, TU H, VAN MARKEN LICHTENBELT WD, HOEKS J, ENERBACK S, SCHRAUWEN P, SPIEGELMAN BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SCHULZ TJ, HUANG P, HUANG TL, XUE R, MCDOUGALL LE, TOWNSEND KL, CYPESS AM, MISHINA Y, GUSSONI E, TSENG YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.POSPISILIK JA, SCHRAMEK D, SCHNIDAR H, CRONIN SJ, NEHME NT, ZHANG X, KNAUF C, CANI PD, AUMAYR K, TODORIC J, BAYER M, HASCHEMI A, PUVIINDRAN V, TAR K, ORTHOFER M, NEELY GG, DIETZL G, MANOUKIAN A, FUNOVICS M, PRAGER G, WAGNER O, FERRANDON D, ABERGER F, HUI CC, ESTERBAUER H, PENNINGER JM. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 6.ATIT R, SGAIER SK, MOHAMED OA, TAKETO MM, DUFORT D, JOYNER AL, NISWANDER L, CONLON RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 7.KONISHI M, MIKAMI T, YAMASAKI M, MIYAKE A, ITOH N. Fibroblast growth factor-16 is a growth factor for embryonic brown adipocytes. J Biol Chem. 2000;275:12119–12122. doi: 10.1074/jbc.275.16.12119. [DOI] [PubMed] [Google Scholar]
- 8.SCHULZ TJ, HUANG TL, TRAN TT, ZHANG H, TOWNSEND KL, SHADRACH JL, CERLETTI M, MCDOUGALL LE, GIORGADZE N, TCHKONIA T, SCHRIER D, FALB D, KIRKLAND JL, WAGERS AJ, TSENG YH. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.TSENG YH, KOKKOTOU E, SCHULZ TJ, HUANG TL, WINNAY JN, TANIGUCHI CM, TRAN TT, SUZUKI R, ESPINOZA DO, YAMAMOTO Y, AHRENS MJ, DUDLEY AT, NORRIS AW, KULKARNI RN, KAHN CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.QIAN SW, TANG Y, LI X, LIU Y, ZHANG YY, HUANG HY, XUE RD, YU HY, GUO L, GAO HD, LIU Y, SUN X, LI YM, JIA WP, TANG QQ. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci U S A. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHITTLE AJ, CAROBBIO S, MARTINS L, SLAWIK M, HONDARES E, VAZQUEZ MJ, MORGAN D, CSIKASZ RI, GALLEGO R, RODRIGUEZ-CUENCA S, DALE M, VIRTUE S, VILLARROYA F, CANNON B, RAHMOUNI K, LOPEZ M, VIDAL-PUIG A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GUPTA RK, ARANY Z, SEALE P, MEPANI RJ, YE L, CONROE HM, ROBY YA, KULAGA H, REED RR, SPIEGELMAN BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SELLAYAH D, BHARAJ P, SIKDER D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–490. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 14.BARTELT A, BRUNS OT, REIMER R, HOHENBERG H, ITTRICH H, PELDSCHUS K, KAUL MG, TROMSDORF UI, WELLER H, WAURISCH C, EYCHMULLER A, GORDTS PL, RINNINGER F, BRUEGELMANN K, FREUND B, NIELSEN P, MERKEL M, HEEREN J. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 15.GUERRA C, NAVARRO P, VALVERDE AM, ARRIBAS M, BRUNING J, KOZAK LP, KAHN CR, BENITO M. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest. 2001;108:1205–1213. doi: 10.1172/JCI13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.STANFORD KI, MIDDELBEEK RJ, TOWNSEND KL, AN D, NYGAARD EB, HITCHCOX KM, MARKAN KR, NAKANO K, HIRSHMAN MF, TSENG YH, GOODYEAR LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VILLARROYA F, VIDAL-PUIG A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 18.CANNON B, NEDERGAARD J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 19.BOSTROM P, WU J, JEDRYCHOWSKI MP, KORDE A, YE L, LO JC, RASBACH KA, BOSTROM EA, CHOI JH, LONG JZ, KAJIMURA S, ZINGARETTI MC, VIND BF, TU H, CINTI S, HOJLUND K, GYGI SP, SPIEGELMAN BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FISHER FM, KLEINER S, DOURIS N, FOX EC, MEPANI RJ, VERDEGUER F, WU J, KHARITONENKOV A, FLIER JS, MARATOS-FLIER E, SPIEGELMAN BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VEGIOPOULOS A, MULLER-DECKER K, STRZODA D, SCHMITT I, CHICHELNITSKIY E, OSTERTAG A, DIAZ MB, ROZMAN J, HRABE DA, NUSING RM, MEYER CW, WAHLI W, KLINGENSPOR M, HERZIG S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 22.BORDICCHIA M, LIU D, AMRI EZ, AILHAUD G, DESSI-FULGHERI P, ZHANG C, TAKAHASHI N, SARZANI R, COLLINS S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SCHULZ TJ, TSENG YH. Brown adipose tissue: development, metabolism and beyond. Biochem J. 2013;453:167–178. doi: 10.1042/BJ20130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WU J, COHEN P, SPIEGELMAN BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BONET ML, OLIVER P, PALOU A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta. 2013;1831:969–985. doi: 10.1016/j.bbalip.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 26.TOWNSEND KL, SUZUKI R, HUANG TL, JING E, SCHULZ TJ, LEE K, TANIGUCHI CM, ESPINOZA DO, MCDOUGALL LE, ZHANG H, HE TC, KOKKOTOU E, TSENG YH. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 2012;26:2187–2196. doi: 10.1096/fj.11-199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CAO L, CHOI EY, LIU X, MARTIN A, WANG C, XU X, DURING MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KLIEWER SA, MANGELSDORF DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr. 2010;91:254S–257S. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SARRUF DA, THALER JP, MORTON GJ, GERMAN J, FISCHER JD, OGIMOTO K, SCHWARTZ MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NGUYEN KD, QIU Y, CUI X, GOH YP, MWANGI J, DAVID T, MUKUNDAN L, BROMBACHER F, LOCKSLEY RM, CHAWLA A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.OOTSUKA Y, BLESSING WW, STEINER AA. Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1294–R1303. doi: 10.1152/ajpregu.00709.2007. [DOI] [PubMed] [Google Scholar]
- 32.AMIR S, SCHIAVETTO A. Injection of prostaglandin E2 into the anterior hypothalamic preoptic area activates brown adipose tissue thermogenesis in the rat. Brain Res. 1990;528:138–142. doi: 10.1016/0006-8993(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 33.MORRISON SF, NAKAMURA K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. 74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.TOWNSEND KL, TSENG YH. Brown adipose tissue: recent insights into development, metabolic function, and therapeutic potential. Adipocyte. 2012;1:13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CLERICO A, GIANNONI A, VITTORINI S, PASSINO C. Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones. Am J Physiol Heart Circ Physiol. 2011;301:H12–H20. doi: 10.1152/ajpheart.00226.2011. [DOI] [PubMed] [Google Scholar]


