Abstract
Background & Aims
Pancreatic ductal adenocarcinoma (PDA) is a leading cause of cancer-related death. Through the process of acinar to ductal metaplasia (ADM), pancreatic acinar cells give rise to pancreatic intraepithelial neoplasia (PanIN), the most common precursor of PDA. However, even when Kras is activated in a majority of acinar cells, ADM and subsequent development of PanINs is inefficient in the absence of additional stresses. Numb regulates cell junctions, integrins and the activity of embryonic signaling pathways, therefore we investigated its effects on acinar cell dedifferentiation, regeneration and metaplasia.
Methods
We used mouse models of pancreatic regeneration and PDA, and also mice with loss-of-function alleles of Numb (p48Cre/p48CreER;Numbf/f and p48Cre/p48CreER;KrasG12D;Numbf/f mice) to study the roles of Numb in pancreatic regeneration and ADM.
Results
Loss of Numb resulted in premature dedifferentiation of acinar cells in response to injury due to administration of the cholecystokinin analog caerulein, and interfered with acinar cell regeneration. Numb was found to regulate multiple signaling pathways in acinar cells during caerulein-induced pancreatitis. Disruption of Numb accelerated and destabilized ADM in the context of oncogenic Kras (in p48Cre;KrasG12D;Numbf/f and p48CreER;KrasG12D;Numbf/f mice).
Conclusions
Numb is an important regulator of acinar cell differentiation and viability during metaplasia. In mice with pancreatitis or pancreatic injury, elimination of Numb causes dedifferentiated acinar cells to undergo apoptosis—a process that is not mitigated by oncogenic Kras.
Keywords: Acinar to ductal metaplasia, PanIN, Pancreatitis, Kras
Introduction
Pancreatic ductal adenocarcinoma (PDA) is one of the most lethal of human malignancies. Pathogenesis is believed to occur through the progression of precursor lesions, the most well described being pancreatic intraepithelial neoplasias (PanINs) 1, 2. Understanding the mechanisms of formation and progression of PanINs is critical for development of early detection techniques, preventative measures and therapeutic intervention.
Mutations in the small GTPase Kras are nearly universal in human PDA 3, and are often detected in PanIN lesions. The notion that Kras is the primary oncogene in PDA is supported by mouse models in which the expression of an activated form of Kras in the pancreas under the endogenous Kras promoter (KrasG12D) results in the development of the full spectrum of PanIN lesions and PDA 4. Although PanINs and PDA appear ductal in nature and express markers of pancreatic ductal cells, studies have shown that multiple pancreatic cell types can give rise to PanINs. In particular, acinar cells can undergo a reprogramming process termed acinar to ductal metaplasia (ADM), to form metaplastic ducts and PanIN lesions 5–9. While oncogenic Kras is sufficient for transformation of adult acinar cells, this is a stochastic process as some cells retain their acinar fate and resist rapid dedifferentiation 4, 7 This suggests that other changes are required in order to generate a permissive environment for Kras to exert its effects. For example, environmental insults, including pancreatitis and inflammation have been shown to promote PanINs in mice expressing mutant Kras 6, 8, 10.
Caerulein-induced pancreatitis has been used as a tool to study the plasticity of the adult acinar cell. In response to acute doses of caerulein, mature acinar cells change their differentiation state resulting in decreased expression of acinar markers, inappropriate expression of ductal markers, a transition to a duct-like morphology11, 12, and re-expression of factors associated with embryonic pancreas development 13, 14. Previous studies have demonstrated that acinar cells undergo dramatic changes in cell adhesion and morphology upon caerulein treatment, including dissociation and subsequent reassembly of E-cadherin and β-catenin at the adherens junction 15. Integrin signaling also maintains acinar differentiation. For example, β1 integrin is required for the maintenance of acinar polarity and in its absence acinar cells become disorganized and display increased sensitivity to caerulein treatment 16.
In wild type mice, the dedifferentiated acinar state is transient and reversible, as demonstrated by cell lineage tracing experiments in which damaged acinar cells re-differentiate into fully functional acinar cells 12. However, increasing evidence suggests that this transient cell state is permissive for the disruptive activity of oncogenic Kras, which diverts the regenerative process towards permanent acinar to ductal metaplasia and leads to the subsequent formation of PanINs 8. Dedifferentiation induces re-activation of embryonic signaling pathways and markers of embryonic progenitor cells, including Pdx1 17 and Hes1 18, a downstream mediator of the Notch signaling cascade. Expression of these factors is sustained throughout neoplasia and cancer formation, which suggests they play important roles in disease initiation and progression. Thus, determining the mechanisms that regulate acinar cell dedifferentiation can provide insights into the nature of the environment that supports the initiation and progression of PDA via oncogenic Kras.
The multifunctional protein Numb plays important roles in cell division, progenitor cell maintenance 19 and regulating cell fate decisions 20, 21, in large part through its negative regulation of Notch 22, 23. Numb also regulates Wnt signaling through regulation of β-catenin protein levels and localization 24, 25, Hedgehog signaling through the degradation of Gli1 26 and p53 signaling via suppression of Mdm2 27. Furthermore, Numb can influence epithelial cell polarity and adhesion through interaction with cadherins and catenins 25, 28 and regulation of localization and recycling of integrins 29.
Given its known functions in regulating cell-cell junctions, integrins and the activity of embryonic signaling pathways, we hypothesized that Numb has crucial roles in regulation of acinar cell fate and morphology during regeneration and cancer initiation. By using conditional deletion of Numb 30 combined with mouse models for pancreatic regeneration, ADM and PanIN initiation, we have uncovered a role for Numb in regulating these processes. We show that Numb restrains acinar cell dedifferentiation and KrasG12D-driven ADM formation in response to caerulein treatment. Furthermore, Numb promotes cell survival during pancreatic regeneration as massive pancreatic atrophy occurs in its absence. Summarily, our findings point to Numb as a critical regulator of the acinar differentiation state during metaplasia.
Results
Numb is required for pancreatic regeneration
We first determined the expression pattern of Numb in pancreatic epithelial cells. Given a lack of antibodies suitable for immunostaining in pancreas, we utilized FACS to enrich for acinar cells based on a previous protocol 31 (Morris and Hebrok, unpublished data). Numb, but not the highly related protein Numb-Like, was detected in isolated acinar cells by quantitative PCR (Supplementary Figure 1A). During caerulein-induced pancreatitis, Numb expression declines during the dedifferentiation phase (day 2 after the last day of caerulein treatment) and is restored upon regeneration. However, in pancreata expressing activated KrasG12D, Numb expression remains low at days 7, 21 and during subsequent PanIN progression (Supplementary Figure 1B). These data suggests that Numb repression contributes to acinar dedifferentiation during caerulein-induced pancreatitis.
In order to determine whether Numb plays a role in pancreatic damage and acinar regeneration, we eliminated Numb in pancreatic epithelium by crossing conditional Numb mutant mice (Numbf/f) 30 with a pancreatic epithelium-specific Cre line driven by the p48 promoter 32. We have previously demonstrated efficient Cre activity in the majority of acinar cells and a subset of duct cells during pancreatic development in these p48Cre transgenic mice 33 and efficient recombination of the Numb allele is observed in six week old p48Cre;Numbf/f mice (Supplementary Figure 2A–B). Remaining transcripts observed in Numb mutant tissue likely originate from subsets of exocrine or endocrine cells and non-epithelial cells within the pancreas not targeted by p48Cre. p48Cre;Numbf/f mice showed no pancreatic abnormalities in pancreas size, morphology and histology as evidenced by comparable levels of E-cadherin, Amylase, Mist1, CK19 and Sox9 expression as well as insulin and amylase areas (Supplementary Figure 2C–H).
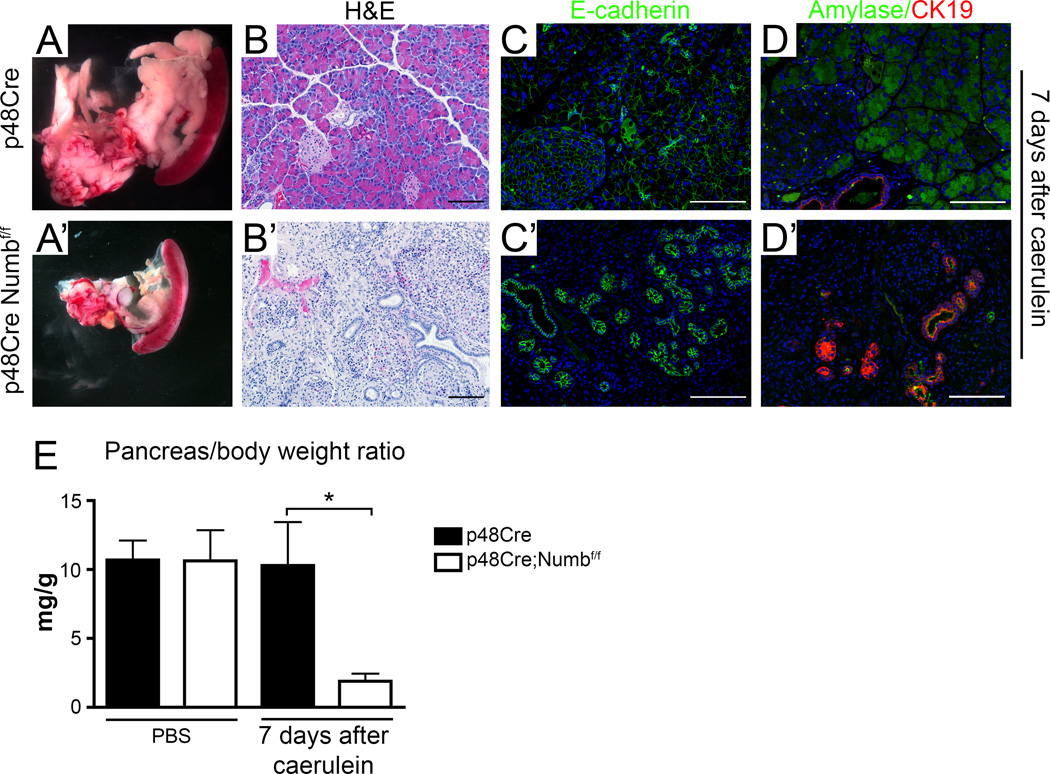
Adult acinar cell dedifferentiation in response to caerulein-induced pancreatitis is a transient process that resolves itself within 7 days post treatment in control animals (Figure 1A–E). In contrast, massive pancreatic atrophy and severe reduction in the pancreas mass to body ratio was observed in mice lacking Numb (Figure 1E). The remaining pancreas tissue contained considerable amounts of non-epithelial tissue interspersed with abundant duct structures and few amylase-expressing acinar cells (Figure 1A’–D’). We then performed lineage tracing and Numb recombination specifically in adult acinar cells using LSL-R26RYFP, where LSL refers to “lox site-transcription stop-lox site” (referred to as YFP from here on) combined with the acinar-specific p48CreERT 34. This confirmed that the reduction in pancreas tissue size was due to the deletion of Numb in acinar cells (Supplementary Figure 3). Therefore, Numb is required for regeneration of acinar tissue in response to caerulein-induced pancreatitis.
Figure 1. Acinar cells lacking Numb are incapable of regeneration and have reduced metaplastic capacity.
(A) Gross morphology, (B) H&E staining, (C) immunostaining for E-cadherin (green), counterstained with DAPI (blue), and (D) immunostaining for Amylase (green) and CK19 (red), counterstained with DAPI (blue) of p48Cre versus p48Cre;Numbf/f 7 days after caerulein treatment. , Scale bars represent 100µm. (E) Pancreas mass:body weight ratio of PBS and caerulein treated mice at day 7. * P<0.05, N=3–5.
Numb restrains acinar cell dedifferentiation
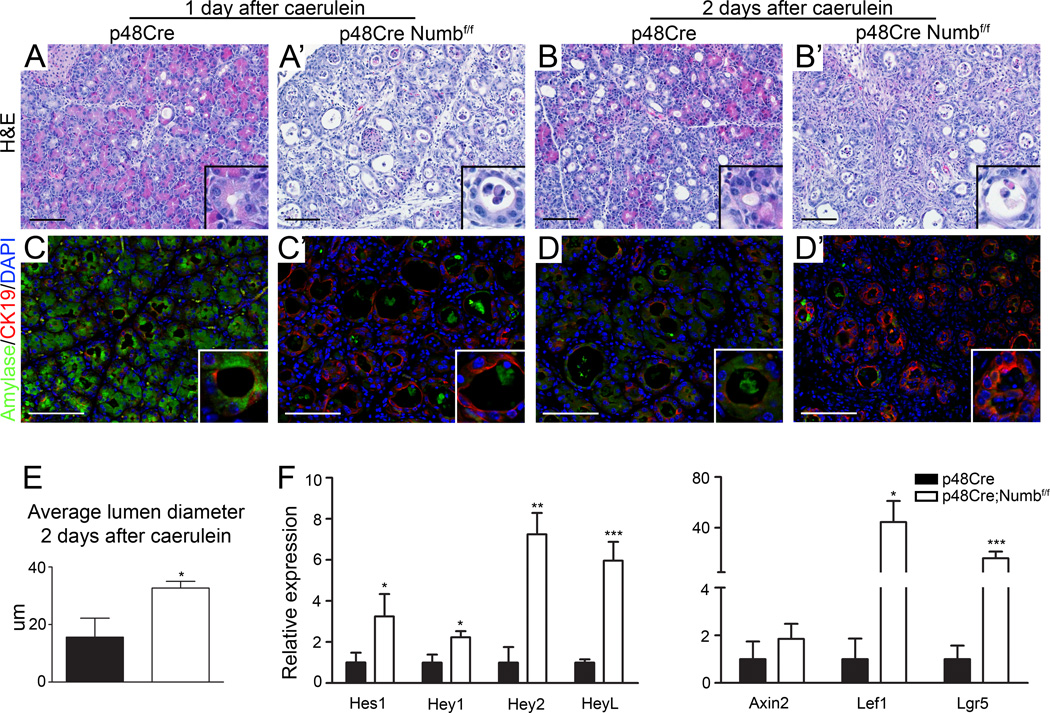
Numb has previously been shown to inhibit the activity of several signaling pathways, including Notch signaling. In PBS treated p48Cre;Numbf/f mice we observed a significant reduction in one of the Wnt target genes Lgr5, and though statistically not significant, there was a trend towards deregulation of Notch and other well-known Wnt target gene expression, but expression of Hedgehog signaling targets was unperturbed (data not shown). The Notch pathway is activated during acinar dedifferentiation and regulates ADM 13, 18. We therefore investigated whether interactions between Numb and Notch signaling mediate acinar cell responses to caerulein treatment. Indeed, 2 days after caerulein treatment, p48Cre;Numbf/f mice presented with enhanced acinar dedifferentiation and increased lumen size (Figure 2A-D’,E), accompanied by significant increases in Notch and Wnt pathway activation (Figure 2F). Furthermore, p48Cre;Numbf/f pancreata showed more apparent stromal expansion and immune cell infiltration, as measured by increased presence of α-Smooth Muscle Actin and CD45 positive cells, respectively (Supplementary Figure 4). Notably, Numb ablation did not prevent proliferation of dedifferentiated acinar cells at these time points (Supplementary Figure 5). These data indicate that acinar cells lacking Numb are more prone to respond to pancreatitis cues that initiate dedifferentiation events.
Figure 2. Acinar cells lacking Numb undergo more rapid and extensive dedifferentiation.
(A–B) H&E staining and (C–D) Amylase (green) and CK19 (red) immunostaining, counterstained with DAPI (blue) 1–2 days following caerulein reveals increased duct structure formation and greater loss of amylase expression in p48Cre;Numbf/f. (E) Lumen diameters of ductal structures at 2 days after caerulein treatment. (F) qPCR analysis of Notch and Wnt target genes 2 days after caerulein treatment. * P<0.05, ** P<0.01, *** P<0.005. N=3–4.
Deletion of Numb accelerates Kras-mediated acinar to ductal metaplasia
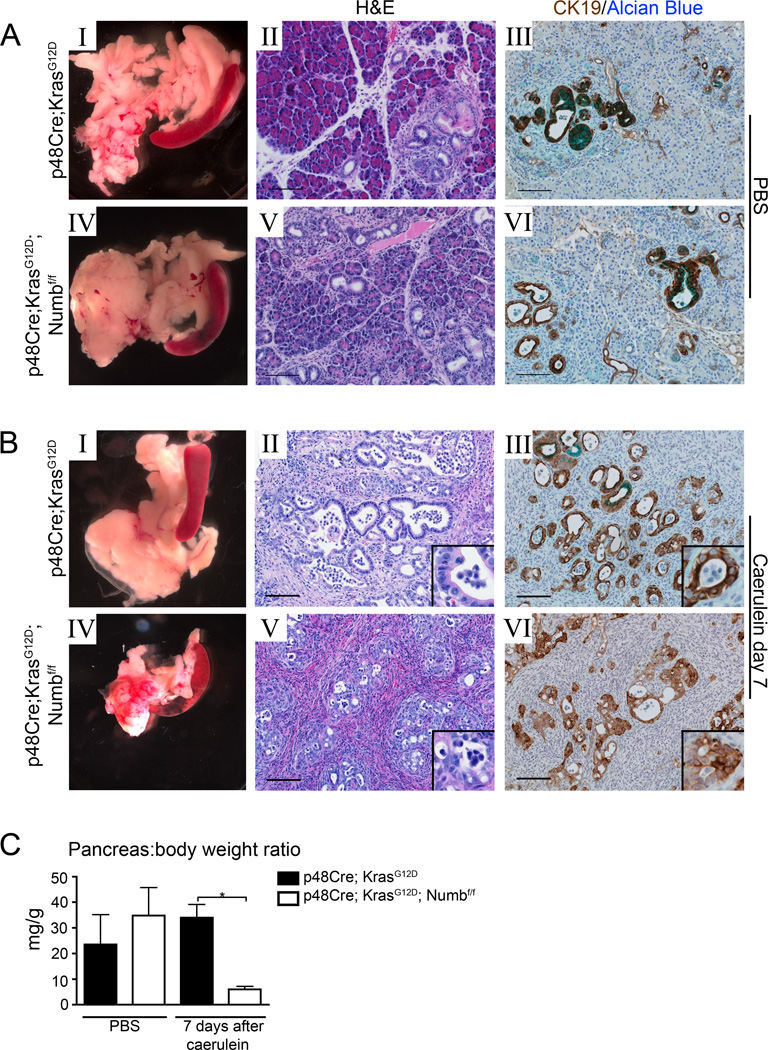
We previously observed that Numb restrains acinar dedifferentiation in response to caerulein pancreatitis. Given these findings, we sought to determine whether Numb also regulates the ADM that occurs in response to expression of oncogenic Kras. To address this question, we crossed a LSL-activated form of KrasG12D 35 with p48Cre and Numbf/f to generate p48Cre;KrasG12D;Numbf/f mice. Similar to p48Cre;Numbf/f, p48Cre;KrasG12D;Numbf/f also undergo significant pancreatic atrophy 7 days after caerulein treatment. Histological examination revealed dense stroma and residual duct structures and a significant reduction in tissue mass (Figure 3B IV–VI, and C).
Figure 3. Acinar cells lacking Numb have reduced viability during caerulein-induced ADM.
(A–B I, IV) Gross morphology, (A–B II, V) H&E and (A–B III, VI) immunostaining for CK19 (brown) counterstained with Alcian Blue (bright blue) p48Cre;KrasG12D and p48Cre;KrasG12D ;Numbf/f pancreata 7 days after PBS or caerulein treatment. Scale bars represent 100µm. (C) Pancreas mass:body weight ratio of PBS and caerulein treated mice at day 7. * P<0.05, N=3–5.
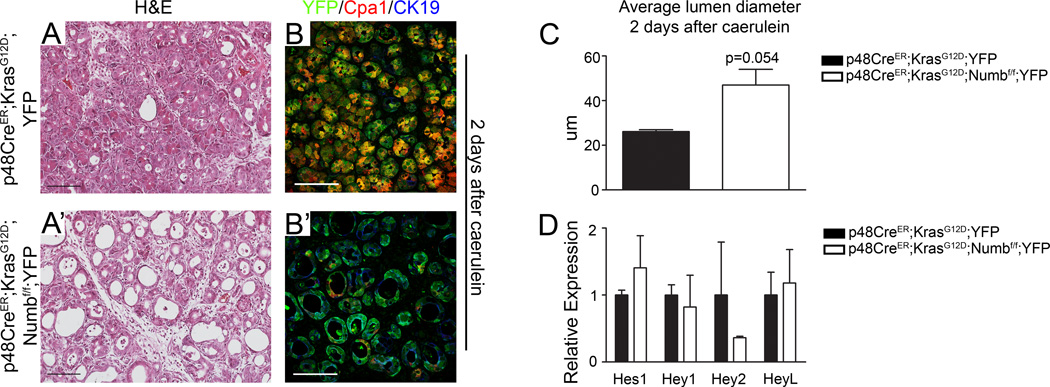
Due to activation of Kras in the embryonic pancreas, a small number of PanIN lesions are already present prior to caerulein injections (Figure 3A IV–VI). In order to better understand ADM and PanIN formation from adult cells, we generated p48CreER;KrasG12D;YFP mice to ensure normal embryonic development and maturation of acinar cells and permit synchronization of Kras activation while examining the effects of Numb loss on the early metaplastic process. Compared to p48Cre;KrasG12D;YFP mutants, p48CreER;KrasG12D;Numbf/f;YFP acini displayed an enhanced response to caerulein as demonstrated by increased lumen size, accelerated loss of the acinar cell marker Cpa1 and significant induction in CK19 expression at day 2 after caerulein (Figure 4A’,B’,C). Costaining of the YFP cell lineage tracing marker with CK19 confirms the acinar origin of duct like structures. Notably, the accelerated ADM was not caused by changes in Notch target expression in p48CreER;KrasG12D;Numbf/f;YFP mice (Figure 4D).
Figure 4. KrasG12D expressing acinar cells lacking Numb undergo more rapid and extensive dedifferentiation.
(A–A’) H&E staining and (B–B’) immunostaining for YFP (green), Cpa1 (red) and CK19 (blue) at 2 days after caerulein in p48CreER;KrasG12D;YFP and p48CreER;KrasG12D;Numbf/f;YFP pancreata. (C) Lumen diameters of ductal structures at 2 days after caerulein treatment. (D) qPCR analysis of Notch target genes 2 days after caerulein treatment. N=3–4.
Numb maintains cell adhesion in response to pancreatic damage
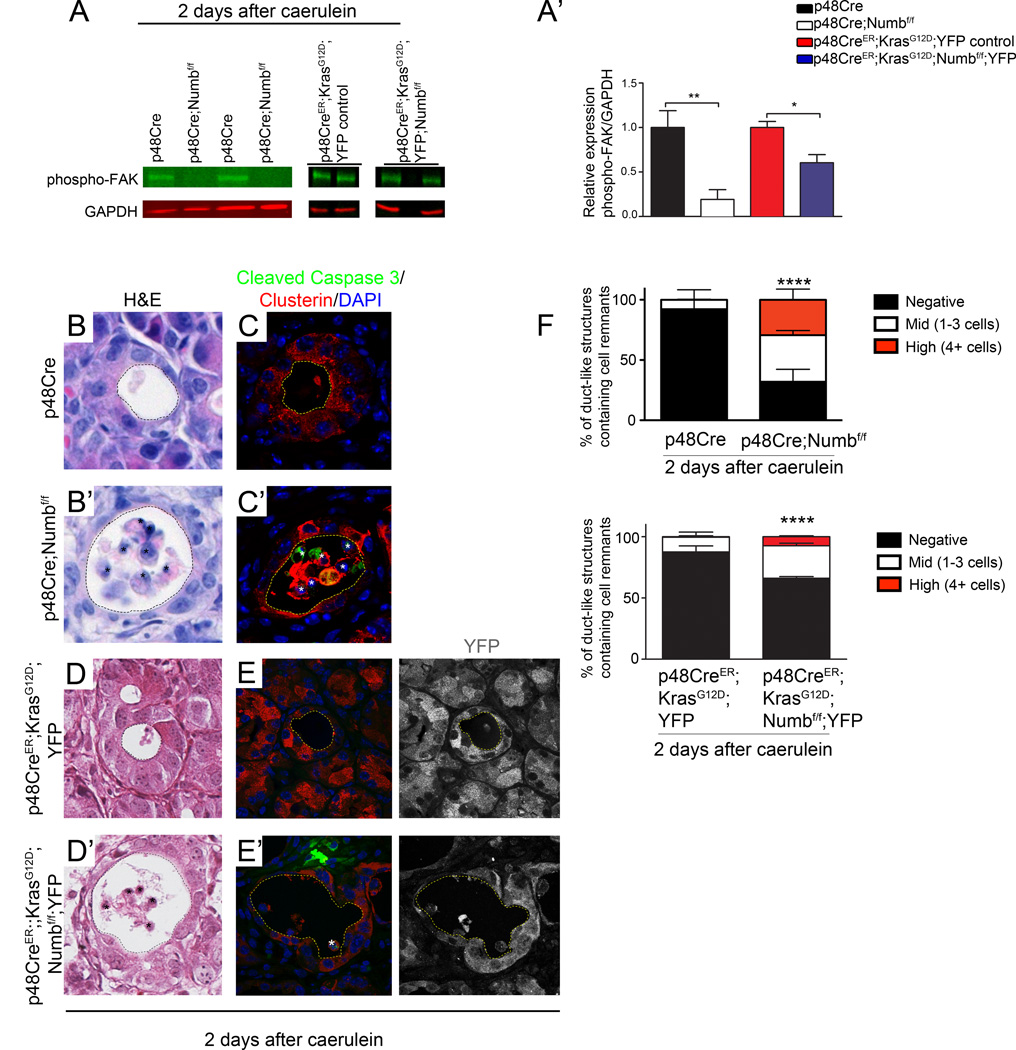
In addition to its modulating activities on embryonic signaling pathways, Numb has also been shown to affect cell adhesion through regulation of integrins, cadherins and catenins 25, 28, 29. Notably, deletion of β1 integrin in the pancreas results in loss of polarity, misdirection of enzyme secretion, increased sensitivity to caerulein-induced damage, and progressive loss of pancreatic tissue mass 16. Therefore, we examined signaling pathways associated with cellular stress and adhesion in p48Cre;Numbf/f pancreata following caerulein treatment. Binding of integrins to basement membrane proteins activates Focal Adhesion Kinase (FAK) through phosphorylation. Phospho-FAK is present in control p48Cre and p48CreER;KrasG12D;YFP, pancreata 2 days after caerulein, but reduced or absent from pancreata lacking Numb (Figure 5A,A’).
Figure 5. Deletion of Numb alters cell adhesion signaling and results in cell extrusion and death.
(A) Western blot of phosphorylated FAK (Tyr-576/577) in p48Cre;Numbf/f and p48CreER;KrasG12D;Numbf/f;YFP and corresponding control mice 2 days after caerulein treatment. All bands for each control/experimental set are taken from the same blot, although separated spatially within the blot where indicated by gaps between images. (A’) Quantification of Western blots, normalized to GAPDH. (B, B’ & D, D’) H&E stainings of p48Cre;Numbf/f and p48CreER;KrasG12D;Numbf/f;YFP pancreata with corresponding controls 2 days post caerulein. (C, C’) Immunostaining of luminally localized cells for Cleaved Caspase 3 (green) and Clusterin (red), counterstained with DAP I (blue) (E, E’) Immunostaining of p48Cre;Numbf/f and p48CreER;KrasG12D;YFP;Numbf/f pancreata for Cleaved Caspase 3 (red) and YFP (green), counterstained with DAPI (blue) 2 days post caerulein. Ductal lumen are outlined with dashed lines, cell remnants contained in lumens are marked with (*). Images in B and C, original magnification 400×. Image D, original magnification 200× (F) Quantification of percent of ductal structures containing either no cells, mid (1–3), or high (4+) numbers of cells within the lumen. Data represented as mean ± SD. ** P<0.01, **** P<0.001. N=3–4 for p48Cre samples and 2 for p48CreER samples.
Disruption of integrin-mediated epithelial cell binding to the extracellular matrix can result in the separation of cells from the epithelial sheet and induction of anoikis, cellular death caused by the loss of cell contact to the basement membrane. Indeed, remnants of cells were observed in the lumens of duct structures in p48Cre;Numbf/f and p48CreER;KrasG12D;Numbf/f;YFP mice at day 2 after caerulein treatment (Figure 5B’,D’), indicating that cells were being extruded from the epithelium. Quantification revealed a significant increase in the percentage of lumens with both mid (1–3) and high (4+) numbers of cells per lumen (Figure 5F). Cells located in the lumen were often, but not always, positive for Cleaved Caspase 3 and co-express the acinar stress marker Clusterin (Figure 5C’,E’). This suggests that cells were undergoing apoptosis while being extruded into the ductal lumen.
Cell death in pancreata lacking Numb is p53 independent
Anoikis can progress either in a p53 dependent or independent manner and to further analyze this process, we examined p53 protein levels and downstream target expression. We observed increased numbers of p53-positive epithelial cells within duct-like structures at day 1 following caerulein treatment in p48Cre;Numbf/f animals compared to controls (Supplementary Figure 6A). The expression of pro-apoptotic genes, including p53 targets Noxa and Puma, were significantly increased during acinar dedifferentiation in the absence of Numb (Supplementary Figure 6B–C). These data suggest that, in the absence of Numb, there is an enhanced apoptotic response upon pancreatitis injury.
To assess the requirement for p53 in the process of cell death during extrusion, we generated compound transgenic p48Cre;p53f/f;Numbf/f mice characterized by the simultaneous loss of Numb and p53 in pancreatic cells. Deletion of p53 alone did not alter regenerative capacities of acinar cells (Supplementary Figure 6D,E, p48Cre;p53f/f;Numbf/wt controls), but the combined loss of p53 and Numb did not rescue the lack of regeneration characteristic of Numb ablation (Supplementary Figure 6D,E). Thus, although p53 accumulation and induction of target genes are increased in p48Cre;Numbf/f mice, cell death in mice lacking Numb can proceed independent of p53 function.
Deletion of Numb accelerates Kras-mediated formation of ductal lesions
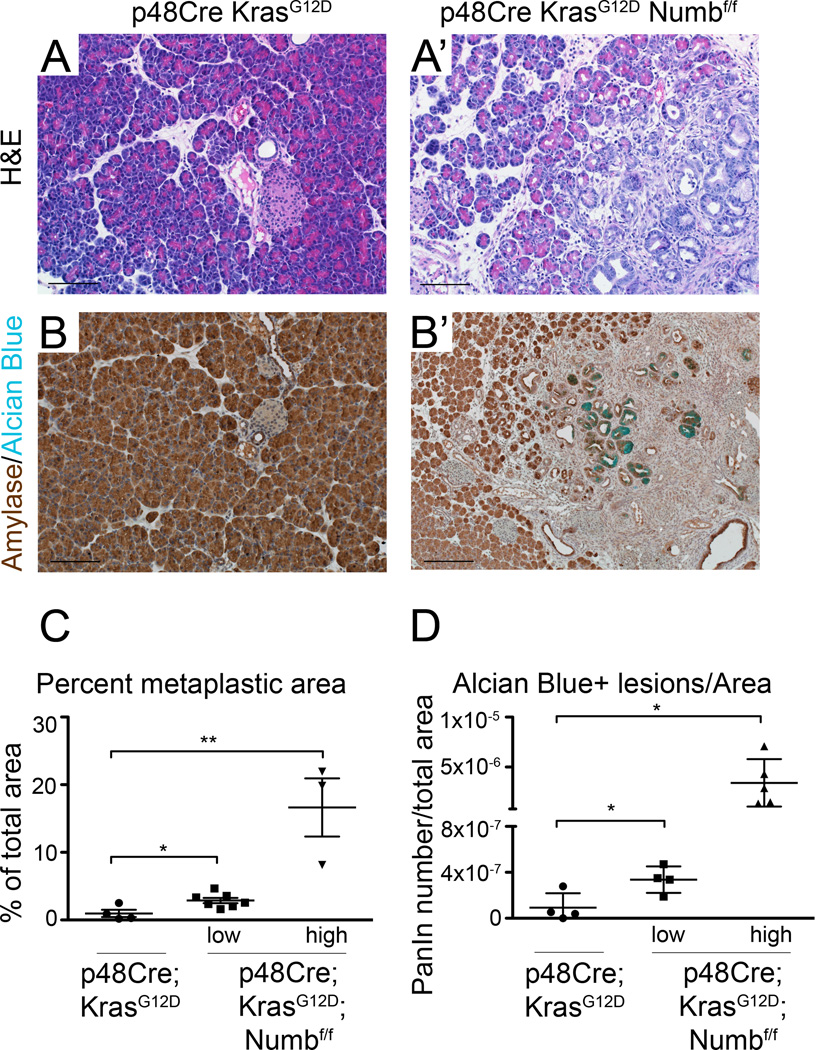
We have previously used caerulein to synchronize Kras-driven metaplasia and subsequent neoplasia. We next asked if Numb loss affects the formation of spontaneous Kras-driven PanIN formation in the absence of caerulein. At postnatal day 2, no differences are observed between p48Cre;KrasG12D and p48Cre;KrasG12D;Numbf/f pancreata, suggesting normal embryonic organ and cell development (Supplemental Figure 7). At 3 weeks of age however, significant areas of ductal lesions and numerous Alcian blue positive lesions are present in p48Cre;KrasG12D;Numbf/f pancreata (Figure 6A’,B’). While, we have observed a high degree of variability between samples, even the low penetrance groups were significantly more impacted than the control tissues (Figure 6C,D). This data demonstrates that Numb normally represses formation of duct-like structures in response to Kras activation.
Figure 6. p48Cre;KrasG12D;Numbf/f mice undergo rapid PanIN formation.
(A,A’) H&E staining and (B,B’) Amylase (brown) and Alcian blue staining shows regions of acinar tissue replaced by ADM and PanIN in p48Cre;KrasG12D;Numbf/f mice at 3 weeks of age. Scale bars represent 100µm. p48Cre;KrasG12D;Numbf/f have significantly higher (C) percent metaplastic and (D) alcian blue positive lesions per area. Data represented as individual mice analyzed, overlaid with mean ± SD. * P<0.05, ** P<0.01.
PanINs and PDA develop in the absence of Numb but pancreatic tissue is lost during progression
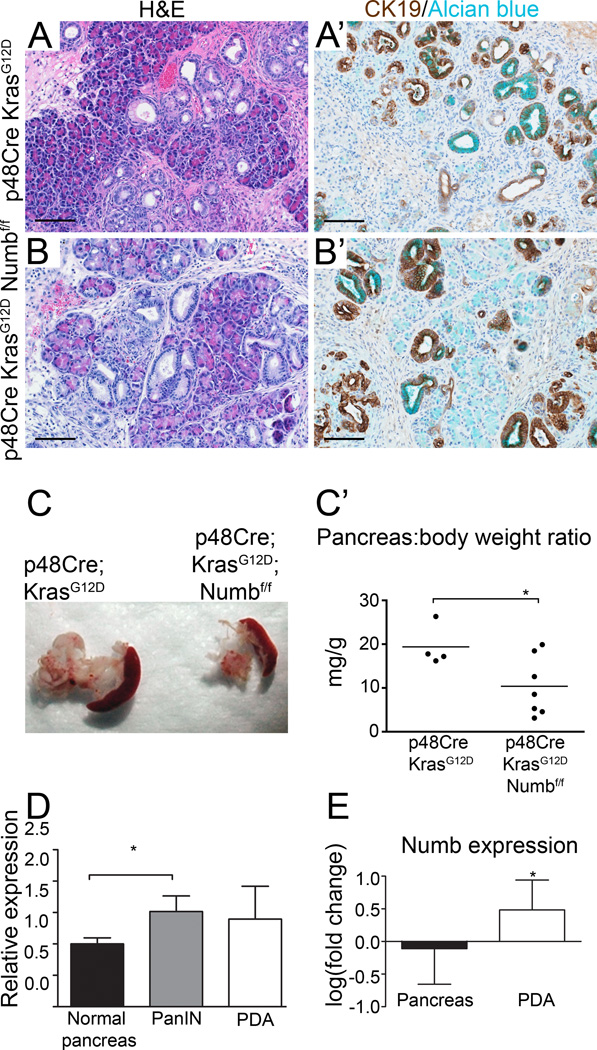
Because metaplasia is initiated earlier and more broadly in p48Cre;KrasG12D;Numbf/f mice, we anticipated a continued acceleration of PanIN formation at later time points. Surprisingly, histological evaluation revealed no differences with regard to ADM and PanIN formation in mutant and control mice at 10 weeks of age (Figure 7A,B). However, pancreata of p48Cre;KrasG12D;Numbf/f mice were significantly smaller than controls as demonstrated by gross morphology and analysis of pancreas to body weight ratio (Figure 7C–C’). Notably, ablation of Numb does not appear to prevent proliferation of metaplastic ducts and PanINs at 6 and 10 weeks of age (Supplementary Figure 5C’,D’). These findings suggest that in addition to restraining early stages of acinar dedifferentiation and metaplasia, Numb sustains cell survival during PanIN progression. Indeed, Numb expression is elevated in mouse PanINs and PDA compared to normal pancreata (Figure 7D). By using meta-analysis of data available in the Oncomine microarray database (Compendia Bioscience, Ann Arbor, MI), we were able to document a similar trend in Numb mRNA expression in humans (combined p value from four independent studies, p=2.02×10−6 (Figure 7E) 36–39, supporting the hypothesis that Numb promotes survival during PanIN progression.
Figure 7. Loss of Numb reduces viability of cells during PanIN progression.
(A, B) H&E staining and (A’, B’) CK19 (brown) and Alcian blue staining reveals similar histology and presence of ADM and PanlNs between the two genotypes. Scale bars represent 100µm. (C,C) Gross morphology and pancreatic weight, normalized to body weight indicate that pancreata of p48Cre;KrasG12D;Numbf/f mice are significantly smaller than controls at 10 weeks of age. (D) Numb expression in normal (p48Cre, 9 weeks old), PanIN (p48Cre; KrasG12D; p53f/+, 12 weeks old) and PDA (p48Cre; KrasG12D; p53f/+ 63–78 weeks old) mouse pancreata. N=3–5. (E) Representative data obtained from the Oncomine™ database showing relative expression of Numb in control versus human PDA samples in a study by Logsdon et al 38. * P<0.05. P=0.0443 for Logdson et al, combined p value of four independent studies, p=2.02×10−6. N=5–39 per study.
Discussion
Upon injury, acinar cells undergo a process of dedifferentiation during which they reduce expression of mature markers and upregulate expression of factors commonly found in duct and embryonic pancreas progenitor cells 13, 40. Understanding the mechanisms underlying acinar cell dedifferentiation is important, as, in the presence of oncogenic Kras, this process is known to give rise to the precursors to pancreatic ductal adenocarcinoma through acinar to ductal metaplasia. Here, we describe a role for the multifunctional protein Numb in the regulation of acinar cell regeneration and acinar to ductal metaplasia.
Numb regulates acinar cell dedifferentiation and regeneration through its regulation of multiple signaling pathways, including Notch
Acinar cells respond to caerulein injury (acute pancreatitis) with rapid, transient dedifferentiation. Following caerulein injury, a regeneration program is launched in wild type mice, during which dedifferentiated acinar cells regain their acinar identity 8, 12. Our data reveal a crucial role for Numb in acinar dedifferentiation. We show that Numb levels are regulated throughout caerulein-induced pancreatitis and PanIN/PDA development. The mechanism for regulation of Numb in this process is unclear, however other studies have shown that Numb is regulated post-transcriptionally by the RNA binding protein Musashi1 as well as through ubiquitination 41, 42. Further study will be required to determine the regulation of Numb during pancreatitis and PDA.
Reduction of Numb during acinar dedifferentiation suggests that lower levels of the protein may be necessary to acquire the dedifferentiated state. Indeed, caerulein-induced pancreatitis is enhanced in Numb mutant mice, as indicated by an increased number of duct-like structures, reduced expression of acinar cell markers and increased expression of duct markers. Thus, our findings suggest that Numb regulates acinar cell response to pancreatic damage and in its absence, acinar cells are more sensitive to the signals that initiate dedifferentiation. In addition, our data demonstrate that Numb governs the regenerative capability of acinar cells in response to damage. Deletion of Numb not only prevents acinar regeneration, it also results in massive pancreatic atrophy following caerulein pancreatitis, which indicates that Numb normally functions to stabilize the dedifferentiated state and allow for proper regeneration of acinar cells
Injury to adult tissues induces a sophisticated repair process that involves activation of developmental signaling pathways such as Notch, Hedgehog, and Wnt. Reactivation of these signaling pathways upon acinar injury has been previously demonstrated 13. Numb can regulate the activities of such signaling pathways including Notch in other tissues 22, 23 and aberrant Notch activity in pancreatic acini promotes their transdifferentiation towards duct-like cells 18. Our data suggests that in the absence of Numb under pancreatitis conditions, deregulation of Notch signaling exacerbates the dedifferentiation of acinar cells and the adoption of the duct- and progenitor-like cell state. Interestingly, Numb does not affect all Notch receptors equally; different combinations of Numb isoforms and Notch receptors determine the signaling output 43. Therefore it is possible that in addition to increasing overall pathway activity levels, deletion of Numb alters the ultimate signaling outcome of the Notch pathway in dedifferentiated acinar cells.
The contribution of increased Notch signaling to cell viability remains unclear. Some evidence demonstrates that excessive Notch signaling can lead to cell death; for example, aberrant Notch activation in neural progenitors, but not post-mitotic neurons, promotes apoptosis 44. We speculate that the dedifferentiated acinar cell state may be sensitive to fluctuations in Notch signaling, requiring some pathway activation, but hindered by excessive levels. Our data may be another instance demonstrating the requirement for a delicate balance of signaling pathway activation in regulating acinar dedifferentiation and regeneration, as has already been demonstrated for Wnt 8.
Numb regulates cell stress responses and cell adhesion during caerulein-induced pancreatitis
We have also gathered evidence that the effects of Numb ablation in the pancreas extend beyond Notch regulation to include perturbed cell adhesion signals, which may be mediated via abrogation of integrin signaling. The lack of FAK activation points to a disruption of cell adhesion that can result in induction of cell death by a process known as anoikis. Numb is known to be required for the maintenance of adherens junctions as well as trafficking of integrins 28, 29, and these activities could be essential for maintenance of tight cell-cell and cell-matrix interactions. Our data is in alignment with recent findings illustrating the connection between cell-cell interactions and acinar differentiation. For example, loss of β1 integrin has been demonstrated to disrupt acinar cell polarity, lead to acinar to ductal metaplasia, and result in the loss of pancreatic tissue mass 16.
Numb regulates and stabilizes Kras mediated acinar to ductal metaplasia
In the absence of additional stresses such as inflammation due to caerulein pancreatitis, Kras is fairly inefficient at reprogramming acini into PanIN lesions. However, the roadblocks that exist to restrain this metaplasia are unclear. In this study, we have revealed that Numb provides such a barrier, as its ablation accelerates metaplasia of acinar cells due to deregulated Kras signaling. The observation of elevated Notch in caerulein-treated Numb mutant pancreas indicates that Numb levels regulate Notch activity. Although significantly elevated Notch is not observed in our system in the presence of activated Kras, these findings as intriguing, as Kras and Notch have been documented to cooperate during metaplasia 5, 45, 46.
Notably, Numb’s effects on cell viability are also observed in the presence of oncogenic Kras. Although ablation of Numb accelerates the process of ADM and PanIN formation that occurs under the control of activated Kras, the viability of cells during these processes is reduced and tissue mass decreases significantly over time. Thus, even the strong neoplastic cues provided by mutant Kras are not sufficient to stabilize the dedifferentiated state generated in the absence of Numb. This is an exciting finding, as it poses possibilities for ways to dampen the transformative properties of Kras. Nonetheless, some PanIN formation still occurs at later stages in the absence of Numb. The notion that PanIN development can proceed under these conditions, albeit with reduced viability during the early metaplastic stage, strongly suggests that the pro-apoptotic cues generated in the absence of Numb can be overcome. Further analysis of the cell survival signals present in these remaining PanINs could provide important insight into critical mechanisms promoting PanIN maintenance and progression.
These findings also indicate that subsequent development of PDA could occur in the absence of Numb. Future studies will address whether this is indeed the case and explore whether Numb elimination in PDA might change the malignant properties of tumor cells. One might speculate that changes in cell adhesion and increases in the activity levels of various signaling pathways that may be detrimental to a cell undergoing metaplasia may instead enhance the tumorigenicity of a fully transformed cell, thus influencing tumor growth, invasion and metastasis. Although no known mutations of Numb have been documented in pancreatic cancer in the COSMIC Database 47, our analysis of gene expression based on the Oncomine datasets showed increased Numb expression in human PanIN and PDA compared to normal pancreata. More in depth analysis is required to fully determine the changes in Numb expression and activity in PDA and how these changes may affect tumorigenicity.
Material and Methods
Mouse lines
Experimental animals were generated by crossing p48Cre (gift of Chris Wright, Vanderbilt University, Nashville, Tennessee, USA) with LSL-KrasG12D (gift of Dave Tuveson, Cancer Research UK Cambridge Research Institute, Cambridge, United Kingdom) and Numbf/f (gift of Yuh Nung Jan, University of California, San Francisco, California, USA). All mice experiments were performed with approval from the UCSF Institutional Use and Care of Animals Committee (IACUC). Littermate controls were used.
Flow cytometry
FACS enrichment of acinar and ductal cells was performed as described by Morris et al (in preparation). A brief description of this method is found in Supplemental Material.
Complete methods can be found in Supplementary information.
Supplementary Material
Acknowledgements
The authors would like to thank Cecilia Austin and Debbie Ngow for histology assistance. Guido Von Figura and Nilotpal Roy for their technical assistance. Also, we thank the following for providing mouse lines: C. Wright (p48Cre); D. Tuveson (LSL-KrasG12D); and Y. Jan (Numbf/f). Work in M. Hebrok’s lab was supported by an NIH grant (RO1 CA112537). Imaging experiments were supported by resources from the UCSF Diabetes and Endocrinology Resource Center (DERC; UO1 DK089541).
Abbreviations
- PDA
Pancreatic ductal adenocarcinoma
- ADM
Acinar to ductal metaplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose.
Author contributions:
M.H. and R.L.G. conceived the study. M.H., R.L.G. and B.K.S. designed the experiments, R.L.G. and B.K.S. performed them and A.L. provided technical assistance. M.H., R.L.G. and B.K.S. wrote the manuscript.
References
Author names in bold designate shared co-first authorship
- 1.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 2.Feldmann G, Maitra A. Molecular genetics of pancreatic ductal adenocarcinomas and recent implications for translational efforts. J Mol Diagn. 2008;10:111–122. doi: 10.2353/jmoldx.2008.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 4.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 5.De La OJ, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JPt, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–173. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, Dor Y, Stoffers D, Jensen J, Leach SD, Maitra A. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Siveke JT, Lubeseder-Martellato C, Lee M, Mazur PK, Nakhai H, Radtke F, Schmid RM. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Lerch MM, Lutz MP, Weidenbach H, Muller-Pillasch F, Gress TM, Leser J, Adler G. Dissociation and reassembly of adherens junctions during experimental acute pancreatitis. Gastroenterology. 1997;113:1355–1366. doi: 10.1053/gast.1997.v113.pm9322531. [DOI] [PubMed] [Google Scholar]
- 16.Bombardelli L, Carpenter ES, Wu AP, Alston N, DelGiorno KE, Crawford HC. Pancreas-specific ablation of beta1 integrin induces tissue degeneration by disrupting acinar cell polarity. Gastroenterology. 2010;138:2531–2540. doi: 10.1053/j.gastro.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyatsuka T, Kaneto H, Shiraiwa T, Matsuoka TA, Yamamoto K, Kato K, Nakamura Y, Akira S, Takeda K, Kajimoto Y, Yamasaki Y, Sandgren EP, Kawaguchi Y, Wright CV, Fujitani Y. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 19.Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- 20.Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat Neurosci. 2004;7:803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- 21.Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
- 22.McGill MA, McGlade CJ. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 2003;278:23196–23203. doi: 10.1074/jbc.M302827200. [DOI] [PubMed] [Google Scholar]
- 23.McGill MA, Dho SE, Weinmaster G, McGlade CJ. Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem. 2009;284:26427–26438. doi: 10.1074/jbc.M109.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, Srivastava D. Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13:1244–1251. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Sandiford S, Wu C, Li SS. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J. 2009;28:2360–2373. doi: 10.1038/emboj.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Marcotullio L, Ferretti E, Greco A, De Smaele E, Po A, Sico MA, Alimandi M, Giannini G, Maroder M, Screpanti I, Gulino A. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol. 2006;8:1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- 27.Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, Pece S, Di Fiore PP. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- 28.Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, Sestan N. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhong W, Jiang MM, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, Jan YN. Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci U S A. 2000;97:6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci U S A. 2007;104:175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 33.Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, Taketo MM, Biankin AV, Hebrok M. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopinke D, Brailsford M, Pan FC, Magnuson MA, Wright CV, Murtaugh LC. Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas. Dev Biol. 2012;362:57–64. doi: 10.1016/j.ydbio.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segara D, Biankin AV, Kench JG, Langusch CC, Dawson AC, Skalicky DA, Gotley DC, Coleman MJ, Sutherland RL, Henshall SM. Expression of HOXB2, a retinoic acid signaling target in pancreatic cancer and pancreatic intraepithelial neoplasia. Clin Cancer Res. 2005;11:3587–3596. doi: 10.1158/1078-0432.CCR-04-1813. [DOI] [PubMed] [Google Scholar]
- 38.Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R, Hanash S. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 39.Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016–2027. [PubMed] [Google Scholar]
- 40.Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, Nakafuku M, Okano H. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21:3888–900. doi: 10.1128/MCB.21.12.3888-3900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pece S, Serresi M, Santolini E, Capra M, Hulleman E, Galimberti V, Zurrida S, Maisonneuve P, Viale G, Di Fiore PP. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004;167:215–221. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beres BJ, George R, Lougher EJ, Barton M, Verrelli BC, McGlade CJ, Rawls JA, Wilson-Rawls J. Numb regulates Notch1, but not Notch3, during myogenesis. Mech Dev. 2011;128:247–257. doi: 10.1016/j.mod.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Mazur PK, Einwachter H, Lee M, Sipos B, Nakhai H, Rad R, Zimber-Strobl U, Strobl LJ, Radtke F, Kloppel G, Schmid RM, Siveke JT. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2010;107:13438–13443. doi: 10.1073/pnas.1002423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci U S A. 2007;104:19327–19332. doi: 10.1073/pnas.0705953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, Teague JW, Campbell PJ, Stratton MR, Futreal PA. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2010;39:D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.