Abstract
Background
Analysis and modeling of vital sign and waveform data in surgical / trauma intensive care unit (STICU) patients may allow for early identification and treatment of patients with evolving respiratory failure.
Methods
Between February 2011 and March 2012, vital sign and waveform data for STICU patients were collected. Every-15-minute calculations (n=172,326) of means and standard deviations of heart rate (HR), respiratory rate (RR), pulse-oxygen saturation (SpO2), cross-correlation coefficients and cross-Sample Entropy for HR-RR, RR-SpO2, and HR-SpO2, and cardiorespiratory coupling were calculated. Urgent intubations were recorded. Univariate analyses were performed for the periods <24 and ≥24 hours prior to intubation. Multivariate predictive models for the risk of unplanned intubation were developed and internally validated by subsequent sample and bootstrapping techniques.
Results
Fifty unplanned intubations (41 patients) were identified from 798 STICU patients. The optimal multivariate predictive model (HR, RR, and SpO2 means and RR-SpO2 correlation coefficient) had a ROC area of 0.770 (95%CI 0.712, 0.841). For this model, relative risks of intubation in the next 24 hours for the lowest and highest quintiles were 0.20 and 2.95 respectively (15-fold increase, baseline risk 1.46%). Adding age and days since previous extubation to this model increased ROC area to 0.865 (0.821, 0.910).
Conclusions
In STICU patients, a multivariate model predicted increases in risk of intubation in the following 24 hours based on vital sign data currently available on bedside monitors. Further refinement could allow for earlier detection of respiratory decompensation and intervention to reduce preventable morbidity and mortality in surgical/trauma patients.
INTRODUCTION
Complex analysis of vital signs to predict outcomes for a given patient population is appealing and intuitive. Heart rate variability (HRV), for example, is a well-known index of illness and a predictor of death in trauma patients (1-3). In addition, decreased HRV is seen as physiologic reserve is depleted and patients approach exhaustion (3). However, while predictors of mortality allow physicians to quantify the severity of a patient’s injuries, they do not guide moment-to-moment decisions regarding patient management. The use of the HeRO® (Heart Rate Observation) score in neonates (4,5) provides a precedent for directing an intervention (in this case, diagnosis and treatment of sepsis) based on a score developed from a complex analysis of vital signs data. A recent large randomized trial showed that display of the HeRO® score was associated with decreased mortality of very low birth weight infants in the neonatal intensive care unit (ICU) by more than 20% (5).
We hypothesized that a similar approach might aid in early detection of respiratory decompensation leading to urgent unplanned intubation. The sequelae of intubation in an ICU population are significant. Ventilator-associated pneumonia (VAP) is associated with prolonged ICU and hospital length of stay, increased costs, and increased mortality (6-10). The incidence of VAP is greater in patients who are ventilator-dependent for longer periods of time (6,7,9,11-13), and reintubation is independently associated with the development of VAP (6,7,11,14). In addition, the need for an urgent intubation is associated with a higher risk of VAP than elective intubation, perhaps due to the increased risk of aspiration of gastric contents during less than ideal conditions (14).
Therefore, identifying patients with progressive respiratory decompensation at an earlier stage has the potential to prevent intubations or, at the least, convert an urgent intubation to a more controlled elective intubation, thereby reducing some of the associated morbidity. Similar to the association of reduced HRV with mortality, it has been shown that measures of HRV are better predictors of prolonged ventilation than mean or median heart rate (15). The aim of this study is to assess complex vital sign analyses for patients who required urgent intubation in a surgical / trauma population for the 24 hours preceding an intubation event to determine whether changes in vital sign and wave form data might allow for earlier identification of evolving respiratory failure in these patients.
METHODS
This study was undertaken in a tertiary-care, level 1 trauma center with a 15-bed combined surgical / trauma intensive care unit (STICU). All patients are managed by a team of board-certified surgical intensivists, residents, and nurse practitioners.
The bedside monitors in all STICU rooms are connected to a BedMasterEx patient monitoring system (Excel Medical, Jupiter, FL) that records vital signs and waveform data, allowing data to be downloaded and warehoused daily on a custom grid computing cluster. (16). Stored data include a coded timestamp and bed assignment, and data are linked to medical record information stored in the Clinical Data Repository to reunite physiologic data with clinical information. These data points include heart rate (HR), respiratory rate (RR), invasive and non-invasive blood pressure measurements, and pulse oximeter saturation reading (SpO2), reported by the monitor every 2 seconds. All analyses were done in MatLab®. We have previously devised and validated multivariable logistic regression modeling using our own scripts (17).
From February 2011 through January 2012, unplanned, urgent intubation events occurring for surgical patients within the STICU were prospectively collected and recorded via daily systematic review of the ‘Adult Ventilator Template’ in the electronic medical record (Epic Systems Corporation, Verona, WI), with confirmation by ICU attending physician notes. In addition, a prospective log of all patients on the general surgery, trauma, emergency general surgery, transplant, or vascular services was maintained. Patients on ancillary services including urology, otolaryngology, obstetrics, or plastic surgery were not included in either event capture or vital signs data collection.
The date, time, and relevant circumstances for the intubation event were recorded. In addition, extubation events were recorded to distinguish between reintubation (defined as return to invasive mechanical ventilation greater than 24 hours from prior extubation) and failed extubation (defined as return to invasive mechanical ventilation within less than 24 hours following extubation). Patients electively intubated for a procedure or operation and extubated in the operating room or post-anesthesia care unit at the conclusion of the case were not considered to have been previously intubated or mechanically ventilated. If a patient received a tracheostomy, an extubation event was considered the time of liberation from the ventilator once a patient had completed a 24-hour period free of mechanical support. If the patient required mechanical ventilatory support via the tracheostomy following that 24-hour period, it was
recorded as a reintubation event. We considered all non-elective intubations to be urgent and unplanned.
Means and standard deviations of vital signs (HR, RR, SpO2) were calculated every 15 minutes as based on the previous 30 minutes of data, resulting in 96 measurements per day. Joint vital signs included the maximum cross-correlation coefficient (Corr) and the cross-Sample Entropy (SampEn, a measure of time-series complexity) of HR-RR, RR-SpO2, and HR-SpO2 pairs (18,19). Cardiorespiratory coupling was measured using phase-based analysis (16). Clinical parameters analyzed were age, days since previous extubation, and length of ICU stay. If a patient had not been previously intubated, a conservative maximum value of 25 days was used for days since extubation for modeling purposes, as it was larger than any value for the previously intubated patients.
Binary logistic regression models were developed by labeling observations during the 24 hours prior to the event as ‘one’, and observations outside this timeframe as ‘zero’. Because features were calculated every 15 minutes, there were many inputs per patient and event. The statistical significance of the model coefficients was adjusted for these repeated measures using the Huber-White method to modify the variance-covariance matrix from the maximum likelihood logistic regression fit (20,21). This approach corrects both for unequal variances and correlated responses from individual patients. More specifically, estimates of regression coefficients and other parameters of the model are obtained in standard fashion, but the p-values are corrected using the "sandwich" estimator of standard errors (22).
The predictive model for intubation was internally validated via two methods. First, a subsequent sample analysis of separate training and test populations was performed. An initial model was developed in a training population prior to the availability of the test set data. Inputs included vital sign features, age and days since extubation. Inputs included vital sign features, age and days since extubation. The performance of this model was then assessed on both the original and subsequent data sets. A second validation utilizing a bootstrapping method was also performed (23). For this method, 1,000 random samples of 798 patients taken with replacement were analyzed. Confidence intervals for the ROC areas were obtained from the 1,000 samples with limits set at the 2.5th and 97.5th percentiles of the sampling distributions.
RESULTS
From 22 February 2011 through 11 January 2012, 873 patient admissions (798 patients) to the STICU met the inclusion criteria above. Demographics are included in Table 1. During this period, 50 unplanned, urgent intubations occurred in 41 patients. Inter-observer agreement for events was high, with a Cohen kappa of 0.9, as expected from the standardized use of the electronic health record. These included 18 intubations in patients not previously intubated during that admission, 21 reintubations, and 11 failed extubations. Of these, one patient had a tracheostomy and required a return to mechanical ventilation following liberation for greater than 24 hours. Those patients requiring intubation differed from those who did not require intubation by ICU length of stay and incidence of ICU-acquired pneumonia.
Table 1.
Demographics. Results are independent samples t-test* or Chi squared test†.
| Intubations n=41 |
Non-Intubations n=757 |
p-value | |
|---|---|---|---|
| Age | 63.3 ± 3.1 | 55.2 ± 0.7 | 0.0090* |
| ICU LOS | 12.8 ± 1.6 | 4.0 ± 0.2 | <0.0001 |
| Trauma Patient | 21 (51%) | 387 (51%) | 1.0† |
| ICU-Acquired Pneumonia | 16 (39%) | 43 (6%) | <0.0001 |
The data analyzed included 172,326 observations of 15-minute interval data for non-intubated patients in the STICU, which translates to 1,795.1 patient days of data. For the patients requiring intubation, there were 2,523 observations, or 26.3 patient days. The overall incidence rate for unplanned intubation in the succeeding 24 hours was 1.46% (2,523 divided by 172,326 observations).
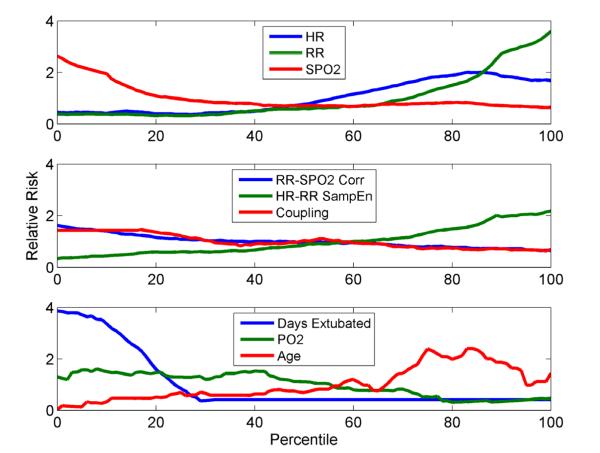
Individual vital signs, joint vital signs measurements, and clinical parameters were assessed by univariate analyses to determine the independent impact of each on unplanned intubations (Figure 1). Increased risk of requiring intubation in the next 24 hours was significantly associated with increasing HR and RR and falling SpO2 (p<0.0005, top panel), decreasing correlation of RR-SpO2 (p<0.0001, middle panel), and increased age and arterial partial pressure of oxygen (PaO2, p<0.022, bottom panel, Table 2). While increasing white blood cell count, increasing HR-RR cross-entropy, and decreasing cardiorespiratory coupling also were associated with increased risk, these did not reach statistical significance. In addition, the length of ICU stay at time of intubation and the time since prior extubation were assessed (Table 2). While overall time in the ICU was not very predictive (p=0.86), time since extubation was highly significant, with a 2.5-fold increased risk in the first 24-48 hours following extubation (p<0.0001, Figure 1). Variables that were statistically significant in the univariate analysis were advanced as candidates in the multivariable analysis.
Figure 1.
Relationship between individual and joint vital sign calculations and selected clinical variables to the relative risk of intubation.
Table 2.
P-values and ROC areas for univariate analyses of individual and joint vital signs and clinical parameters assessed.
| p-value | ROC Area | |
|---|---|---|
| Heart Rate | 0.0005 | 0.664 |
| Respiratory Rate | <0.0001 | 0.743 |
| Pulse Oxygen Saturation (SpO2) | 0.0004 | 0.613 |
| RR-SpO2 Correlation | <0.0001 | 0.571 |
| HR-RR Sample Entropy | 0.172 | 0.654 |
| Cardiorespiratory Coupling | 0.26 | 0.573 |
| White Blood Cell Count | 0.052 | 0.596 |
|
Partial Pressure of Oxygen (PaO2) |
0.022 | 0.618 |
| Age | 0.0042 | 0.660 |
| Days Since Extubation | <0.0001 | 0.742 |
| Days in STICU | 0.90 | 0.480 |
The predictive models were developed and validated internally using two methods. The model was validated on a separate test data set consisting of 209 patients admitted to the STICU from 4/17/2012 to 8/07/2012 where 20 unplanned intubations were identified in 19 patients. The vital-signs only model was calculated every 15 minutes in unventilated patients totaling 42,022 observations (438 days). The ROC area for predicting episodes < 24 hours prior to the event was .729 which was within the 95% confidence interval estimated from the training set.
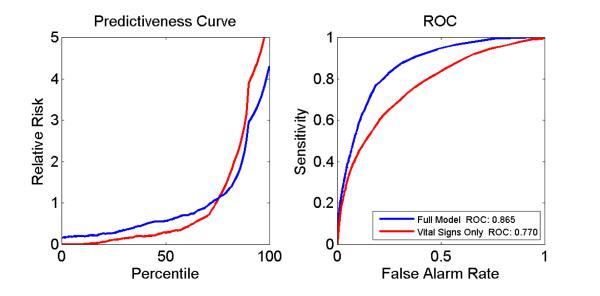
For the second, we utilized the bootstrapping technique (23). The predictive model analyzing all 50 intubation events with inclusion of individual and joint vital sign measures had a ROC curve area of 0.770 (95% confidence interval 0.712 - 0.841). There was a sharp increase in relative risk for the upper quintile, with a 15-fold overall increased risk from the lowest (0.20) to highest quintiles (2.95) for the vital sign model. Adding the clinical features of age and time since extubation increased the ROC curve area to an even higher value of 0.865 (95% CI 0.821-0.910, Figure 2). The relative risk for the lower quintile was 0.006 and for the upper quintile was 3.87. The full model parameters are presented in Table 3.
Figure 2.
Predictiveness and ROC curves for vital signs only and full model.
Table 3.
Multivariate analysis for full model including the regression coefficient ± standard error, Wald chi square statistic, and p-value.
| Coefficient | Wald Chi Square | p-value | |
|---|---|---|---|
| Heart Rate | 0.032 ± 0.01 | 17.19 | 0.00003 |
| Respiratory Rate | 0.056 ± 0.01 | 15.19 | 0.00010 |
| Pulse Oxygen Saturation (SpO2) | −0.081 ± 0.02 | 16.09 | 0.00006 |
| RR-SpO2 Correlation Coefficient | −0.967 ± 0.31 | 9.69 | 0.0019 |
| Age | 0.035 ± 0.01 | 10.12 | 0.0015 |
| Days Since Extubation | −0.086 ± 0.02 | 24.25 | <0.00001 |
In order to assess the model’s performance prior to the time of intubation, the vital signs model was performed on sliding 24-hour windows of available data in one-hour increments. It continued to demonstrate good predictive performance, with ROC curve areas for the vital signs only model of 0.755, 0.737, and 0.712 at −2, −6, and −12 hours, respectively. Thus, even vital signs collected from 6 to 12 hours prior to intubation were acceptable predictors of intubation.
DISCUSSION
The current study evaluated the application of vital sign and waveform analyses in a predictive model to assess the risk of urgent, unplanned intubation in the next 24 hours in a population of surgical and trauma intensive care patients. The resulting models demonstrated very good predictive performance in this representative population of nearly 800 patients with 50 urgent intubation events.
Much work has focused on heart rate variability as the complex vital-sign measurement of choice in further assessing patient status from bedside monitor data. The notion that organ signaling systems are uncoupled during illness was advanced by Godin and Buchman in 1996 (24), and is supported by data suggesting autonomic dysregulation of heart rate and respiratory rate coupling is disrupted in states of infection and inflammation or physiologic exhaustion (1-3,25,26).
This loss of heart rate variability (HRV) in response to respiration when the heart and lungs are coupled in health has long been interpreted as a sign of illness (2,15,24,26-28). For example, Grogan and colleagues showed that a measure of cardiac volatility-related dysfunction, a 5-minute heart rate variability metric recorded over the initial 24 hours of stay in trauma patients, predicts both in-hospital and 30-day mortality with a ROC curve area of 0.81 and sensitivity and specificity of 70.1 and 80.0, respectively (1).
Norris and colleagues have shown that this same predictive property extends through the course of the entire ICU stay (2). In addition, they showed that the pattern of cardiac uncoupling is altered according to the cause of death, with infection causing uncoupling at 6-10 days compared to the early uncoupling seen with multiple organ failure or traumatic brain injury. There are now multiple measures of physiologic variability that can be applied to heart rate and other time series data obtained from bedside monitors (29). The ability to notice such patterns suggests that data analysis at this level may be able to discriminate between other events as well, such as respiratory failure versus septic shock.
The current data demonstrate the feasibility of a developing an internally validated predictive model from complex vital sign analysis in patients requiring urgent, unplanned intubation in a surgical trauma population. However, the relatively small number of events in this single center study may limit the ability to generalize these findings, and the specific vital sign parameters may vary in a larger or more diverse patient population. Additionally, while this analysis was restricted to a surgical ICU patient population, there was still heterogeneity among patients with regards to their trauma status. Larger, multicenter studies are required.
The addition of clinical variables improved the performance of the model, as defined by the predictiveness curves and ROC area (Figure 2). However, the clinical significance of the full model should be interpreted cautiously, as the relative contribution of the days since extubation variable to the model potentially overshadows the more clinically relevant vital sign variables.
The analysis utilized in this study follows the standards set by Moss (30) based on previous work by Concato (31). The internal validation via bootstrapping included odds ratios and confidence intervals (23) for the final model. The MatLab® analysis scripts have been verified against SAS routines. Additionally, interaction was testing utilizing cross-correlation of the vital signs, though collinearity was not explicitly tested. The goodness of fit was demonstrated by the predictiveness curve and ROC area, and overfitting was avoided by using eight to nine outcomes per variable as recommended (23,32). Conformity to linear gradients was analyzed in the univariate predictiveness curve, and univariate significance levels determined inclusion of variables in the multivariate model. Both subsequent samples and bootstrapping techniques were used for internal validation.
The utility of this and similar methods for early detection of subacute and potentially catastrophic illnesses (such as respiratory failure) must be analyzed in the context of the clinician’s own suspicion of respiratory decline. If a physician is already concerned about the patient’s status due to overt clinical changes when the vital sign-based algorithm turns positive, then the utility of such a system is limited. If, however, that system reports an increasing risk of respiratory failure before a physician becomes concerned, then intubation may be prevented by initiating pre-emptive testing (such as chest x-ray and arterial blood gas assay) and interventions (such as increasing oxygen supplementation or other non-invasive ventilatory support measures). Assessment of sliding 24-hour windows of the model provides some indication that changes in vital signs occur well in advance of clinical deterioration, but should be validated in a prospective setting.
The broader goal of such predictive monitoring systems is to describe patient trajectories from health to illness in order to identify those patients with reversible illness in subclinical states who would benefit from earlier interventions (33). An additional and potentially greater value of this methodology lies in monitoring patients on the acute care wards, as those patients tend to have a higher nurse-to-patient ratio and less frequent collection of routine vital signs. This population would hence benefit greatly from an automated system that alerts clinicians to the early changes associated with respiratory decompensation and provide time for such interventions to be initiated, potentially altering the patient’s outcome.
LIMITATIONS
Though the results of these investigations are suggestive of real-time clinical utility in predicting clinical adverse events prospectively (in real time) in the same manner that such efficacy has been demonstrated in predicting neonatal sepsis, no such evaluation has yet been performed in adults. In addition, the authors acknowledge that larger studies, external validation, and prospective multi-center trials will be needed to make these findings generalizable and useful for clinical decision making.
CONCLUSION
This clinical and mathematical study demonstrates detectable changes in vital signs in the 24 hours preceding an unplanned intubation event in STICU patients. Internally-validated predictive models based on logistic regression have impressive statistical performance, especially when combined with clinical parameters, with ROC area greater than 0.8. Multicenter clinical trials are required to determine whether such monitoring detects changes far enough in advance of clinical suspicion to affect outcomes.
Acknowledgments
Financial support: T32AI078875 - financial support for ADP and LMR Research Fund, Department of Surgery, Division of Acute Care Surgery and Outcomes Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Grogan EL, Morris JA, Jr, Norris PR, France DJ, Ozdas A, Stiles RA, Harris PA, Dawant BM, Speroff T. Reduced heart rate volatility: an early predictor of death in trauma patients. Ann Surg. 2004 Sep;240(3):547–54. doi: 10.1097/01.sla.0000137143.65540.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norris PR, Ozdas A, Cao H, Williams AE, Harrell FE, Jenkins JM, Morris JA., Jr Cardiac uncoupling and heart rate variability stratify ICU patients by mortality: a study of 2088 trauma patients. Ann Surg. 2006 Jun;243(6):804–12. doi: 10.1097/01.sla.0000219642.92637.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris JA, Jr, Norris PR, Ozdas A, Waitman LR, Harrell FE, Jr, Williams AE, Cao H, Jenkins JM. Reduced heart rate variability: an indicator of cardiac uncoupling and diminished physiologic reserve in 1,425 trauma patients. J Trauma. 2006 Jun;60(6):1165–73. doi: 10.1097/01.ta.0000220384.04978.3b. [DOI] [PubMed] [Google Scholar]
- 4.Moorman JR, Delos JB, Flower AA, Cao H, Kovatchev BP, Richman JS, Lake DE. Cardiovascular oscillations at the bedside: early diagnosis of neonatal sepsis using heart rate characteristics monitoring. Physiol Meas. 2011 Nov;32(11):1821–32. doi: 10.1088/0967-3334/32/11/S08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, Bancalari E, Aschner JL, Whit Walker M, Perez JA, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr. 2011 Dec;159(6):900–906. e1. doi: 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres A, Aznar R, Gatell JM, Jiménez P, González J, Ferrer A, Celis R, Rodriguez-Roisin R. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990 Sep;142(3):523–8. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001 Aug;120(2):555–61. doi: 10.1378/chest.120.2.555. [DOI] [PubMed] [Google Scholar]
- 8.Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, Cohen MM, Fraser VJ. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003 May;31(5):1312–7. doi: 10.1097/01.CCM.0000063087.93157.06. [DOI] [PubMed] [Google Scholar]
- 9.Hugonnet S, Eggimann P, Borst F, Maricot P, Chevrolet JC, Pittet D. Impact of ventilator-associated pneumonia on resource utilization and patient outcome. Infect Control Hosp Epidemiol. 2004 Dec;25(12):1090–6. doi: 10.1086/502349. [DOI] [PubMed] [Google Scholar]
- 10.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005 Oct;33(10):2184–93. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 11.Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, Jaeschke RZ, Brun-Buisson C. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998 Sep 15;129(6):433–40. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Apostolopoulou E, Bakakos P, Katostaras T, Gregorakos L. Incidence and risk factors for ventilator-associated pneumonia in 4 multidisciplinary intensive care units in Athens, Greece. Respir Care. 2003 Jul;48(7):681–8. [PubMed] [Google Scholar]
- 13.Joseph NM, Sistla S, Dutta TK, Badhe AS, Parija SC. Ventilator-associated pneumonia: a review. Eur J Intern Med. 2010 Oct;21(5):360–8. doi: 10.1016/j.ejim.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Joseph NM, Sistla S, Dutta TK, Badhe AS, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: incidence and risk factors. J Infect Dev Ctries. 2009 Dec 15;3(10):771–7. doi: 10.3855/jidc.396. [DOI] [PubMed] [Google Scholar]
- 15.Grogan EL, Norris PR, Speroff T, Ozdas A, France DJ, Harris PA, Jenkins JM, Stiles R, Dittus RS, Morris JA., Jr Volatility: a new vital sign identified using a novel bedside monitoring strategy. J Trauma. 2005 Jan;58(1):7–12. doi: 10.1097/01.ta.0000151179.74839.98. [DOI] [PubMed] [Google Scholar]
- 16.Clark MT, Rusin CG, Hudson JL, Lee H, Delos JB, Guin LE, Vergales BD, Paget-Brown AO, Kattwinkel J, Lake DE, et al. Breath-by-breath analysis of cardiorespiratory interaction in premature infants. J Appl Physiol. 2012 Mar;112(5):859–67. doi: 10.1152/japplphysiol.01152.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin MP, O’Shea TM, Bissonette EA, Harrell FE, Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatric Research. 2003;53:920–926. doi: 10.1203/01.PDR.0000064904.05313.D2. [DOI] [PubMed] [Google Scholar]
- 18.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000 Jun;278(6):H2039–49. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 19.Richman JS, Lake DE, Moorman JR. Sample entropy analysis. Methods in Enzymology. 2004;384:172–84. doi: 10.1016/S0076-6879(04)84011-4. [DOI] [PubMed] [Google Scholar]
- 20.Huber PJ. The behavior of maximum likelihood estimation under nonstandard conditions. Proc Fifth Berkeley Symposium Math Stat. 1967;1:221–33. [Google Scholar]
- 21.White H. Maximum likelihood estimation of misspecified models. Econometrica. 1982;50:1–25. [Google Scholar]
- 22.Wei LJ, Lin DY. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J. Am. Stat. Assoc. 1989;84:1065–73. [Google Scholar]
- 23.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001 Aug;54(8):774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 24.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24:1107–16. doi: 10.1097/00003246-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Curr Opin Crit Care. 2002 Aug;8(4):311–5. doi: 10.1097/00075198-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad S, Ramsay T, Huebsch L, Flanagan S, McDiarmid S, Batkin I, McIntyre L, Sundaresan SR, Maziak DE, Shamji FM, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One. 2009 Aug 14;4(8):e6642. doi: 10.1371/journal.pone.0006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancio LC, Batchinsky AI, Salinas J, Kuusela T, Convertino VA, Wade CE, Holcomb JB. Heart-rate complexity for prediction of prehospital lifesaving interventions in trauma patients. J Trauma. 2008 Oct;65(4):813–9. doi: 10.1097/TA.0b013e3181848241. [DOI] [PubMed] [Google Scholar]
- 28.Riordan WP, Jr, Norris PR, Jenkins JM, Morris JA., Jr Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. J Surg Res. 2009 Oct;156(2):283–9. doi: 10.1016/j.jss.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 29.Bravi A, Longtin A, Seely AJ. Review and classification of variability analysis techniques with clinical applications. Biomed Eng Online. 2011;10:90. doi: 10.1186/1475-925X-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss M, Wellman DA, Cotsonis GA. An appraisal of multivariable logistic models in the pulmonary and critical care literature. Chest. 2003 Mar;123(3):923–8. doi: 10.1378/chest.123.3.923. [DOI] [PubMed] [Google Scholar]
- 31.Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993 Feb 1;118(3):201–10. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]
- 32.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996 Dec;49(12):1373–9. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 33.Buchman TG. Novel representation of physiologic states during critical illness and recovery. Crit Care. 2010;14:127. doi: 10.1186/cc8868. [DOI] [PMC free article] [PubMed] [Google Scholar]