Abstract
Few tools are available to determine the structure of large integral membrane proteins such as intracellular Ca2+ release channels, RyRs and IP3Rs. Single particle cryo-EM can readily determine the structure of such channels to intermediate resolution, and can be used to quantitatively assess conformational variability. However, due to the, often low, image contrast of these cryospecimens, methods for validation are critical to insure the accuracy of such structures, and to put limits on their interpretability. The low-resolution structure of RyR has been well established for some time, but high-resolution has been slow to emerge. The structure of IP3R channel by cryo-EM had a number of false-starts, but improved validation methods have recently lead to a demonstrably accurate reconstruction.
Introduction
Cell function depends on the regulated flow of specific ions across the biological membrane in and out the cell as well as between intracellular compartments. Ion channels form a class of integral membrane proteins permitting regulated but passive transfer of ions across membranes. Understanding the structural basis for ion transport is one of the most important frontiers in structural biology. Integral membrane proteins are the most difficult targets for structural analysis due to their tight association with lipid membranes in vivo and their intrinsic dynamic nature. While X-ray crystallography has begun making some strides in this area, ion channels remain one of the most complex and structurally least understood of the cellular proteins. It is estimated that membrane proteins represent 20–30% of the genome [1], and yet of the 82,224 structures currently listed in the PDB, only ~5800 (7%) are reported as membrane proteins, and the PDBTM, a subset of the PDB containing only structures believed to contain transmembrane domains, currently has only 1827 entries (2%). Large sections of ion channels such as the Ca2+ release channels have virtually no sequence homology with any known structures. Moreover, some membrane proteins form unusually large macromolecular assemblies, whose enormous size makes X-ray crystallography or NMR difficult to apply.
At present, single-particle electron cryomicroscopy (cryo-EM) has emerged as one of the most effective and straightforward techniques for the study of large membrane proteins and their interactions. Among these are the intracellular Ca2+ release channels also known as ryanodine and inositol-1,4,5-trisphosphate receptors (RyRs and IP3Rs) that form exceptionally large integral membrane protein complexes of over 2.3 and 1.2 MDa, respectively. These channels mobilize Ca2+ from intracellular stores such as endoplasmic/sarcoplasmic reticulum (ER/SR) into cytoplasm and play important roles in generation of Ca2+ signals regulating a wide array of cellular processes, including muscle contraction and brain function.
To date, the best 3D structures of complete tetrameric RyR and IP3R channels have been solved by single particle cryo-EM at 10–17 Å resolution [2–5], and high-resolution crystal structures of both channel proteins are currently limited to soluble cytoplasmic fragments representing only ~10–15 % of the entire protein [6–13]. While attempts have been made to dock these crystal structures into existing cryo-EM maps [9,12,13], it is important to note that the accuracy of these efforts depends on the resolution of the cryo-EM map, and assumes that the fragment structures retain an identical conformation as in the full assembly [14]. The reliability of docking improves dramatically when the cryo-EM maps have achieved sufficient resolution to unambiguously localize α-helices (~7–8 Å), explaining why further resolution improvement in cryo-EM studies of entire channels is such a critical goal.
Cryo-EM of soluble proteins has recently achieved resolutions approaching those provided by X-ray crystallography (3–5 Å) [15], but the apparent quality of these structures varies. This general perception has prompted the cryo-EM community to more aggressively develop tools for the critical assessment of maps at all resolution ranges [16]. At the high resolution extreme, existing methods developed over decades for x-ray crystallography are being adapted to cryo-EM. However, in the low-resolution regime (worse than 5–7 Å), completely different validation and assessment tools are required. As membrane protein structures reported to date fall almost entirely in this resolution range, in this manuscript we survey some of the methods that have been applied to controversial structures in the field, and discuss the growing consensus that, as cryo-EM is maturing as a field, these validation methods should be required of all new structures [16]. This review briefly summarizes our current knowledge about the 3D structure of Ca2+ release channels and outlines the emerging ideas on structural validation of cryo-EM density maps.
From discovery to structure of Ca2+ Release Channels
The story of Ca2+ release channels begins with discovery that physiological responses, such as striated muscle contraction, are triggered by release of Ca2+ from ER/SR [17,18]. Subsequent studies established that specific proteins tightly bound to SR/ER membranes function as Ca2+-permeable ion channels, which are activated by selective ligands, inositol-1, 4, 5-trisphosphate or ryanodine [19–22]. Cloning of receptor proteins established that both RyR and IP3R are unusually large membrane proteins, comprising four subunits of ~5,000 amino acids for RyR [23] or ~2,700 amino acids for IP3R [24,25]. Furthermore, mammalian genes have been identified encoding three homologous isoforms (types 1–3) of each receptor.
Structure determination of Ca2+ release was not possible until RyR and IP3R proteins had been purified in detergent-solubilized form [26–29]. Structural studies have been focused primarily on type 1 RyR (RyR1) and type 1 IP3R (IP3R1), which are the best functionally characterized isoforms and are abundant in skeletal muscle and cerebellum, respectively. Our current knowledge of the quaternary structure of Ca2+ release channels is based solely on TEM studies [30,31]. Both channels have a common architectural arrangement: the four subunits form a single central ion conduction pathway with an overall characteristic mushroom shape (Figure 1) [2–4]. The cytoplasmic (CY) domains are targets of multiple interactions with large array of intracellular regulatory molecules including proteins that are linked to the channel gating activity. Through low-resolution cryo-EM analysis, some important sites for protein-protein interactions and disease-related “hotspot” amino acids in the protein sequence have been mapped to 3D structure of the tetrameric RyR. Furthermore, conformational changes associated with channel activation have been identified in both the transmmembrane (TM and CY regions of RyR [30,31]. Cryo-EM structural data in combination with recent crystallographic studies of small soluble domains [6–13] has provided important structural insights into our understanding of how Ca2+ release channels work at molecular level. However, the lack of atomic details for the entire channel molecule limits the conclusions that we can currently draw thus leaving significant functional and structural ambiguities. Clearly, the field faces challenges related with interpretation and validation of currently available structural data.
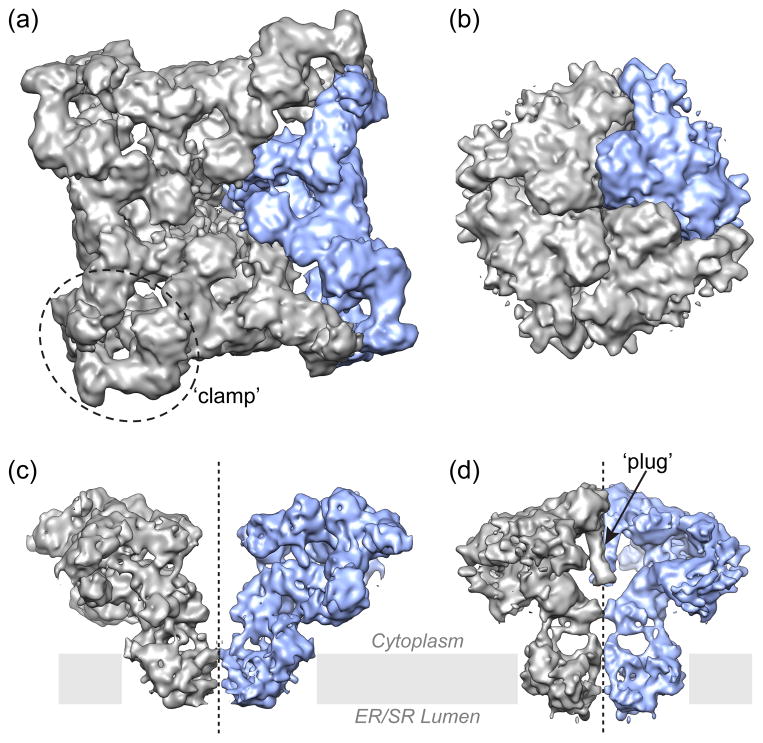
Figure 1. Surface representation of 3D maps of Ca 2+ release channels determined by single-particle cryo-EM.
(a, c) RyR1 (EMD-1275) [2]; (b, d) IP3R1 (EMD-5278) [4]. (a, b) Structures of the tetrameric channels are viewed from the cytoplasmic side with one putative subunit is depicted in blue; (c, d) the two opposing subunits are viewed along membrane plane to allow visualization of internal features in the channel structure. The TM regions of RyR1 and IP3R1 channels are quite similar, however the CY portions, aside from the difference in their overall dimensions, have quite different architectural arrangements including the most notable features such as a central plug in the IP3R1 structure, and distinctive clamp-shaped regions in the RyR1 structure.
Validation of EM structure
The first 3D structure of RyR1 channel was determined from a random conical tilt series of images of negatively stained channel particles [32]. This study provided an initial platform for future EM studies of RyR1 in a frozen-hydrated state [31]. The fact that two EM groups using different approaches (random conical tilt vs. angular reconstitution) achieved essentially the same 3D structure of the channel is a strong indication of accuracy within the resolution limits of these early studies (~35 Å). Subsequent cryo-EM studies at higher resolution continued to elucidate structural features thus expanding our mechanistic understanding of RyR channel (Figure 2a). It is worth noting, that RyR1 was among first non-icosahedral structures solved by single particle cryo-EM [33,34 ]. In contrast to RyR1, early structures of IP3R1 did not agree [30], and it took more than 15 years to produce a reliable 3D reconstruction of this difficult channel [4,5].
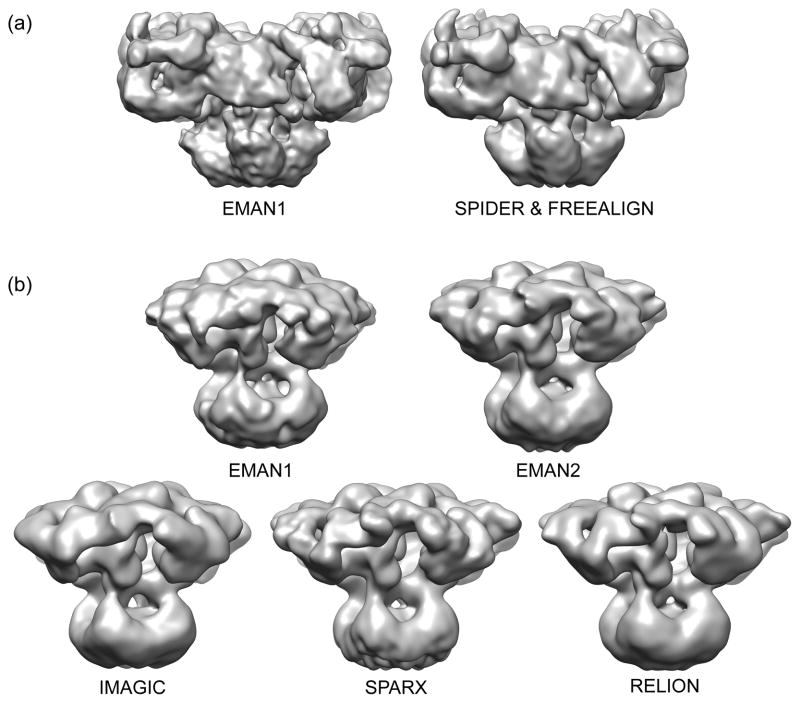
Figure 2. Validation of cryo-EM structures with different software packages.
(a) 3D reconstructions of RyR1 (EMD-1275 [2] and EMD-5014) [3]) were filtered to the same ~12 Å resolution and rendered at a contour levels corresponding to a molecular mass of ~2.4 MDa. (b) 3D reconstructions of IP3R1 were calculated in each software package using the same cryo-EM images [5], the final maps were filtered to match (~20 Å) and rendered at equivalent thresholds.
This raises the more general question of how to assess the overall reliability of a cryo-EM reconstruction. Over a decade ago, a method was proposed for validating structures at low resolution through varying the data collection geometry [35]. Specifically, a small set of single particle data is collected wherein each set of particles is imaged in two different orientations, with a known rotation relating them. These tilt-pairs of particle images are then compared to a putative 3-D reconstruction to insure that the same relative rotation is produced computationally as experimentally (Figure 3a). If the orientations are substantially inconsistent, then the structure is incorrect (Figure 3b–d). Due to the requirement for additional experiments, this technique has been slow to gain acceptance, but in recent years it has emerged as a standard [36,37 ].
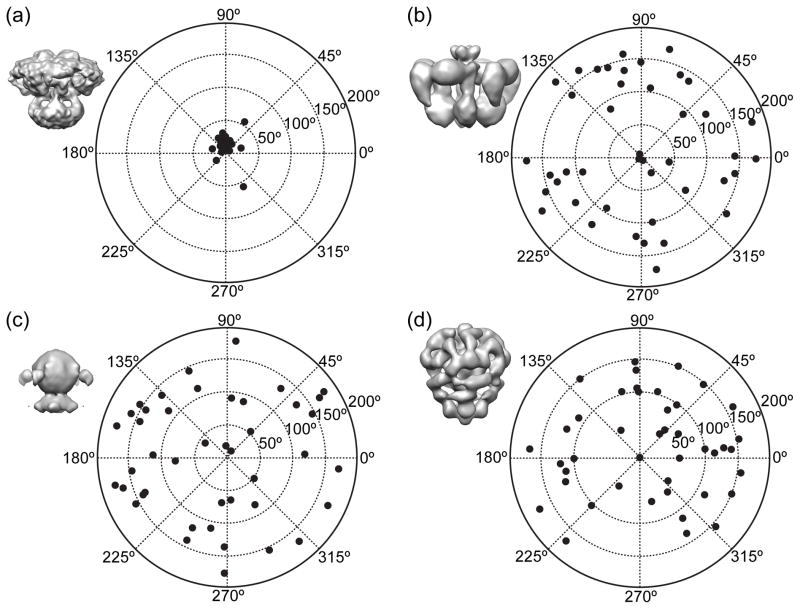
Figure 3. Tilt-pair analysis of images from vitrified IP3R1.
The tilt-pair parameter plots for 42 tilt pairs of particle images, recorded with a tilt angle of 10° in recently published study [5]. Surface representations of 3D maps used to determined orientations of particles from cryo-EM images: (a) EMDB-5278 [4]; (b) from [51]; (c) from [52]; (d) EMDB-1061 [53]. The plot in (a) clearly shows that the particles are clustered at the expected location based on the experimental tilt geometry, thus confirming veracity of cryo-EM map and its self-consistency [4]. The same tilt-pair validation failed with three cryo-EM structures (b–d) published a decade earlier thus demonstrating that these maps are in no way consistent with the current particle images.
Once the accuracy of the quaternary structure has been established, it is important to establish the limiting resolution for reliable interpretation of features in the map. Resolution is generally assessed by splitting the raw particle image data into two halves, then refining the two halves independently, and assessing resolution based on the similarity of the two independent results at each length scale. Historically, however, the definition of “independent” was interpreted different ways by different groups. A more rigorous standard for resolution assessment (the so-called “gold standard” method), which defines what is required to claim such independence, has recently been proposed [38], and reviewers in the cryo-EM community are beginning to enforce this standard in new publications. After participating in designing this standard, we recently showed that the resolution we believed to be ~10 Å [4] for our cryo-EM structure of IP3R1 channel, is actually, closer to 17 Å, due to intrinsic flexibility of the assembly, though it was possible to achieve ~14 Å by classifying the data [5]. When the same test was performed for RyR1, its estimated resolution falls only from ~10 Å to ~12 Å, again, due to flexibility rather than refinement bias.
Very often flexibility is an integral part of the biological function of the molecules themselves, and rigidly locking them into a single conformation would actually distort the true picture of how they function. There are a variety of methods for quantitatively assessing such structural variability [39], including variance maps for localizing regions of variation [40], simultaneous multi-model refinement [41,42], maximum likelihood [43] and biochemical manipulation of the specimen such as binding a specific ligand to the molecule of interest would restrict dynamic behavior of ligand-binding domains.
Furthermore, at low resolution, it can also be useful to refine the same data in multiple software packages (Figure 2). In the case of IP3R, it was shown that 5 different software packages all produced the same basic quaternary structure (Figure 2b), and that the cross-resolution among these structures was comparable to the “gold standard” resolution determined for the original map [5]. This self-consistency is reassuring, and small visible differences observed among the structures can help give guidance when trying to avoid over-interpreting visual features of a map.
Conclusions and perspectives
Understanding molecular machinery of ion channels at the atomic level remains a major challenge. The current cryo-EM structures of Ca2+ release channels have been unambiguously demonstrated to be accurate to intermediate resolution. Debate continues over the architecture of the ion conduction pathway. Structural homology among tetrameric cation ion channels suggests a common architecture for the ion-conduction pathway. The TM region of Ca2+ release channels and that of certain K+ channels [2–4,44] bear a striking similarity, indicating they may share a similar structural motif. While such overall similarity can be observed at the current resolution (12–15 Å), it is probable that the α-helices currently observed in these domains will require adjustment with improving resolution, as happened, for example, with early studies of the nicotinic acetylcholine receptor [45,46]. Ultimately, channel gating requires a structural rearrangement of the ion conduction pathway, but the precise molecular nature of these conformational changes and how ligand-binding communicated to the channel pore will remain largely speculative without a sufficiently high-resolution reconstruction [47]. Furthermore, at the current marginal resolution limit, when no secondary structure elements can be rigorously identified in the majority of the density map, no mechanism exists to unambiguously validate fits of crystal structures of small domains into the large cryo-EM map. These limitations can lead to ambiguities in fitting, such as in recent docking studies of RyR1 [12,48].
It has been demonstrated that single-particle cryo-EM is a powerful method for producing reliable structures of macromolecular assemblies at resolutions as high as 3–5 Å [15], however integral membrane proteins such as ligand-gated Ca2+ release channels have struggled just to reach intermediate resolutions (10–15 Å). Certainly the imaging of these specimens themselves pose a challenge, as the presence of detergent or other solubilizing agents has the side-effect of reducing image contrast, and other experimental issues such as preferred orientation and particle distribution problems abound. However, emerging evidence indicates that the primary reason for this limited resolution is the structural flexibility of the specimen. A feature shared by both of these channels, is a large number of molecular modulators, each of which is clearly going to modify the structure of the channel. Recent studies with, for example, the ribosome, have demonstrated that with a suitably large population of particle images, it is possible to characterize the entire dynamic pathway of a complex molecular assembly [49]. Such methods can readily be applied to ion channels as well, and may prove to be the path forward to moderately better resolutions.
While it has been demonstrated that solubilized ion channels can be reconstituted into lipid vesicles and remain functional, it is still quite possible that the solubilized form of the channels is undergoing more conformational variability. The lipid bilayer may act as a stabilizing agent. Study of reconstituted channels thus offers another potential way forward for this field [50].
With recent advances, such as direct electron detectors and phase plates, more rigorous data analysis strategies, and new specimen preparation methods, cryo-EM technology continues to improve rapidly, offering hope that high-resolution structures of these important ion channels may finally be achieved in the near future.
Highlights.
The complete 3D structures of IP3R/RyR channels have been solved by single-particle cryo-EM at 10–17 Å resolution
Structure validation is critical for low-contrast images of detergent-containing cryospecimens.
Tilt-pair analysis is a powerful tool for validating the low-resolution veracity of structures.
Subclassification of particles can characterize molecular motion, but very large data sets may be required.
The “gold-standard” FSC resolution test is not susceptible to exaggeration due to bias or conformational variability
Acknowledgments
The authors acknowledge support from the National Institutes of Health (R01GM072804, R21AR063255 and R01GM080139) and the America Heart Association (12GRT10510002).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
- 1.Tusnady GE, Dosztanyi Z, Simon I. Transmembrane proteins in the Protein Data Bank: identification and classification. Bioinformatics. 2004;20:2964–2972. doi: 10.1093/bioinformatics/bth340. http://dx.doi.org/10.1093/bioinformatics/bth340. [DOI] [PubMed] [Google Scholar]
- 2.Ludtke SJ, Serysheva II, Hamilton SL, Chiu W. The pore structure of the closed RyR1 channel. Structure (Camb) 2005;13:1203–1211. doi: 10.1016/j.str.2005.06.005. http://dx.doi.org/10.1016/j.str.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samso M, Wagenknecht T, Allen PD. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo-EM. Nat Struct Mol Biol. 2005;12:539–544. doi: 10.1038/nsmb938. http://dx.doi.org/10.1038/nsmb938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Ludtke SJ, Tran TP, Ngo QT, Moiseenkova-Bell VY, Chiu W, Serysheva II. Flexible architecture of IP3R1 by Cryo-EM. Structure. 2011;19:1192–1199. doi: 10.1016/j.str.2011.05.003. http://dx.doi.org/10.1016/j.str.2011.05.003 Reports a 3D cryo-EM structure of a fully functional type 1 IP3R channel from rat recebellum in the closed state. High structural variance has been identified in the cytoplasmic region. This study puts to rest the long-standing debates about the molecular architecture of IP3R1 channel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Murray SC, Flanagan J, Popova OB, Chiu W, Ludtke SJ, Serysheva II. Validation of Cryo-EM Structure of IP3R1 Channel. Structure. 2012 doi: 10.1016/j.str.2013.04.016. in press. 3D structure of tetrameric IP3R1 channel obtained using single-particle cryo-EM (see ref. 4 in the current review) has been validated by use five reconstruction algorithms (EMAN1, EMAN2, Relion, Imagic, SPARX), tilt-pair analysis and class-average/map comparisons. The map resolution and feature resolvability have been re-assessed using the “gold standard” criterion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. http://dx.doi.org/10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 7.Bosanac I, Yamazaki H, Matsu-Ura T, Michikawa T, Mikoshiba K, Ikura M. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol Cell. 2005;17:193–203. doi: 10.1016/j.molcel.2004.11.047. http://dx.doi.org/10.1016/j.molcel.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 8•.Lin CC, Baek K, Lu Z. Apo and InsP-bound crystal structures of the ligand-binding domain of an InsP receptor. Nat Struct Mol Biol. 2011;18:1172–1174. doi: 10.1038/nsmb.2112. The first crystal structures of the ligand-binding region (the first ~600 amino acid residues) of a rat inositol 1,4,5-trisphosphate receptor in its apo- and IP3-bound conformations were reported at 3.8 Å resolutions. Comparison of these two conformations have revealed that binding of IP3 changes the relative orientations of the domains comprising the IP3-binding region. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, et al. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. http://dx.doi.org/10.1038/nature10751 This paper reports crystal structures of a Cys-less form of the N-terminal region (residues 1-604) of rat IP3R1 with and without IP3 bound at 3.6 Å and 3.0 Å, respectively. Two interfaces (α and β) between the IP3-binding core (IBC, residues 224-604) and the supressor domain (SD, residues 1-223) were identified to undergo conformational change upon IP3-binding. Putative location of the N-terminal domains were determined by docking of crystal structures into the cryo-EM density map of the complete terameric IP3R1 determined in [4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amador FJ, Liu S, Ishiyama N, Plevin MJ, Wilson A, MacLennan DH, Ikura M. Crystal structure of type I ryanodine receptor amino-terminal beta-trefoil domain reveals a disease-associated mutation “hot spot” loop. Proc Natl Acad Sci U S A. 2009;106:11040–11044. doi: 10.1073/pnas.0905186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobo PA, Van Petegem F. Crystal structures of the N-terminal domains of cardiac and skeletal muscle ryanodine receptors: insights into disease mutations. Structure. 2009;17:1505–1514. doi: 10.1016/j.str.2009.08.016. http://dx.doi.org/10.1016/j.str.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;468:585–588. doi: 10.1038/nature09471. http://dx.doi.org/10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- 13.Yuchi Z, Lau K, Van Petegem F. Disease mutations in the ryanodine receptor central region: crystal structures of a phosphorylation hot spot domain. Structure. 2012;20:1201–1211. doi: 10.1016/j.str.2012.04.015. http://dx.doi.org/10.1016/j.str.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 14•.Egelman EH. Problems in fitting high resolution structures into electron microscopic reconstructions. HFSP J. 2008;2:324–331. doi: 10.2976/1.2992221. http://dx.doi.org/10.2976/1.2992221 This paper discusses the strengths and limitations of docking high-resolution crystal or NMR structures into low- or intermediate resoutions cryo-EM reconstructions of assemblies in order to build pseudoatomic models for complete assembly quaternary structures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grigorieff N, Harrison SC. Near-atomic resolution reconstructions of icosahedral viruses from electron cryo-microscopy. Curr Opin Struct Biol. 2011;21:265–273. doi: 10.1016/j.sbi.2011.01.008. http://dx.doi.org/10.1016/j.sbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Henderson R, Sali A, Baker ML, Carragher B, Devkota B, Downing KH, Egelman EH, Feng Z, Frank J, Grigorieff N, et al. Outcome of the first electron microscopy validation task force meeting. Structure. 2012;20:205–214. doi: 10.1016/j.str.2011.12.014. http://dx.doi.org/10.1016/j.str.2011.12.014 This paper descibes the outcomes of the inaugural meeting of the Electron Microscopy Validation Task Force organized by the EMDatabank. The workshop participants discussed standards which could be used to validate cryoEM derived maps and models. The first discussions of the new gold standard method for resolution assessment occurred at this meeting, and a variety of other good practices were proposed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. http://dx.doi.org/10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 18.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. http://dx.doi.org/10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988;336:583–586. doi: 10.1038/336583a0. http://dx.doi.org/10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- 20.Iino M. Calcium dependent inositol trisphosphate-induced calcium release in the guinea-pig taenia caeci. Biochem Biophys Res Commun. 1987;142:47–52. doi: 10.1016/0006-291x(87)90449-9. http://dx.doi.org/10.1016/0006-291X(87)90449-9. [DOI] [PubMed] [Google Scholar]
- 21.Smith JS, Coronado R, Meissner G. Single-channel measurements of Calcium Release Channel from Skeletal Muscle Sarcoplasmic Reticulum. J Gen Physiol. 1986;88:573–588. doi: 10.1085/jgp.88.5.573. http://dx.doi.org/10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutko JL, Kenyon JL. Ryanodine modification of cardiac muscle responses to potassium-free solutions. Evidence for inhibition of sarcoplasmic reticulum calcium release. J Gen Physiol. 1983;82:385–404. doi: 10.1085/jgp.82.3.385. http://dx.doi.org/10.1085/jgp.82.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeshima H, Nishi M, Iwabe N, Miyata T, Hosoya T, Masai I, Hotta Y. Isolation and characterization of a gene for a ryanodine receptor/calcium release channel in Drosophila melanogaster. FEBS Lett. 1994;337:81–87. doi: 10.1016/0014-5793(94)80634-9. http://dx.doi.org/10.1016/0014-5793(94)80634-9. [DOI] [PubMed] [Google Scholar]
- 24.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5- trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. http://dx.doi.org/10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 25.Mignery GA, Sudhof TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. http://dx.doi.org/10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- 26.Supattapone S, Worley PF, Baraban JM, Snyder SH. Solubilization, purification and characteriztion of an inositol triphosphate receptor. J Biol Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- 27.Imagawa T, Smith JS, Coronado R, Campbell KP. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987;262:16636–16643. [PubMed] [Google Scholar]
- 28.Inui M, Saito A, Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987;262:1740–1747. [PubMed] [Google Scholar]
- 29.Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987;262:15637–15642. [PubMed] [Google Scholar]
- 30.Serysheva II, Ludtke SJ. 3D Structure of IP(3) Receptor. Curr Top Membr. 2010;66C:171–189. doi: 10.1016/S1063-5823(10)66008-5. http://dx.doi.org/10.1016/S1063-5823(10)66008-5. [DOI] [PubMed] [Google Scholar]
- 31.Wagenknecht TC, Liu Z. Electron microscopy of ryanodine receptors. Curr Top Membr. 2010;66C:27–47. doi: 10.1016/S1063-5823(10)66002-4. http://dx.doi.org/10.1016/S1063-5823(10)66002-4. [DOI] [PubMed] [Google Scholar]
- 32.Wagenknecht T, Grassucci R, Frank J, Saito A, Inui M, Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989;338:167–170. doi: 10.1038/338167a0. http://dx.doi.org/10.1038/338167a0. [DOI] [PubMed] [Google Scholar]
- 33.Radermacher M, Rao V, Grassucci R, Frank J, Timerman AP, Fleischer S, Wagenknecht T. Cryo-electron microscopy and three-dimensional reconstruction of the calcium release channel/ryanodine receptor from skeletal muscle. J Cell Biol. 1994;127:411–423. doi: 10.1083/jcb.127.2.411. http://dx.doi.org/10.1083/jcb.127.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serysheva II, Orlova EV, Chiu W, Sherman MB, Hamilton SL, van Heel M. Electron cryomicroscopy and angular reconstitution used to visualize the skeletal muscle calcium release channel. Nat Struct Biol. 1995;2:18–24. doi: 10.1038/nsb0195-18. http://dx.doi.org/10.1038/nsb0195-18. [DOI] [PubMed] [Google Scholar]
- 35••.Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J Mol Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. http://dx.doi.org/10.1016/j.jmb.2003.07.013 This paper introduced the tilt pair validation procedure for assessing the accuracy of low resolution cryo-EM reconstructions. It also contains some important derivations related to cryo-EM resolution assessment. [DOI] [PubMed] [Google Scholar]
- 36•.Henderson R, Chen S, Chen JZ, Grigorieff N, Passmore LA, Ciccarelli L, Rubinstein JL, Crowther RA, Stewart PL, Rosenthal PB. Tilt-pair analysis of images from a range of different specimens in single-particle electron cryomicroscopy. J Mol Biol. 2011;413:1028–1046. doi: 10.1016/j.jmb.2011.09.008. http://dx.doi.org/10.1016/j.jmb.2011.09.008 Authors analyze tilt-pair images recorded from range of specimens with different symmetries and molecular masses. It has been demonstarted that tilt-pair analysis enables assessment of orientation acuracy, map quality, speicmen motion, and conformational heterogeneity. Tilt-pair plot, where the particles are clustered around the expected tilt axis and tilt angle, provides a strong evidence of correctness of a 3D reconstruction detremined using electron microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson R, McMullan G. Problems in obtaining perfect images by single-particle electron cryomicroscopy of biological structures in amorphous ice. J Electron Microsc (Tokyo) 2013;62:43–50. doi: 10.1093/jmicro/dfs094. http://dx.doi.org/10.1093/jmicro/dfs094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Scheres SH, Chen S. Prevention of overfitting in cryo-EM structure determination. Nat Methods. 2012;9:853–854. doi: 10.1038/nmeth.2115. http://dx.doi.org/10.1038/nmeth.2115 The use of “gold standard FSC” has been demonstrated to provide a realistic estimate of signal and eliminates overfitting of noise using suboptimal FSCs thus providing the “true” resolution estimate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leschziner AE, Nogales E. Visualizing flexibility at molecular resolution: analysis of heterogeneity in single-particle electron microscopy reconstructions. Annu Rev Biophys Biomol Struct. 2007;36:43–62. doi: 10.1146/annurev.biophys.36.040306.132742. http://dx.doi.org/10.1146/annurev.biophys.36.040306.132742. [DOI] [PubMed] [Google Scholar]
- 40•.Penczek PA, Kimmel M, Spahn CM. Identifying conformational states of macromolecules by eigen-analysis of resampled cryo-EM images. Structure. 2011;19:1582–1590. doi: 10.1016/j.str.2011.10.003. http://dx.doi.org/10.1016/j.str.2011.10.003 The paper presents the codimensional component analysis (PCA) for resolving heterogeneity within a complex data set such as cryo-EM 2D images of Thermus thermophilus 70S ribosome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen DH, Song JL, Chuang DT, Chiu W, Ludtke SJ. An expanded conformation of single-ring GroEL-GroES complex encapsulates an 86 kDa substrate. Structure. 2006;14:1711–1722. doi: 10.1016/j.str.2006.09.010. http://dx.doi.org/10.1016/j.str.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Menetret JF, Schaletzky J, Clemons WM, Jr, Osborne AR, Skanland SS, Denison C, Gygi SP, Kirkpatrick DS, Park E, Ludtke SJ, et al. Ribosome binding of a single copy of the SecY complex: implications for protein translocation. Mol Cell. 2007;28:1083–1092. doi: 10.1016/j.molcel.2007.10.034. http://dx.doi.org/10.1016/j.molcel.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 43.Scheres SH. Classification of structural heterogeneity by maximum-likelihood methods. Methods Enzymol. 2010;482:295–320. doi: 10.1016/S0076-6879(10)82012-9. http://dx.doi.org/10.1016/S0076-6879(10)82012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samso M, Feng W, Pessah IN, Allen PD. Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol. 2009;7:e85. doi: 10.1371/journal.pbio.1000085. http://dx.doi.org/10.1371/journal.pbio.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unwin N. Nicotinic acetylcholine receptor at 9 Å resolution. J Mol Biol. 1993;229:1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- 46.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. http://dx.doi.org/10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- 47.Baker ML, Zhang J, Ludtke SJ, Chiu W. Cryo-EM of macromolecular assemblies at near-atomic resolution. Nat Protoc. 2010;5:1697–1708. doi: 10.1038/nprot.2010.126. http://dx.doi.org/10.1038/nprot.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serysheva II, Ludtke SJ, Baker ML, Cong Y, Topf M, Eramian D, Sali A, Hamilton SL, Chiu W. Subnanometer-resolution electron cryomicroscopy-based domain models for the cytoplasmic region of skeletal muscle RyR channel. Proc Natl Acad Sci U S A. 2008;105:9610–9615. doi: 10.1073/pnas.0803189105. http://dx.doi.org/10.1073/pnas.0803189105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466:329–333. doi: 10.1038/nature09206. http://dx.doi.org/10.1038/nature09206 While a number of groups have performed structural variability analysis using cryo-EM, this study stands out in the large number of different substructures it produced. 50 different 3-D structures were obtained, documenting the process of translocation in ribosome. [DOI] [PubMed] [Google Scholar]
- 50•.Wang L, Sigworth FJ. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 2009;461:292–295. doi: 10.1038/nature08291. http://dx.doi.org/10.1038/nature08291 This paper describes the first single-particle cryo-EM study of the voltage-activated K+ channel (BK) in a lipid environment. BK channels were reconstituted at low density into lipid vesicles, and their 3D structure has been determined using a new random spherically constrained (RSC) single-particle reconstruction technique. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serysheva II, Bare DJ, Ludtke SJ, Kettlun CS, Chiu W, Mignery GA. Structure of the type 1 inositol 1,4,5-trisphosphate receptor revealed by electron cryomicroscopy. J Biol Chem. 2003;278:21319–21322. doi: 10.1074/jbc.C300148200. http://dx.doi.org/10.1074/jbc.C300148200. [DOI] [PubMed] [Google Scholar]
- 52.Jiang QX, Thrower EC, Chester DW, Ehrlich BE, Sigworth FJ. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 A resolution. Embo J. 2002;21:3575–3581. doi: 10.1093/emboj/cdf380. http://dx.doi.org/10.1093/emboj/cdf380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato C, Hamada K, Ogura T, Miyazawa A, Iwasaki K, Hiroaki Y, Tani K, Terauchi A, Fujiyoshi Y, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor contains multiple cavities and L-shaped ligand-binding domains. J Mol Biol. 2004;336:155–164. doi: 10.1016/j.jmb.2003.11.024. http://dx.doi.org/10.1016/j.jmb.2003.11.024. [DOI] [PubMed] [Google Scholar]