Abstract
Objective
To determine the association of increased kyphosis with declines in mobility, balance and disability among community-living older adults.
Design
18-month follow-up visit data from 2006–2009 for 620 participants from the population-based MOBILIZE Boston Study of older adults was used. Cross-sectional multivariable regression analyses were performed to assess the relationship between kyphosis (measured using the kyphosis index (KI)) and measures of mobility performance (Short Physical Performance Battery (SPPB)), balance (Berg Balance Score (BBS)) and disability (self-reported difficulty walking a quarter-mile or climbing a flight of stairs). We then evaluated men and women separately. Adjustment variables included demographic factors (age, gender, race, education), body-mass index, self-rated health, comorbidities (heart disease, diabetes, stroke, depressive symptoms), back pain, knee pain and falls self-efficacy.
Results
After full adjustment, greater KI was associated with lower SPPB scores (adj. β = −0.08, p = 0.01), but not BBS (adj. β = −0.09, p = 0.23) or self-reported disability (adj. β = 1.00, 95% CI, 0.93 –1.06). In gender-specific analyses, KI was only associated with SPPB in women.
Conclusions
Greater kyphosis is associated with poorer mobility performance, but not balance or self-reported disability. This association with SPPB was only observed among women. Mechanisms by which increased kyphosis influence physical performance should be explored prospectively.
Keywords: Kyphosis, Mobility, Balance, Disability
Increased kyphosis is a common condition among older adults that may, directly or indirectly, be a risk factor for poor mobility and disability. Epidemiologic studies have demonstrated that age-related hyperkyphosis, which some authors define as having a kyphosis angle greater than 40 degrees,1,2 affects 20–40% of older adults.3 This condition has been associated with other medical problems, such as falls,4 osteoarthritis, osteoporosis, impaired pulmonary function, and mortality3. In addition, kyphosis may affect physical function and disability through both its effect on standing posture and the aforementioned associated medical problems.
Previous studies regarding the effect of kyphosis on physical function have not been consistent. Some studies have found greater kyphosis to be associated with worse physical function, balance or mortality5–9. However, two studies reported no relationship between increased kyphosis and balance10,11. Of the few studies that have evaluated kyphosis and mobility, most are limited by small sample size and the exclusion of men6,8–10,12–16. Few have focused primarily on the geriatric population; one demonstrated that the association between kyphosis and physical function was stronger in women than men7 and the other showed that seniors with greater degrees of kyphosis were more likely to have poorer physical function5. The differing results observed among studies of increased kyphosis may be due to multiple reasons, including the method used to measure kyphosis or the specific outcomes used. Kado and colleagues concluded that there is a need to better understand these relationships within a population-based sample of older adults3.
To address this concern we conducted an analysis of kyphosis using the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly (MOBILIZE) Boston Study data, a population-based study of community-living older adults. We hypothesized that increased kyphosis would be associated with declines in balance performance, and both observed and self-reported mobility.
METHODS
Participants
We used data from the 18-month follow-up visit of the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly (MOBILIZE) Boston Study cohort. Study participants included women and men aged 70 years and older living within 5 miles of the study clinic at Hebrew SeniorLife in Boston; spouses aged 64 to 69 of participants were also enrolled. People were considered eligible if they were able to walk 20 feet without the personal assistance of another person, able to communicate in English, and expected to stay in the Boston area for 2 years. Participants were excluded if they had a terminal disease, severe visual deficits (i.e., unable to read large print), severe hearing deficits (i.e., unable to converse over a telephone), or moderate to severe cognitive impairment (defined as a Mini-Mental State Examination score < 18). Details of the study methods have been published previously17. All protocols for the study and consent procedures were approved by the Institutional Review Boards of Hebrew SeniorLife (HSL) and Spaulding Rehabilitation Hospital.
Extensive health assessments were conducted at the baseline and 18-month follow-up visits. Kyphosis measurements were obtained only at the 18-month follow-up, at which 620 of the initial 765 participants had assessments including kyphosis measurements. Before the 18-month visit, 36 (4.7%) participants died and 98 (12.8%) withdrew from the study for health and other reasons. There were 11 (1.4%) people missing kyphosis index data, and thus 620 (81.0%) participants were included in our analysis.
Kyphosis
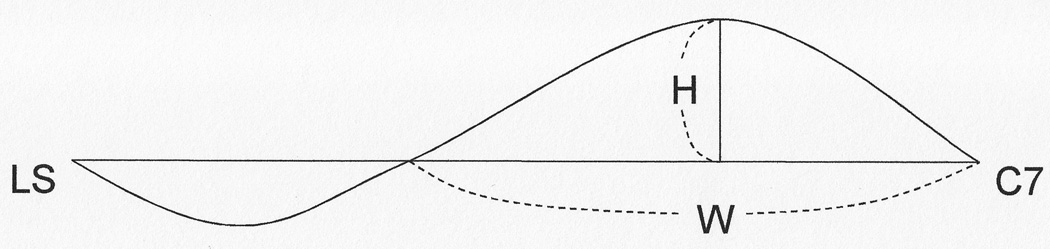
Thoracic kyphosis was quantified through determination of the kyphosis index using a flexicurve ruler. This very reliable measurement,18,19, was obtained by molding a flexicurve ruler along a standing participant’s spine after placing one end of the ruler at the level of C7 spinous process. The location on the ruler that was level with the lumbosacral joint was marked, and the curve was traced onto graph paper. A straight line was then drawn from the level of C7 to the lumbosacral joint20. The index of kyphosis was represented by the height of the thoracic curve divided by the spinal length, times 100, with higher KI indicating greater kyphosis (See Figure 1.)8. In several different studies, the reliability of KI measurement was found to range from 0.86–0.99 in populations of post-menopausal women18,19 and older men and women.21 This mode of kyphosis measurement has been validated radiographically.21
Figure 1.
The curvature of the spine is measured by placing a flexicurve ruler along the participant’s spine and measuring the curve from C7 to the lumbosacral junction (represented here by LS). A horizontal line is then drawn from C7 to LS, and the height and width of the thoracic portion of the curve is measured. The kyphosis index (KI) is calculated by dividing the thoracic height by the thoracic width and multiplying the result by 100 (KI = (H/W)*100).
Mobility and Balance Measures
Lower extremity mobility performance was measured using the Short Physical Performance Battery (SPPB), consisting of a 4-meter usual-paced walk, standing balance, and time to stand up and down from a chair 5 times (“chair stand time”)22,23. The SPPB has been well validated, and varies from 0–12 with lower scores associated with an increased risk of disability, nursing home admission and mortality22,23. A difference of at least 0.5 on the SPPB score is considered clinically meaningful24,25. The Berg Balance Scale is a multi-component test of standing balance, consisting of 14 balance tasks (each scored from 0–4) with a score range of 0–5626. This scale has been validated in community-living older adults and lower scores predict greater risk of falls27. A clinically meaningful difference is 4 units on the BBS28. Disability (1 = yes, 0 = no) was defined as any self-reported difficulty walking a quarter of a mile or climbing one flight of stairs or 10 steps. Self-reported mobility disability is a well-validated measure associated with functional mobility performance.29–31
Demographic and Health Characteristics
Demographic characteristics included age, gender, race and years of education. General self-rated health was measured on a 1 to 5 scale of excellent, very good, good, fair, or poor, respectively.32 Medical conditions were determined both through self-report (e.g., for strokes, heart disease, diabetes), a diabetes algorithm33, measurement (e.g., for body mass index (BMI)) and previously validated scales (such as the Eaton method using the Center for Epidemiologic Studies Depression scale)33. Pain was measured using the Brief Pain Inventory34 and the McGill Pain Map35. Falls self-efficacy was measured using the Tinetti Falls Efficacy Scale (FES), a 10-item instrument assessing degree of self-confidence in performing daily activities on a scale of 0 to 100; a cut-off of 90 was used to separate likely fallers from non-fallers36.
Statistical Methods
We first looked at descriptive statistics, scatter plots and the distribution of KI and the outcomes. We performed three iterative regression models to evaluate the relation between KI and both the SPPB and BBS. The initial model adjusted for demographic factors (age, gender, race, and education). Subsequently, we added adjustment for BMI, self-rated health and important comorbidities (heart disease, diabetes, stroke, depressive symptoms). Lastly, in our fully adjusted model, we also adjusted for back pain, knee pain and falls self-efficacy.
We first modeled KI as a continuous variable and performed linear regressions to evaluate the relationship between KI and both SPPB and BBS. We evaluated the relationship of KI to mobility-related disability using a logistic regression model. Next we modeled KI as a categorical variable, dividing the population into quintiles by KI and evaluated the trend across the five levels for the SPPB and BBS. If we observed a clinically significant trend, we then evaluated KI using multiple dummy variables with Quintile 1 (including participants with the lowest KI’s) as the referent category to find the quintile at which a significant difference in the outcome occurred.
Then we did a series of post hoc analyses. We first explored differences by gender status, checking the interaction terms between KI and gender for our outcomes, modeling KI as a continuous variable. Where a statistically significant interaction term was found, we looked at gender stratified models and assessed differences between quintiles and our referent Quintile 1 using dummy variables. In these gender-specific analyses, participants were stratified into the same KI categories as were used in the analyses of all participants. In addition, we examined the relationship between KI and gender for the three SPPB components (balance, gait speed, and chair time) among all participants and in gender-stratified models. Data were analyzed using SAS Version 9.1 (Cary, NC).
RESULTS
Of the 620 participants (226 men; 394 women), the mean age was 79.2 years (standard deviation 5.4), 22% were non-white, and 68% were college graduates. When compared to the 620 participants in the 18-month follow-up, the 145 MOBILIZE Boston Study enrollees who did not participate in follow-up were similar according to age (mean 79.4±5.6 at baseline), gender (65.5% female) and race (25.5% non-white). Fewer non-participants graduated from college (37.2%) and more had fair or poor self-rated health (fair or poor health: 25.5% of non-participants versus 12.4% of participants) at baseline.
The KI scores ranged from 4 to 26 (mean 10.7 ± 3.1). We observed differences according to KI quintile; participants who were older (p < 0.001), white (p < 0.01), had worse self-reported health (p = 0.04), or history of stroke (p = 0.04) tended to have higher KI. [Table 1.] The mean SPPB score over our entire population was 9.2 ± 2.5 (range: 1–12). The unadjusted mean values for SPPB across Quintiles 1–5 was: Q1 – 9.5, Q2 – 9.5, Q3 – 9.4, Q4 – 9.1, Q5 – 8.4. The mean BBS score over our entire population was 49.5 ± 6.6 (range: 12–56). The unadjusted mean values for BBS across Quintiles 1–5 was: Q1 – 50.0, Q2 – 50.1, Q3 – 50.4, Q4 – 49.2, Q5 – 47.8. The percent of participants in each quintile that reported difficulty walking a quarter mile or climbing stairs for the overall population was 38.1% with the following values across Quintiles 1–5: Q1 – 40.2%, Q2 – 29.9%, Q3 – 36.1%, Q4 – 34.4%, Q5 – 49.6%. The highest KI scores for Quintiles 1–4 were: Q1 – 8.187; Q2 – 9.783; Q3 – 11.278; Q4 – 13.105.
Table 1.
Participant Characteristics According to Kyphosis Indice
| Kyphosis Index | |||||||
|---|---|---|---|---|---|---|---|
| Distribution by Kyphosis Index Quintile | |||||||
| Adjustment Variables | All [N(%)] |
Quintile 1 [N(%)] |
Quintile 2 [N(%)] |
Quintile 3 [N(%)] |
Quintile 4 [N(%)] |
Quintile 5 [N(%)] |
Trend p-value |
| Age (years) | < 0.001 | ||||||
| 65–69 | 5(1) | 0(0) | 4(3) | 0(0) | 0(0) | 1(1) | |
| 70–74 | 129(21) | 27(22) | 36(29) | 29(23) | 27(22) | 10(8) | |
| 75–79 | 224(36) | 46(37) | 44(35) | 47(38) | 47(38) | 40(32) | |
| 80–84 | 155(25) | 31(25) | 24(19) | 27(22) | 32(26) | 41(33) | |
| 85+ | 107(17) | 20(16) | 16(13) | 21(17) | 18(15) | 32(26) | |
| Female | 394(64) | 81(65) | 73(59) | 86(69) | 74(60) | 80(65) | 0.953 |
| Race | 0.002 | ||||||
| White | 485(78) | 88(71) | 89(72) | 94(76) | 105(85) | 109(88) | |
| Black | 94(15) | 28(23) | 26(21) | 19(15) | 12(10) | 9(7) | |
| Other | 40(6) | 8(6) | 8(7) | 11(9) | 7(6) | 6(5) | |
| Education (years) | 0.118 | ||||||
| <12 | 60(10) | 17(14) | 6(5) | 19(15) | 11(9) | 7(6) | |
| 12–15 | 140(23) | 31(25) | 30(24) | 24(19) | 24(19) | 31(25) | |
| >15 | 419(68) | 76(61) | 87(71) | 81(65) | 89(72) | 86(69) | |
| BMI (kg/m2) | 0.087 | ||||||
| <25 | 173(28) | 38(31) | 42(34) | 32(26) | 27(22) | 34(28) | |
| 25–29 | 252(41) | 50(40) | 50(41) | 52(42) | 53(43) | 47(39) | |
| ≥30 | 190(31) | 36(29) | 30(25) | 40(32) | 43(35) | 41(34) | |
| Self-rated health: Fair, Poor* | 73(12) | 16(13) | 6(5) | 20(16) | 18(15) | 13(11) | 0.038 |
| Heart disease | 283(46) | 54(44) | 62(50) | 50(40) | 59(48) | 58(47) | 0.776 |
| Diabetes | 107(17) | 24(19) | 21(17) | 21(17) | 26(21) | 15(12) | 0.329 |
| Stroke | 60(10) | 7(6) | 15(12) | 8(6) | 9(7) | 21(17) | 0.035 |
| Depressive symptoms | 54(9) | 10(8) | 9(8) | 11(9) | 12(10) | 12(10) | 0.499 |
| Knee pain | 164(27) | 32(26) | 24(21) | 37(30) | 37(30) | 34(28) | 0.322 |
| Back pain | 166(27) | 45(36) | 23(19) | 30(25) | 37(30) | 31(26) | 0.369 |
| Tinetti FES < 90** | 80(13) | 16(13) | 14(12) | 16(13) | 14(11) | 20(16) | 0.527 |
Quintile 1 refers to the participants whose KI fell in the lowest 20% of our population, and Quintile 5 refers to the participants with the highest KI's. The highest KI scores for Quintiles 1–4 were: Q1 – 8.187; Q2 – 9.783; Q3 – 11.278; Q4 – 13.105.
Percentages were based on non-missing data. No single variable had more than 2% data missing in the overall population.
Percentages within KI quintiles were based on totals within KI quintiles for each variable.
Self-rated health was rated on a scale of 1–5 with Excellent = 1 and Poor = 5.
The Tinetti Falls Efficacy Scale (FES) is graded on a scale of 0–100; fallers are more likely to score less than 90.
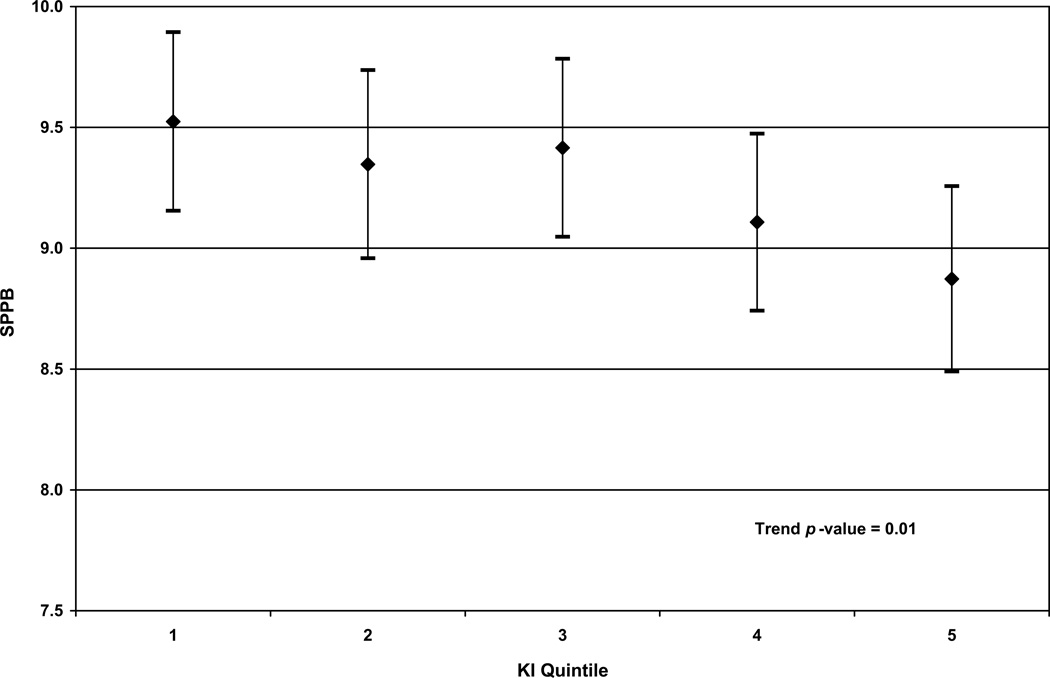
KI was significantly associated with worse performance on the SPPB. When we modeled KI as a continuous variable, the association remained significant (fully adjusted β = −0.08, p < 0.01) even after adjusting for demographic factors, health conditions, pain and falls self-efficacy. Similarly, we found that the test for trend across KI quintiles was significant (p = 0.01) in the fully adjusted model. [See Figure 2a.] When we modeled KI as a categorical variable, we observed a statistically significant difference between Quintiles 1 and 5 (p < 0.01).
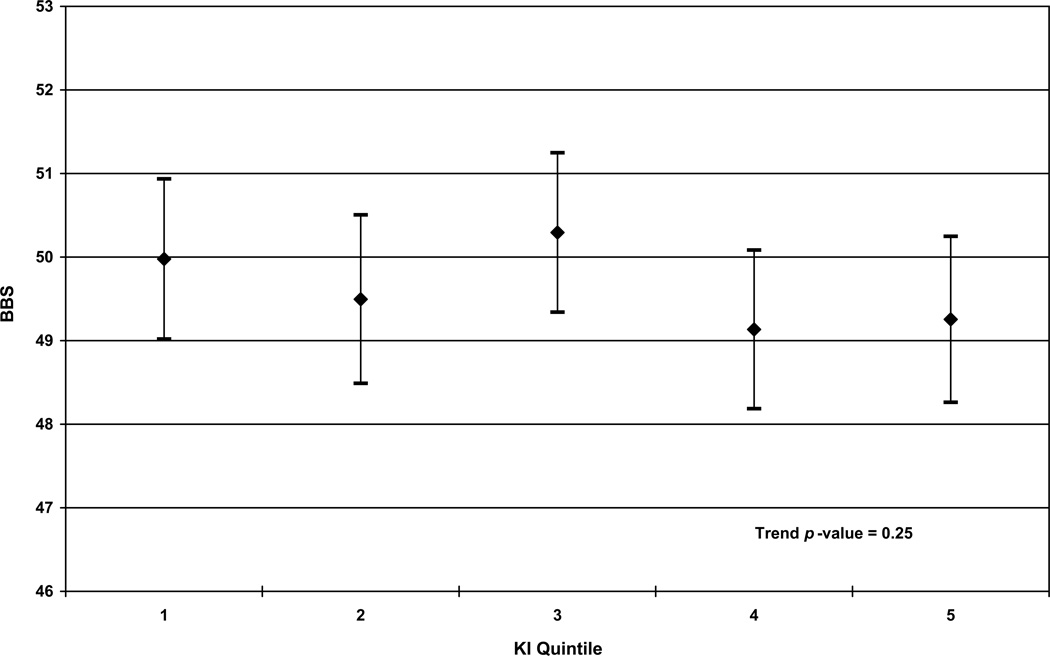
Figure 2.
In Figures (a) and (b), fully adjusted least-squares means and 95% confidence intervals of (a) Short Physical Performance Battery (SPPB) scores and (b) Berg Balance Scale (BBS) scores by kyphosis index (KI) quintiles in multivariable linear regression models are shown. In Figure (c), the fully adjusted odds ratios of multivariable logistic regression model predicting self-reported disability for all participants is shown; Quintile 1 is used as the referent in (c). Full adjustment includes adjustment for demographic factors (age, gender, race, and education), BMI, self-rated health, comorbidities (heart disease, diabetes, stroke, depressive symptoms), back pain, knee pain and falls self-efficacy. In these models, the entire study population was used.
KI was not significantly associated with BBS. This was observed when we modeled KI as a continuous variable, (fully adjusted β = −0.09, p = 0.23) and across quintiles of KI (p = 0.25). [See Figure 2b] When we modeled KI as a categorical variable we did not observe a significant difference between Quintile 1 and any other quintile (p ranging from 0.06–0.80).
In our models of self-reported mobility disability, we found no association between KI and our outcome. KI was not significant when modeled as a continuous variable (fully adjusted model: 95% CI = (0.93–1.06)) or as a five-level variable (fully adjusted model: trend p-value = 0.99). [See Figure 2c.]
In our post-hoc analyses of KI and gender, we found a statistically significant interaction (fully adjusted β = 0.04, p = 0.01) only in our models of SPPB. In our gender stratified analyses, KI was only associated with SPPB; specifically, higher KI was associated with worse performance on the SPPB (fully adjusted β = −0.09, p = 0.01, for continuous KI) among women. Again we found that, among women, the trend across KI quintiles was significant (p = 0.01) and that there was a significant difference between Quintile 5 and Quintile 1 (p = 0.02) even after full adjustment. We did not observe significant associations between KI and SPPB in men when KI was modeled continuously (p = 0.28) or as a five-level variable (p = 0.49).
In our post-hoc analysis of KI and the SPPB components, we found that higher KI was significantly associated with slower gait speed and longer chair time. This relationship was demonstrated when KI was modeled continuously for both gait speed (fully adjusted β = −0.01, p = 0.01) and chair time (fully adjusted β = 0.32, p = 0.001). The trend across KI quintiles was also significant for gait speed (fully adjusted β = −0.02, p = 0.01) and chair time (fully adjusted β = 0.61, p < 0.01). However, higher KI was not associated with the balance component of the SPPB when modeled continuously (p = 0.90) or as a five-level variable (p = 0.97). The interaction term for KI and gender was not significant for gait speed (p = 0.41) or chair time (p = 0.80). However, as in our SPPB models, gender-stratified models revealed statistically significant associations between KI and chair time and gait speed only among women. When KI was modeled as a continuous variable, a significant association was noted with gait speed in women (fully adjusted β = −0.01, p < 0.001) but not in men (fully adjusted β = −0.01, p = 0.31). Similarly when KI was modeled continuously, a significant association was noted with chair time in women (fully adjusted β = 0.36, p < 0.01) but not in men (fully adjusted β = 0.28, p = 0.09). [Results describing the association between KI and these SPPB components when KI is modeled categorically are shown in Table 2 and Table 3.] Among women, both Quintile 4 and Quintile 5 were found to be significantly different from Quintile 1 for both gait speed (p ranging from 0.01–0.02) and chair time (p = 0.01) after full adjustment.
Table 2.
Multivariable Linear Regression Models Demonstrating the Association Between Kyphosis Index Quintiles and Gait Speed in the MOBILIZE Boston Study 2006–2009
| All participants (N = 595) | Fully Adjusted Model | ||
|---|---|---|---|
| Kyphosis Index Quintile | Beta | SE | p |
| Quintile 2 | −0.01 | 0.03 | 0.85 |
| Quintile 3 | −0.01 | 0.03 | 0.61 |
| Quintile 4 | −0.04 | 0.03 | 0.17 |
| Quintile 5 | −0.06 | 0.03 | 0.02 |
| Trend p-value | 0.01 |
||
| Women (N = 375) | |||
| Kyphosis Index Quintile | |||
| Quintile 2 | −0.04 | 0.03 | 0.22 |
| Quintile 3 | > −0.01 | 0.03 | 0.88 |
| Quintile 4 | −0.08 | 0.03 | 0.02 |
| Quintile 5 | −0.09 | 0.03 | 0.01 |
| Trend p-value | < 0.01 | ||
| Men (N = 220) | |||
| Kyphosis Index Quintile | |||
| Quintile 2 | 0.06 | 0.05 | 0.22 |
| Quintile 3 | −0.05 | 0.05 | 0.34 |
| Quintile 4 | 0.01 | 0.05 | 0.87 |
| Quintile 5 | −0.02 | 0.05 | 0.62 |
| Trend p-value | 0.33 | ||
Notes: In these models, KI was modeled as a categorical variable. Quintile 1 refers to the participants whose KI fell in the lowest 20% of our population. Fully adjusted models include adjustment for age, gender, race, education, BMI, self-rated health, heart disease, diabetes, stroke, depressive symptoms, back pain, knee pain and falls efficacy.
Table 3.
Multivariable Linear Regression Models Showing The Association Between Kyphosis Index Quintiles and Chair Time in the MOBILIZE Boston Study 2006–2009
| All participants (N = 596) | Fully Adjusted Model | ||
|---|---|---|---|
| Beta | SE | p | |
| Kyphosis Index Quintile | |||
| Quintile 2 | 1.68 | 0.95 | 0.08 |
| Quintile 3 | 0.84 | 0.92 | 0.36 |
| Quintile 4 | 1.95 | 0.92 | 0.03 |
| Quintile 5 | 2.86 | 0.95 | < 0.01 |
| Trend p-value | < 0.01 | ||
| Women (N = 376) | |||
| Kyphosis Index Quintile | |||
| Quintile 2 | 1.67 | 1.25 | 0.18 |
| Quintile 3 | 1.26 | 1.16 | 0.28 |
| Quintile 4 | 3.05 | 1.22 | 0.01 |
| Quintile 5 | 3.30 | 1.23 | 0.01 |
| Trend p-value | < 0.01 | ||
| Men (N = 220) | |||
| Kyphosis Index Quintile | |||
| Quintile 2 | 1.32 | 1.52 | 0.39 |
| Quintile 3 | 0.10 | 1.57 | 0.95 |
| Quintile 4 | 0.49 | 1.50 | 0.75 |
| Quintile 5 | 2.26 | 1.56 | 0.15 |
| Trend p-value | 0.32 | ||
Notes: In these models, KI was modeled as a categorical variable. Quintile 1 refers to the participants whose KI fell in the lowest 20% of our population. Fully adjusted models include adjustment for age, gender, race, education, BMI, self-rated health, heart disease, diabetes, stroke, depressive symptoms, back pain, knee pain and falls efficacy.
DISCUSSION
The major findings of our study were that: 1) greater kyphosis was associated with worse physical performance among community dwelling older adults, 2) that the influence varied by gender, 3) that the influence of greater KI on physical performance was only observed among women and that 3) no relationship was observed between greater KI and the outcomes of balance or self reported disability.
Consistent with our hypothesis, we found that older adults who had greater kyphosis performed worse on the SPPB, a measure of lower extremity mobility. There was a clinically meaningful difference of 0.65 in the fully adjusted least squares mean SPPB score between Quintile 1 (adjusted mean: 9.52) and Quintile 5 (adjusted mean: 8.87). Specifically, increased kyphosis was associated with slower gait speed and increased time needed to stand up from a chair repeatedly. Participants in Quintile 1 had KI ≤ 8.187, Participants in Quintile 5 had KI > 13.105. Of note, clinically relevant cut points for KI have not been previously established in the literature. Our study suggests that 13.1 (the cut-point before Quintile 5) may be a clinically relevant cut-point.
These findings are consistent with previous studies. Antonelli-Incalzi and colleagues found a stronger relationship between physical function and kyphosis in women as compared to men in a population-based study of home-dwelling people ages 65 and older7. In contrast, Kado and colleagues also found that greater kyphosis was associated with difficulty with chair stand time, but observed no evidence that gender modified the association between kyphotic posture and physical function in a population-based study of people ages 55 and older5. Kado and colleagues used 1.7 cm blocks to measure kyphosis, a cruder measure than KI. Interestingly, Kado’s study also found a significant association between kyphosis and self-reported disability5. In contrast to our study, their self-reported disability measure included difficulty with bending in addition to difficulty with walking or stair climbing. Clinical experience suggests that difficulty with bending reflects an activity that may be more vulnerable to increased kyphosis. This may explain the observed differences regarding disability between Kado’s findings and our own. This also highlights the point that a measure representing a broader range of disability might be a better measure to evaluate the influence of increased kyphosis among older adults. The association between increased kyphosis and SPPB may be related to unmeasured attributes, including force production of back extensor muscles as well as the presence of spinal compression fractures. Increased kyphosis has been linked to back extensor weakness38–40. Also, Suri and colleagues demonstrated that SPPB scores are linked to trunk extensor muscle endurance41. Thus, decreased trunk extension endurance may contribute to both increased kyphosis and poorer performance on the SPPB. Greater kyphosis has also been associated with decreased bone mineral density in the spine42, and osteoporotic vertebral compression fractures have been associated with impaired functional status among women43. Thus vertebral compression fractures, which are in themselves associated with back extension strength, may also contribute to the association of greater kyphosis and poorer performance on the SPPB. The MOBILIZE Boston Study did not measure back strength, back endurance, bone density or radiographic evidence of spinal compression fractures, which would have enabled us to confirm these possible relationships. However, in recent study evaluating kyphosis, bone status and mobility, Katzman and colleagues have found that increasing kyphosis is associated with worse mobility among older women independent of spinal osteoporosis.44,45
Our gender-specific analyses showed that the relationship between kyphosis and physical performance was only evident among women. Sinaki and colleagues demonstrated that women had less muscle strength than men regardless of age with women’s back extensor strength ranging from 54% to 76% compared to men’s back extensor strength across decades of life46. Among women, it has been shown that osteoporotic women have significantly lower back extensor strength than healthy women40, which is important since both osteoporosis and back extensor weakness are associated with greater kyphosis. Osteoporosis and spinal compression fractures are more common among older women than older men and thus may account for our observed gender differences. It could be hypothesized that our findings may in part be due the smaller percentage of older men in the study; however among the results that were statistically significant for women the corresponding effect estimates were always smaller for men.
It is interesting to note that kyphosis was not associated with balance as measured by the BBS or within the balance component of the SPPB. Other studies have reported conflicting findings. Antonelli- Incalzi and colleagues found that increased kyphosis was associated with impaired balance in women, where balance was measured using a testing procedure that is similar to the balance component of the SPPB7. In a study involving 22 osteoporotic women, Greig and colleagues found vertebral fracture to be related to impaired balance characteristics, rather than thoracic kyphosis10. This might suggest that a history of fracture is a factor that is influencing balance rather than kyphosis and could explain the positive association observed in other investigations. While we acknowledge that balance measures varied methodologically between our study and some of these other studies observing positive findings, both the BBS and the SPPB balance component are considered to be clinically relevant balance outcomes.
Also greater kyphosis was not associated with self-reported disability. It is well established that SPPB performance is predictive of disability, especially using the definition of disability utilized within this study. Our cross-sectional findings suggest that while kyphosis may interfere with physical function it does not do so to a degree that would lead to individuals reporting mobility difficulty.
Of note, the ideal kyphosis index has not yet been agreed upon. There is a natural kyphotic curve, and its distribution has rarely been studied. In the community-based population studied by Milne and Lauder, the average KI among young adults (ages 20 to 49) was approximately 8 for men and 6–8 for women, and KI increased after age 60 in men and age 50 in women37. In another recent study by Kado and colleagues, KI among 610 community-dwelling white women, ages 67–93 years, was normally distributed with a mean value of 12.3 and a standard deviation of 3.447. Among women in the MOBILIZE-Boston cohort, KI was also normally distributed with a mean value of 10.7 and a standard deviation of 3.3. Since we are hypothesizing that a high degree of KI has an adverse effect on mobility and function, we analyzed KI both continuously and categorically and chose the participants with the 20% lowest KI’s as our referent group for the categorical analysis.
Our study does have limitations. In addition to those previously mentioned, the analyses are cross-sectional and therefore cannot determine causation. A prospective analysis would be needed to clarify relationships between KI and outcomes, such as falls or nursing home admissions. Additionally, we were not able to evaluate changes in kyphosis over time, which might have a more deleterious impact on our outcomes. Furthermore, we looked at thoracic curvature in only one plane; there may be other aspects of spinal curvature in other planes or other spinal segments that may affect our outcomes that we did not measure.
Strengths of our study include its large sample size, the inclusion of both men and women, the use of a community-based study population, and the use of a clinically simple kyphosis measure that shows important variations in kyphosis.
CONCLUSION
Even after adjustment, increased kyphosis is significantly associated with measures of physical function, particularly gait speed, chair-time and SPPB score. This association holds true in women, but not men. Our findings provide support for further study evaluating the relevance of kyphosis among older adults, especially for women. Ideally, these future studies would include information about kyphosis, compression fractures and trunk extension strength to help clarify the contributions of these interrelated matters.
Acknowledgments
Research was supported by grant P01AG004390 from the National Institute on Aging Research Nursing Home Program Project. Dr. Jonathan Bean's time was funded through grant 1K24HD070966 from the National Institute of Child and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentations include: Eum R, Leveille SG, Kiely DK, Kiel DP, Samelson EJ, Bean JF. “Is Kyphosis Relevant to Mobility and Disability?” Paper Presentation. Association of Academic Physiatrists 2011 Annual Meeting. Phoenix, AZ. Apr. 12–16, 2011. Abstract published: American Journal of Physical Medicine & Rehabilitation. 90(4): a11, April 2011. Eum R, Leveille SG, Kiely DK, Kiel DP, Samelson EJ, Bean JF. “Is Kyphosis Relevant to Mobility or Disability Among Older Adults?” Grand Rounds. Spaulding Rehabilitation Hospital-Boston. Boston, MA. March 11, 2011. Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
References
- 1.Katzman WB, Wanek L, Shepherd JA, Sellmeyer DE. Age-related hyperkyphosis: its causes, consequences, and management. J Orthop Sports Phys Ther. 2010;40(6):352–360. doi: 10.2519/jospt.2010.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katzman W, Cawthon P, Hicks GE, Vittinghoff E, Shepherd J, Cauley JA, Harris T, Simonsick EM, Strotmeyer E, Womack C, Kado DM. Association of spinal muscle composition and prevalence of hyperkyphosis in healthy community-dwelling older men and women. J Gerontol A Biol Sci Med Sci. 2012;67(2):191–195. doi: 10.1093/gerona/glr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kado DM. The rehabilitation of hyperkyphotic posture in the elderly. Eur J Phys Rehabil Med. 2009 Dec;45(4):583–593. 2009. [PubMed] [Google Scholar]
- 4.Kasukawa Y, Miyakoshi N, Hongo M, Ishikawa Y, Noguchi H, Kamo K, Sasaki H, Murata K, Shimada Y. Relationships between falls, spinal curvature, spinal mobility and back extensor strength in elderly people. J Bone Miner Metab. 2010;28(1):82–87. doi: 10.1007/s00774-009-0107-1. [DOI] [PubMed] [Google Scholar]
- 5.Kado DM, Huang MH, Barrett-Connor E, Greendale GA. Hyperkyphotic posture and poor physical functional ability in older community-dwelling men and women: the Rancho Bernardo study. J Gerontol A Biol Sci Med Sci. 2005 May 5;60(5):633–637. doi: 10.1093/gerona/60.5.633. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Balance disorder and increased risk of falls in osteoporosis and kyphosis: significance of kyphotic posture and muscle strength. Osteoporos Int. 2005 Aug;16(8):1004–1010. doi: 10.1007/s00198-004-1791-2. 2005. [DOI] [PubMed] [Google Scholar]
- 7.Antonelli-Incalzi R, Pedone C, Cesari M, Di Iorio A, Bandinelli A, Ferrucci L. Relationship between the occiput-wall distance and physical performance in the elderly: a cross sectional study. Aging Clin Exp Res. 2007 Jun;19(3):207–212. doi: 10.1007/bf03324691. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold CM, Busch AJ, Schachter CL, Harrison L, Olszynski W. The relationship of intrinsic fall risk factors to a recent history of falling in older women with osteoporosis. J Orthop Sports Phys Ther. 2005;35(7):452–460. doi: 10.2519/jospt.2005.35.7.452. [DOI] [PubMed] [Google Scholar]
- 9.Lynn SG, Sinaki M, Westerlind KC. Balance characteristics of persons with osteoporosis. Arch Phys Med Rehabil. 1997 Mar;78(3):273–277. doi: 10.1016/s0003-9993(97)90033-2. 1997. [DOI] [PubMed] [Google Scholar]
- 10.Greig AM, Bennell KL, Briggs AM, Wark JD, Hodges PW. Balance impairment is related to vertebral fracture rather than thoracic kyphosis in individuals with osteoporosis. Osteoporos Int. 2007 Apr;18(4):543–551. doi: 10.1007/s00198-006-0277-9. 2007. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa Y, Miyakoshi N, Kasukawa Y, Hongo M, Shimada Y. Spinal curvature and postural balance in patients with osteoporosis. Osteoporos Int. 2009 Dec;20(12):2049–2053. doi: 10.1007/s00198-009-0919-9. 2009. [DOI] [PubMed] [Google Scholar]
- 12.Chow RK, Harrison JE. Relationship of kyphosis to physical fitness and bone mass on post-menopausal women. Am J Phys Med. 1987 Oct;66(5):219–227. 1987. [PubMed] [Google Scholar]
- 13.Lombardi I, Jr, Oliveira LM, Monteiro CR, Confessor YQ, Barros TL, Natour J. Evaluation of physical capacity and quality of life in osteoporotic women. Osteoporos Int. 2004 Jan;15(1):80–85. doi: 10.1007/s00198-003-1512-2. 2004. [DOI] [PubMed] [Google Scholar]
- 14.Ryan PJ, Blake G, Herd R, Fogelman I. A clinical profile of back pain and disability in patients with spinal osteoporosis. Bone. 1994 Jan-Feb;15(1):27–30. doi: 10.1016/8756-3282(94)90887-7. 1994. [DOI] [PubMed] [Google Scholar]
- 15.Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Significant reduction in risk of falls and back pain in osteoporotic-kyphotic women through a Spinal Proprioceptive Extension Exercise Dynamic (SPEED) program. Mayo Clin Proc. 2005 Jul;80(7):849–855. doi: 10.4065/80.7.849. 2005. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien K, Culham E, Pickles B. Balance and skeletal alignment in a group of elderly female fallers and nonfallers. J Gerontol A Biol Sci Med Sci. 1997 Jul;52(4):B221–B226. doi: 10.1093/gerona/52a.4.b221. 1997. [DOI] [PubMed] [Google Scholar]
- 17.Leveille SG, Kiel DP, Jones RN, Roman A, Hannan MT, Sorond FA, Kang HG, Samelson EJ, Gagnon M, Freeman M, Lipsitz LA. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatrics. 2008 Jul 18;8:16. doi: 10.1186/1471-2318-8-16. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold CM, Beatty B, Harrison L, Olszynski W. The reliability of five clinical postural alignment measures for women with osteoporosis. Physiother Canada. 2000;54:286–294. [Google Scholar]
- 19.Lundon KM, Li AM, Biberschtein S. Interrater and intrarater reliability in the measurement of kyphosis in postmenopausal women with osteoporosis. Interrater and intrarater reliability in the measurement of kyphosis in postmenopausal women with osteoporosis. Spine. 1998;23:1978–1985. doi: 10.1097/00007632-199809150-00013. [DOI] [PubMed] [Google Scholar]
- 20.Milne JS, Lauder IJ. The relationship of kyphosis to the shape of vertebral bodies. Ann Hum Biol. 1976 Mar;3(2):173–179. doi: 10.1080/03014467600001281. 1976. [DOI] [PubMed] [Google Scholar]
- 21.Greendale GA, Nili NS, Huang MH, Seeger L, Karlamangla AS. The reliability and validity of three non-radiological measures of thoracic kyphosis and their relations to the standing radiological Cobb angle. Osteoporos Int. 2011;22(6):1897–1905. doi: 10.1007/s00198-010-1422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332(9):556–561. doi: 10.1056/NEJM199503023320902. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000 Apr;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. 2000. [DOI] [PubMed] [Google Scholar]
- 24.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 25.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13(6):538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg KO, Maki BE, Williams JI, Holliday PJ, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992 Nov;73(11):1073–1080. 1992. [PubMed] [Google Scholar]
- 27.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997 Aug;77(8):812–819. doi: 10.1093/ptj/77.8.812. 1997. [DOI] [PubMed] [Google Scholar]
- 28.Donoghue D. Physiotherapy Research and Older People (PROP) group, Stokes EK. How much change is true change? The minimum detectable change of the Berg Balance Scale in elderly people. J Rehabil Med. 2009 Apr;41(5):343–346. doi: 10.2340/16501977-0337. [DOI] [PubMed] [Google Scholar]
- 29.Alexander NB, Guire KE, Thelen DG, Ashton-Miller JA, Schultz AB, Grunawalt JC, Giordani B. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48(11):1408–1413. doi: 10.1111/j.1532-5415.2000.tb02630.x. [DOI] [PubMed] [Google Scholar]
- 30.Verghese J, Wang C, Xue X, Holtzer R. Self-reported difficulty in climbing up or down stairs in nondisabled elderly. Arch Phys Med Rehabil. 2008;89(1):100–104. doi: 10.1016/j.apmr.2007.08.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26(2):130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982;72(8):800–808. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leveille SG, Jones RN, Kiely DK, Hausdorff JM, Shmerling RH, Guralnik JM, Kiel DP, Lipsitz LA, Bean JF. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009 Nov 25;302(20):2214–2221. doi: 10.1001/jama.2009.1738. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cleeland C. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Adv Pain Res Ther. Vol 12. New York, NY: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 35.Escalante A, Lichtenstein MJ, Lawrence VA, Roberson M, Hazuda HP. Where does it hurt? Stability of recordings of pain location using the McGill Pain Map. J Rheumatol. 1996 Oct;23(10):1788–1793. 1996. [PubMed] [Google Scholar]
- 36.Tinetti ME, Mendes de Leon CF, Doucette JT, Baker DI. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. J Gerontol. 1994;49(3):M140–M147. doi: 10.1093/geronj/49.3.m140. [DOI] [PubMed] [Google Scholar]
- 37.Milne JS, Lauder IJ. Age effects in kyphosis and lordosis in adults. Ann Hum Biol. 1974 Jul;1(3):327–337. doi: 10.1080/03014467400000351. 1974. [DOI] [PubMed] [Google Scholar]
- 38.Sinaki M, Itoi E, Rogers JW, Bergstralh EJ, Wahner HW. Correlation of back extensor strength with thoracic kyphosis and lumbar lordosis in estrogen-deficient women. Am J Phys Med Rehabil. 1996 Sep-Oct;75(5):370–374. doi: 10.1097/00002060-199609000-00013. 1996. [DOI] [PubMed] [Google Scholar]
- 39.Mika A, Unnithan VB, Mika P. Differences in thoracic kyphosis and in back muscle strength in women with bone loss due to osteoporosis. Spine. 2005 Jan;1530(2):241–246. doi: 10.1097/01.brs.0000150521.10071.df. 2005. [DOI] [PubMed] [Google Scholar]
- 40.Hongo M, Itoi E, Sinaki M, Miyakoshi N, Shimada Y, Maekawa S, Okada K, Mizutani Y. Effect of low-intensity back exercise on quality of life and back extensor strength in patients with osteoporosis: a randomized controlled trial. Osteoporos Int. 2007 Oct;18(10):1389–1395. doi: 10.1007/s00198-007-0398-9. 2007. [DOI] [PubMed] [Google Scholar]
- 41.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PM R. 2009 Oct;1(10):916–924. doi: 10.1016/j.pmrj.2009.09.009. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinaki M, McPhee MC, Hodgson SF, Merritt JM, Offord KP. Relationship between bone mineral density of spine and strength of back extensors in healthy postmenopausal women. Mayo Clin Proc. 1986 Feb;61(2):116–122. doi: 10.1016/s0025-6196(12)65197-0. 1986. [DOI] [PubMed] [Google Scholar]
- 43.Lyles KW, Gold DT, Shipp KM, Pieper CF, Martinez S, Mulhausen PL. Association of Osteoporotic Vertebral Compression Fractures with Impaired Functional Status. Am J Med. 1993;94(6):595–601. doi: 10.1016/0002-9343(93)90210-g. [DOI] [PubMed] [Google Scholar]
- 44.Katzman WB, Vittinghoff E, Ensrud K, Black DM, Kado DM. Increasing kyphosis predicts worsening mobility in older community-dwelling women: a prospective cohort study. J Am Geriatr Soc. 2011;59(1):96–100. doi: 10.1111/j.1532-5415.2010.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katzman WB, Vittinghoff E, Kado DM. Age-related hyperkyphosis, independent of spinal osteoporosis, is associated with impaired mobility in older community-dwelling women. Osteoporos Int. 2011;22(1):85–90. doi: 10.1007/s00198-010-1265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinaki M, Nwaogwugwu NC, Philips BE, Mokri MP. Effect of gender, age, and anthropometry on axial and appendicular muscle strength. Am J Phys Med Rehabil. 2001 May;80(5):330–338. doi: 10.1097/00002060-200105000-00002. 2001. [DOI] [PubMed] [Google Scholar]
- 47.Kado DM, Lui LY, Ensrud KE, Fink HA, Karlamangla AS, Cummings SR. Hyperkyphosis predicts mortality independent of vertebral osteoporosis in older women. Ann Intern Med. 2009 May 19;150(10):681–687. doi: 10.7326/0003-4819-150-10-200905190-00005. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]