Abstract
Accelerated atherosclerosis remains a major cause of death in late systemic lupus erythematosus (SLE). Omega-3 has been reported to have benefit for endothelial dysfunction, one of the earliest stages of atherosclerosis, and to reduce disease activity in SLE. We performed a randomized, double-blind placebo-controlled trial to examine the effect of Omega-3 on endothelial function, disease activity, inflammatory markers and lipids in SLE. SLE patients (n = 85, mean age 47, 55 % Caucasian, 38 % African-American, 94 % female) were randomly assigned to 3 g of Omega-3 (Lovaza, GSK) versus placebo for 12 weeks. Endothelial function was measured at baseline and at 12 weeks using flow-mediated dilation, calculated using high-resolution B-mode ultrasound of the brachial artery diameter in response to vasoactive stimuli (hyperemia). Disease activity was measured using the physician global assessment and SELENA-SLEDAI score. Inflammatory markers (sICAM-1, sVCAM-1, IL-6) and fasting lipid profile were done at baseline and 12-week follow-up. There was no difference between the treatment groups with respect to changes in flow-mediated dilation parameters or disease activity. An average increase in LDL cholesterol of 3.11 mg/dL (±21.99) was found with Omega-3 versus a decrease of 1.87 mg/dL (±18.29) with placebo (p = 0.0266). In this trial, Omega-3 did not improve endothelial function, disease activity, nor reduce inflammatory markers in SLE. Longer trials might be required if there are delayed clinical effects. There was evidence that Omega-3 may increase LDL cholesterol, but not the LDL/HDL ratio.
Keywords: Omega-3, LDL cholesterol, Flow-mediated dilation, Systemic lupus erythematosus
Introduction
Omega-3-fatty acids are polyunsaturated fatty acids which include eicosapentaenoic acid, docosahexaenoic acid and alpha-linolenic acid. Eicosapentaenoic acid and docosahexaenoic acid are present in fatty fish, while alpha-linolenic acid is found in vegetable oils. Omega-3 fatty acids have become one of the most popular over the counter supplements. In the 2000–2002 Minnesota Heart Survey, Omega-3 was established as one of the most common nonprescription medications for perceived cardiovascular health [1]. Potential health benefits have been reported in cancer [2, 3], cognitive function [4], depression [5], immune and inflammatory processes [6, 7], and the cardiovascular system (dyslipidemia and cardiac function [8– 10]. Early ecological studies of the Greenland Inuit Tribe and Okinawa Islanders of Japan attributed their low risk of coronary artery disease morbidity and mortality to their fatty fish diet [11, 12].
Though survival in systemic lupus erythematosus (SLE) patients has improved over the last decades [13–15], cardiovascular disease remains a major cause of death [16, 17]. Urowitz et al. [18] found that SLE had a bimodal mortality pattern, with later deaths attributed largely to atherosclerotic cardiovascular disease. Cardiovascular disease morbidity and mortality in SLE is greatly increased compared to the general population. Manzi et al. [19] reported a 50-fold increase in the risk of myocardial infarction among premenopausal SLE women compared to controls, a Canadian study reported a fivefold increased risk of coronary artery disease in SLE [20], and recently, the Nurses’ Health Study, a prospective cohort study, showed a greater than twofold increased risk of cardiovascular disease among participants with SLE [21].
Jolly et al. [22] showed that a fish oil-supplemented diet, in combination with a 40 % food restriction, had an impact on extending the life span in lupus-prone (NZB × NZW) F1 mice, partly by delaying disease-associated immune dysregulation. Fernandes et al. [23] reported that daily dietary Omega-3 fatty acid supplementation reduced the chance of cardiovascular disease and delayed the onset of autoimmune disorders in mice.
Possible therapeutic effects of Omega-3 fatty acids in human SLE have been explored. A double-blind, double-placebo-controlled factorial trial performed in 52 SLE patients in Northern Ireland over 24 weeks reported a significant decline in disease activity in the fish oil treatment group. The patients were randomized to one of the 4 treatment groups: fish oil and copper, fish oil and placebo copper, copper and placebo fish oil or placebo fish oil and placebo copper. Disease activity was then measured serially by the revised systematic lupus activity measure (SLAM-R) [24]. A significant decline in the SLAM-R score from 6.12 to 4.69 was reported in the patients taking fish oil compared to placebo [25]. In a second study, Wright et al. conducted a 24-week randomized double-blind placebo-controlled parallel trial of the effect of 3 g of Omega-3-polyunsaturated fatty acids in 60 SLE patients. Disease activity was recorded using the revised SLAM-R and the British Isles Lupus Assessment Group (BILAG) measure at baseline, 12 and 24 weeks [24, 26]. They also measured endothelial function using flow-mediated dilation (FMD) of the brachial artery and oxidative stress using platelet 8-isoprostanes at baseline, 12 and 24 weeks. They reported a significant improvement in SLAM-R (from 9.4 to 6.3, p < 0.001), in BILAG (from 13.6 to 6.7, p< 0.001), in FMD (from 3.0 to 8.9 %, p < 0.001) and in platelet 8-isoprostanes (from 177 to 90 pg/mg, p = 0.007). Wright et al. [27] concluded that low-dose dietary supplementation with fish oil in SLE had therapeutic benefit on disease activity, endothelial function and oxidative stress and might have cardiovascular benefits.
A randomized, double-blind, placebo-controlled 3 months clinical trial of Omega-3-polyunsaturated fatty acids in SLE patients drawn from the Hopkins Lupus Cohort was performed, using the only FDA-approved formulation, Omega-3-acid ethyl esters, Lovaza (Glaxo-SmithKline, St. Petersburg, FL). We investigated the effects of Omega-3-fatty acids on endothelial function, disease activity, lipids and inflammatory markers.
Patients and methods
Study participants
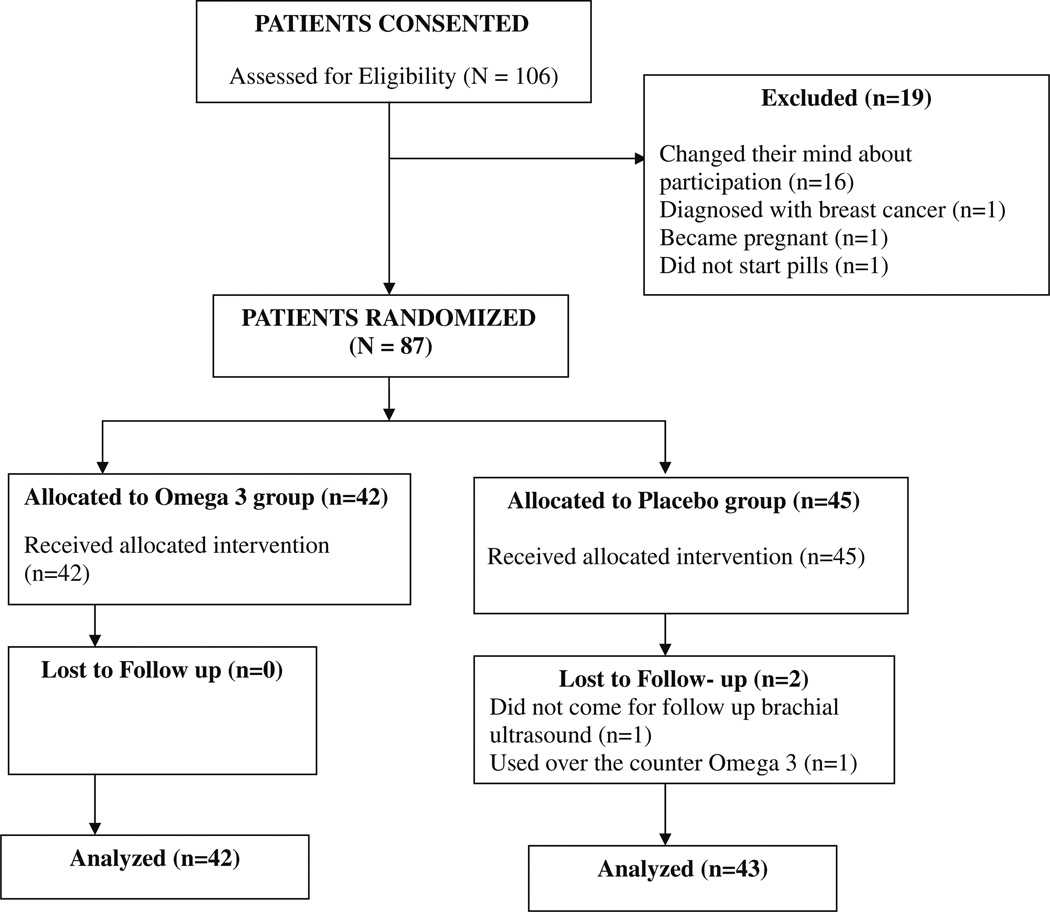
This randomized, double-blind, placebo-controlled clinical trial of Omega-3-polyunsaturated fatty acids was conducted in SLE patients drawn from the Hopkins Lupus Cohort. The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. All patients gave informed consent (clinical trial: NCT00828178). All patients met the revised American College of Rheumatology (ACR) classification criteria for SLE [28, 29]. Eligibility for the study was determined at screening. Exclusion criteria for the study included the following: pregnancy, pregnancy plans or nursing; warfarin or heparin use; increased liver enzymes (AST or ALT) more than two times the upper limit of normal; allergy to fish, fish oil or any Omega-3 product; Omega-3 use within the previous 6 months; and established coronary artery disease. Patients recruited into the trial continued to take their regular SLE medications. No patients were taking statins. A total of 106 patients were consented for the trial, 87 patients were randomized, and 85 had both their first and 12-week visit (Fig. 1).
Fig. 1.
Study design
Study design
A 12-week randomized, double-blind, placebo-controlled clinical trial of Omega-3-polyunsaturated fatty acids was conducted. Participants were seen at baseline and at week 12. Participants were also called at week 6 to ask about compliance and any adverse events. Randomization occurred via the Hopkins Investigational Drug Pharmacy. Patients were randomized in a 1:1 ratio to receive either 3 g of Omega-3 (1.8 g eicosapentaenoic acid, 1.2 g docosahexaenoic acid ethyl esters) or placebo (corn starch). They were all instructed not to take any other Omega-3 products or any supplements that contained Omega-3 fatty acids.
Variables measured at each visit included: disease activity measured using the physician’s global assessment (PGA) and the SELENA Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) [30]; fasting lipid profile; standard laboratory tests to assess SLE (white blood count, platelet count, creatinine, urinalysis, urine protein/creatinine ratio, anti-dsDNA, anticardiolipin, complement C3 and C4 levels); inflammatory markers (IL-6, sICAM-1, sVCAM-1); and FMD.
Flow-mediated dilation
Endothelial function was measured by brachial artery vasoactivity testing using high-resolution B-mode ultrasound to image and measure brachial artery diameter in response to hyperemia-induced vasoactive stimuli [31–33]. All participants were instructed to fast for 8–12 h before the procedure. They were also told to avoid vasoactive factors including exercise, caffeine, smoking, high-fat foods and nitrate-based medications on the morning of the procedure.
A blood pressure cuff was inflated on the upper arm for 5 min. B-mode images of the brachial artery and flow upon release of the cuff were then recorded for up to 2-min post-cuff release. The brachial ultrasounds were all performed in the Johns Hopkins Hospital Noninvasive Cardiovascular Ultrasound Research Lab in the Cardiology Division, under the direction of Corretti [31, 32]. Results obtained from the imaging were used in calculating % FMD by incorporating them into the equation below:
% FMD = (Post-hyperemic diameter − Baseline diameter) × 100/Baseline diameter.
Outcome variables
The primary outcome was the effect on brachial artery flow dilation measured at 30, 60 and 90 s. Secondary outcome variables were as follows: disease activity and markers of inflammation such as IL-6, I-CAM1, V-CAM1 and fasting lipid profile.
Statistical analysis
Statistical analysis system (SAS) software was used (SAS Institute Inc. Cary, North Carolina, SAS 9.2). Baseline demographic and clinical characteristics were summarized using appropriate descriptive statistics and compared across treatment groups using Chi-square. Two-sample t tests were used in the statistical analysis of the FMD outcomes. ANCOVA was used to compare the groups with respect to changes in clinical variables adjusting for baseline values.
Results
Two participants, out of the 87 randomized, did not complete the trial. One of them used an over the counter Omega-3 supplement, and the other failed to come for her follow-up FMD measurement. There were a total of 6 adverse events in the trial. In the Omega-3 treatment group, one patient had palpitations, a second patient had hives, a third patient had diarrhea, and another had abdominal pain due to pancreatitis. In the placebo treatment group, one patient had a hip fracture and a second patient had a lupus flare. None of the adverse events was attributed to Omega-3, and no patient withdrew from the trial due to an adverse event.
Baseline demographics and clinical characteristics
The 85 SLE patients who completed the study had a mean age of 47.2 years and were 55 % Caucasian, 38 % African-American and 94 % female. Cumulative ACR revised classification were as follows: malar rash 52.9 %, discoid rash 15.3 %, photosensitivity 60 %, oral ulcers 51.8 %, arthritis 80 %, serositis 41.2 %, renal disorder 23.5 %, neurologic disorder 7.1 %, hematologic disorder 71.8 %, immunologic disorder 76.5 % and ANA positivity 98.8 % (Table 1). Baseline demographic characteristics of participants by treatment groups were not statistically different (Table 2). Most of the patients had low disease activity.
Table 1.
Comparison of the Omega-3 versus placebo groups with respect to percentage satisfying ACR SLE classification criteria
| All (N = 85) n (%) |
Omega-3 (N = 42) n (%) |
Placebo (N = 43) n (%) |
p value | |
|---|---|---|---|---|
| Malar rash | 45 (52.9) | 27 (64.3) | 18 (41.9) | 0.0384 |
| Discoid rash | 13 (15.3) | 3 (7.1) | 10 (23.3) | 0.0391 |
| Photosensitivity | 51 (60.0) | 25 (59.5) | 26 (60.5) | 0.9294 |
| Oral ulcers | 44 (51.8) | 22 (52.4) | 22 (51.2) | 0.9105 |
| Arthritis | 68 (80.0) | 36 (85.7) | 32 (74.4) | 0.1930 |
| Serositis | 35 (41.2) | 12 (28.6) | 23 (53.5) | 0.0196 |
| Renal disorder | 20 (23.5) | 13 (31.0) | 7 (16.3) | 0.1108 |
| Neurologic disorder | 6 (7.1) | 4 (9.5) | 2 (4.7) | 0.3805 |
| Hematologic disorder | 61 (71.8) | 28 (66.7) | 33 (76.7) | 0.3021 |
| Immunologic disorder | 65 (76.5) | 31 (73.8) | 34 (79.1) | 0.5676 |
| ANA positivity | 84 (98.8) | 42 (100) | 42 (97.7) | 0.3201 |
Table 2.
Baseline characteristics, by treatment group
| Omega-3 N = 42 n (%) |
Placebo N = 43 n (%) |
|
|---|---|---|
| Age in years (mean ± SD) | 48.9 ± 10.6 | 45.5 ± 10.8 |
| Gender (%) | ||
| Female | 41 (97.6) | 39 (90.7) |
| Male | 1 (2.4) | 4 (9.3) |
| Ethnicity (%) | ||
| Caucasian | 27 (64.3) | 20 (46.5) |
| African-American | 13 (31.0) | 19 (44.2) |
| Asian | 0 (0.0) | 1 (2.3) |
| Other | 2 (4.8) | 3 (7.0) |
| History of smoking (%) | 13 (31.0) | 19 (44.2) |
| Any anti-hypertensive (%) | 30 (71.4) | 22 (51.2) |
| Diabetes (%) | 4 (9.5) | 3 (7.0) |
| Disease activity (mean ± SD) | ||
| SELENA-SLEDAIa | 1.5 ± 2.0 | 1.5 ± 2.5 |
| PGAb | 0.49 ± 0.52 | 0.41 ± 0.49 |
| Systolic BP (mean ± SD) | 119.4 ± 14.6 | 119.4 ± 11.9 |
| Cumulative prednisone dose in last year (mg/d, mean ± SD) |
1.6 ± 2.4 | 3.4 ± 4.5 |
| BMI (mean ± SD) | 28.5 ± 6.0 | 29.0 ± 6.6 |
| BMI (median, IQR) | 27.8 (7.6) | 28.9 (9.8) |
SELENA-SLEDAI—SELENA revision of the Systemic Lupus Erythematosus Disease Activity Index
PGA—physician global assessment on a 0–3 visual analog scale
There was no significant difference in the brachial artery diameter (cm) results before treatment between the Omega-3 treatment group and the placebo group (Table 3). The mean brachial artery diameter at baseline was 0.32 cm (±0.06) in the Omega-3 group and 0.33 cm (±0.06) in the placebo group; p = 0.52. There was no significant difference in the FMD between the 2 groups at baseline (Table 3).
Table 3.
Brachial artery diameter results, pre- and post-treatment
| Period | Variable | Omega-3 mean (SD) | Placebo mean (SD) | p valuea |
|---|---|---|---|---|
| Pre-treatment | Baseline, cm | 0.32 (0.06) | 0.33 (0.06) | 0.52 |
| 30 s, cm | 0.34 (0.06) | 0.35 (0.06) | 0.58 | |
| 30 s % change | 6.53 (5.51) | 6.33 (6.63) | 0.88 | |
| 60 s, cm | 0.36 (0.06) | 0.37 (0.06) | 0.43 | |
| 60 s % change | 12.02 (6.00) | 12.31 (7.23) | 0.84 | |
| 90 s, cm | 0.36 (0.06) | 0.37 (0.06) | 0.50 | |
| 90 s % change | 12.30 (6.83) | 11.94 (7.24) | 0.82 | |
| Post-treatment | Baseline, cm | 0.32 (0.04) | 0.33 (0.06) | 0.38 |
| 30 s, cm | 0.34 (0.05) | 0.35 (0.06) | 0.43 | |
| 30 s % change | 5.97 (6.10) | 5.73 (6.54) | 0.87 | |
| 60 s, cm | 0.35 (0.04) | 0.36 (0.06) | 0.32 | |
| 60 s % change | 9.59 (6.93) | 9.52 (6.60) | 0.97 | |
| 90 s, cm | 0.35 (0.04) | 0.36 (0.06) | 0.23 | |
| 90 s % change | 8.48 (8.63) | 9.00 (7.82) | 0.78 | |
| Pre–post differences | Baseline, cm | 0.00 (0.04) | 0.00 (0.05) | 0.99 |
| 30 s % change | –0.60 (7.80) | –0.89 (0.83) | 0.87 | |
| 60 s % change | –2.36 (10.01) | –2.73 (8.72) | 0.86 | |
| 90 s % change | –3.96 (11.73) | –2.76 (9.68) | 0.62 |
Based on a two-sample t test
Post-treatment results
Flow-mediated dilation results
Analysis of the brachial artery diameter results comparing the two treatment groups using a two-sample t test found that there was no significant difference at any time point in the 2 groups after 12 weeks of treatment. The mean brachial artery diameter at baseline measured during the post-treatment visit was 0.32 cm (±0.04) in the Omega-3 group and 0.33 cm (±0.06) in the placebo group (p = 0.38). There was also no significant difference in the FMD percent change between the two groups at 30, 60 and 90 s post-cuff deflation (Table 3).
Inflammatory markers
Statistical analysis of the inflammatory markers (IL-6, sICAM-1 and sVCAM-1), comparing the 2 treatment groups before and after treatment, showed no significant differences (Table 4). However, while sVCAM-1 declined in the Omega-3 group, it increased slightly in the placebo group (p = 0.092).
Table 4.
Inflammatory markers, lipids and disease activity by treatment group
| Mean change in Omega-3 group (SD) (N = 42) |
Mean change in placebo group (SD) (N = 43) |
p value* | Adjusted p value** | |
|---|---|---|---|---|
| sICAM-1, ng/mL | −1.61 (40.27) | −5.56 (48.74) | 0.2456 | 0.2429 |
| sVCAM-1, ng/mL | −17.52 (101.65) | 26.57 (135.98) | 0.0918 | 0.1218 |
| IL-6, pg/mL | −1.95 (11.16) | −27.60 (186.37) | 0.2447 | 0.5847 |
| Cholesterol, mg/dL | 3.36 (27.24) | −6.48 (22.95) | 0.0228 | 0.0099 |
| Triglycerides, mg/dL | −43.45 (71.71) | −29.48 (61.81) | 0.7041 | 0.8441 |
| HDL, mg/dL | 2.55 (8.49) | −2.21 (11.87) | 0.0929 | 0.1934 |
| LDL, mg/dL | 3.11 (21.99) | −1.87 (18.29) | 0.0266 | 0.0093 |
| LDL/HDL ratio | −0.003 (0.39) | 0.004 (0.40) | 0.3756 | 0.2132 |
| PGAa | 0.07 (0.54) | 0.21 (0.44) | 0.2914 | 0.3300 |
| SLEDAIb | −0.17 (1.87) | 0.51 (2.18) | 0.1122 | 0.1801 |
Based on ANCOVA model using baseline values as a covariate
Based on ANCOVA modeling using baseline values, use of any anti-hypertensives, diabetes, average daily use of prednisone during the follow-up period, weight, urine protein-to-creatinine ratio and systolic BP as covariates
PGA—physician global assessment on a 0–3 visual analog scale
SLEDAI—SELENA revision of Systemic Lupus Erythematosus Disease Activity Index
Disease activity
Those in the Omega-3 group had an average decline in SELENA-SLEDAI of 0.17 points, while those in the placebo group had an average increase in SELENA-SLEDAI of 0.51 points (p = 0.11). The 95 % CI for the difference between the groups with respect to mean change in SELENA-SLEDAI ranged from –1.5 (i.e., mean SELENA-SLEDAI declined 1.5 points greater in the Omega-3 group) to 0.2 (i.e., mean SELENA-SLEDAI increased 0.2 points greater in the placebo group). There was no statistical difference between the groups in either change in SELENA-SLEDAI or change in PGA (Table 4).
Fasting lipid profile
Analysis of the changes in fasting lipid profile comparing the two treatment groups found statistically significant results. An average increase in LDL cholesterol of 3.11 mg/dL (±21.99) was experienced in the Omega-3 treatment group, compared to an average decrease in LDL cholesterol of 1.87 mg/dL (±18.29) in the placebo group (p = 0.0266), based on an ANCOVA model. The ANCOVA analysis also showed a statistically significant average increase in total cholesterol in the Omega-3 treatment group compared to the placebo treatment group (Table 4). The LDL/HDL ratio was not statistically different between the groups.
Discussion
Studies have shown that brachial endothelium function is impaired in SLE patients compared to matched healthy controls [34, 35]. Mak et al. [36] found significantly lower endothelium-dependent FMD in 580 SLE patients compared to 381 matched controls (p < 0.001), but there was no difference in endothelium-independent FMD between the groups. Our clinical trial used endothelium-dependent FMD as our primary outcome. We picked Omega-3 as the intervention, because of the trial done by Wright et al. that found it improved endothelium-dependent dilation in SLE.
In this trial, we did not find strong evidence that Omega-3 improved endothelial function, reduced disease activity, nor reduced inflammatory markers in SLE. The greater decline in sVCAM-1 and disease activity in the Omega-3 group did not reach statistical significance. Given the size of our confidence intervals around differences between the groups, our data cannot completely rule out the possibility of a mild effect of Omega-3 on these outcomes. Because we were limited to suing the FDA-approved formulation of Omega-3 (Lovaza), we cannot rule out the possibility that other doses or other formulations might have an effect on endothelial function [37]. Furthermore, a short trial cannot assess actual cardiovascular outcomes. However, recently, Rizos et al. [38] published a meta-analysis of Omega-3 trials in the general population and concluded that it had no benefit on cardiovascular outcomes.
Our results differ from the finding of Wright et al. who reported a significant improvement in SLAM-R (from 9.4 (SD 3.0) to 6.3 (2.5), p < 0.001); in BILAG (from 13.6 (6.0) to 6.7 (3.8), p < 0.001); and in FMD (from 3.0 % (−0.5 to 8.2) to 8.9 % (1.3–16.9), p < 0.001) in the Omega-3 group at 24 weeks. We cannot explain the conflicting results by the differing treatment lengths, as they also measured disease activity and FMD at their 12-week follow-up, and still found significant reductions in SLAM-R (p = 0.009) and BILAG (p = 0.001), and improved FMD at the 12-week follow-up visit (p = 0.002) [32].
The statistical comparison done by Wright et al. was a within-group “before and after” analysis of the treatment groups separately. In contrast, we performed a more stringent analysis, comparing changes in outcome measures between the two treatment groups. We failed to find any difference with either a before–after comparison or even when the treatment groups were directly compared (Table 4). We measured disease activity using the SELENA-SLEDAI and PGA, compared to the SLAM-R and BILAG used by Wright et al. BILAG requires monthly visits (our trial was baseline and 3 months). We think it unlikely that the BILAG instrument would record the improvement missed by both the PGA and the SELENA-SLEDAI. SLAM-R includes several variables (headache, myalgia, erythrocyte sedimentation rate) not related to clinical SLE activity. The strength of our trial was that all disease activity measures were completed by one investigator.
In this randomized, double-blind, placebo-controlled clinical trial of Omega-3 polyunsaturated fatty acids in the Hopkins Lupus Cohort, there was evidence that Omega-3 may increase total cholesterol and LDL cholesterol. The LDL/HDL ratio was not significantly different. The increase in LDL cholesterol has been documented in multiple studies [39, 40]. This is a clinically important finding, because of the known detrimental role that LDL cholesterol plays in cardiovascular disease. In a recent study, cholesterol was associated with the progression of coronary artery calcium in SLE over just 2 years [41]. Cardiovascular disease prevalence is increased in SLE patients and is also a leading cause of mortality in SLE [18–21]. This trial found that Omega-3 supplementation leads to an increase in LDL cholesterol in SLE, which could further worsen the already increased risk of cardiovascular disease in SLE. In fact, the FDA has now modified the Omega-3 label to include a warning that Omega-3 may increase the levels of LDL cholesterol [42]. In a pooled analysis from two randomized, placebo-controlled, double-blind, parallel-group studies of 84 adult patients (42 on Omega-3, 42 on placebo), a 44.5 % increase in LDL cholesterol was reported in the Omega-3 group compared to a 4.8 % decrease in the placebo group [43]. Wright et al. [27] found a smaller, not statistically significant increase in LDL cholesterol in the Omega-3 group (2.5–2.7 mmol/L) compared to the placebo group (2.7–2.7 mmol/L). Some studies reported an increase in LDL cholesterol particle size with Omega-3 supplementation [43–45]. Because small, dense LDL particles are associated with coronary artery disease [46], the impact of the increase in LDL cholesterol with Omega-3 is not yet completely understood. We were not able to measure other lipids of interest in SLE, such as pro-inflammatory HDL [47].
Simopoulos reported the importance of a low Omega-6-to-Omega-3 fatty acids ratio in reducing cardiovascular diseases and other chronic diseases [48]. Omega-6 fatty acids have been shown to inhibit the incorporation of eicosapentaenoic acid in humans [49]. A limitation in our study is that we did not take diet histories, so we were not able to calculate the Omega-6-to-Omega-3 ratio. We are unable to comment on whether excessive intake of dietary Omega-6-rich foods might have affected the incorporation of Omega-3 into the cell membranes.
If there are delayed beneficial clinical effects, longer trials might be required to further investigate the effect of Omega-3 on disease activity in SLE. However, the earlier but smaller trial by Wright et al. did find benefit at 12 weeks. The evidence of significantly increased LDL cholesterol in the Omega-3 treatment group in this clinical trial, however, is a potential cause of reluctance for longer trials. Patients in our study tended to have low disease activity, so we cannot rule out a potential effect in a higher disease activity subset. However, atherosclerosis in SLE is not just associated with high disease activity. Roman et al. [50] found that accelerated atherosclerosis in SLE was not more common in those on prednisone or cyclophosphamide. An earlier study in the Hopkins Lupus Cohort found that SLE patients that did not require high-dose steroids (stable low disease activity SLE patients) were still at the highest risk for coronary artery disease [51]. We are left with the frustrating result of no proven effective preventive therapy for accelerated atherosclerosis in SLE.
Acknowledgments
Supported by NIAMS AR43727, the Stabler/ P30/P30 and the Johns Hopkins University School of Medicine General Clinical Research Center ULIRR025005.
Contributor Information
Kayode J. Bello, Division of Rheumatology, Johns Hopkins University School of Medicine, 1830 East Monument Street, Suite 7500, Baltimore, MD 21205, USA, bellojibril@yahoo.co.uk
Hong Fang, Division of Rheumatology, Johns Hopkins University School of Medicine, 1830 East Monument Street, Suite 7500, Baltimore, MD 21205, USA, hfang6@jhmi.edu.
Parastoo Fazeli, Division of Rheumatic and Autoimmune Diseases, University of Minnesota, Saint Paul, MN, USA, pfazeli@umn.edu.
Waleed Bolad, Department of Internal Medicine/Rheumatology Division, University of Virginia, Charlottesville, VA, USA, wb4t@virginia.edu.
Mary Corretti, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, mcorret1@jhmi.edu.
Laurence S. Magder, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, USA, lmagder@epi.umaryland.edu
Michelle Petri, Division of Rheumatology, Johns Hopkins University School of Medicine, 1830 East Monument Street, Suite 7500, Baltimore, MD 21205, USA, mpetri@jhmi.edu.
References
- 1.Artz MB, Harnack LJ, Duval SJ, Armstrong C, Arnett DK, Luepker RV. Use of nonprescription medications for perceived cardiovascular health. Am J Prev Med. 2006;30:78–81. doi: 10.1016/j.amepre.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 3.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer. 1996;74:159–164. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taepavarapruk P, Song C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J Neurochem. 2010;112:1054–1064. doi: 10.1111/j.1471-4159.2009.06524.x. [DOI] [PubMed] [Google Scholar]
- 5.da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111:351–359. doi: 10.1016/j.jad.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Iwami D, Nonomura K, Shirasugi N, Niimi M. Immunomodulatory effects of eicosapentaenoic acid through induction of regulatory T cells. Int Immunopharmacol. 2011;11:384–389. doi: 10.1016/j.intimp.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Hao W, Wong OY, Liu X, Lee P, Chen Y, Wong KK. ω-3 fatty acids suppress inflammatory cytokine production by macrophages and hepatocytes. J Pediatr Surg. 2010;45:2412–2418. doi: 10.1016/j.jpedsurg.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 9.Pignier C, Revenaz C, Rauly-Lestienne I, Cussac D, Delhon A, Gardette J, Le Grand B. Direct protective effects of poly-unsaturated fatty acids, DHA and EPA, against activation of cardiac late sodium current: a mechanism for ischemia selectivity. Basic Res Cardiol. 2007;102:553–564. doi: 10.1007/s00395-007-0676-x. [DOI] [PubMed] [Google Scholar]
- 10.Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 11.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 12.Kagawa Y, Nishizawa M, Suzuki M, Miyatake T, Hamamoto T, Goto K, et al. Eicosapolyenoic acids of serum lipids of Japanese islanders with low incidence of cardiovascular diseases. J Nutr Sci Vitaminol. 1982;28:441–453. doi: 10.3177/jnsv.28.441. [DOI] [PubMed] [Google Scholar]
- 13.Urowitz MB, Gladman DD, Abu-Shakra M, Farewell VT. Mortality studies in systemic lupus erythematosus. Results from a single center. III. Improved survival over 24 years. J Rheumatol. 1997;24:1061–1065. [PubMed] [Google Scholar]
- 14.Uramoto KM, Michet CJ, Jr, Thumboo J, Sunku J, O’Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum. 1999;42:46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine. 2006;85:147–156. doi: 10.1097/01.md.0000224709.70133.f7. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Shakra M, Urowitz MB, Gladman DD, Gough J. Mortality studies in systemic lupus erythematosus. Results from a single center. I. Causes of death. J Rheumatol. 1995;22:1259–1264. [PubMed] [Google Scholar]
- 17.Bjo¨rnådal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964–95. J Rheumatol. 2004;31:713–719. [PubMed] [Google Scholar]
- 18.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 19.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 20.Urowitz MB, Gladman DD. Accelerated atheroma in lupus–background. Lupus. 2000;9:161–165. doi: 10.1191/096120300678828271. [DOI] [PubMed] [Google Scholar]
- 21.Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum. 2009;61:1396–1402. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolly CA, Muthukumar A. Reddy Avula CP, Fernandes G. Maintenance of NF-kappaB activation in T-lymphocytes and a naive T-cell population in autoimmune-prone (NZB/NZW)F(1) mice by feeding a food-restricted diet enriched with n-3 fatty acids. Cell Immunol. 2001;213:122–133. doi: 10.1006/cimm.2001.1866. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes G, Bysani C, Venkatraman JT, Tomar V, Zhao W. Increased TGF-beta and decreased oncogene expression by omega-3 fatty acids in the spleen delays onset of autoimmune disease in B/W mice. J Immunol. 1994;152:5979–5987. [PubMed] [Google Scholar]
- 24.Liang MH, Socher SA, Larsen MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 25.Duffy EM, Meenagh GK, McMillan SA, Strain JJ, Hannigan BM, Bell AL. The clinical effect of dietary supplementation with omega-3 fish oils and/or copper in systemic lupus erythematosus. J Rheumatol. 2004;31:1551–1556. [PubMed] [Google Scholar]
- 26.Hay EM, Bacon PA, Gordon C, Isenberg DA, Maddison P, Snaith ML, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86:447–458. [PubMed] [Google Scholar]
- 27.Wright SA, O’Prey FM, McHenry MT, Leahey WJ, Devine AB, Duffy EM, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841–848. doi: 10.1136/ard.2007.077156. [DOI] [PubMed] [Google Scholar]
- 28.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 29.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 30.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. OC-SELENA Trial. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 31.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 32.Corretti MC, Plotnick GD, Vogel RA. Technical aspects of evaluating brachial artery vasodilatation using high-frequency ultrasound. Am J Physiol. 1995;268:1397–1404. doi: 10.1152/ajpheart.1995.268.4.H1397. [DOI] [PubMed] [Google Scholar]
- 33.Vogel RA, Corretti MC, Plotnick GD. A comparison of brachial artery flow-mediated vasodilation using upper and lower arm arterial occlusion in subjects with and without coronary risk factors. Clin Cardiol. 2000;23:571–575. doi: 10.1002/clc.4960230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima DS, Sato EI, Lima VC, Miranda F, Hatta FH., Jr Brachial endothelial function is impaired in patients with systemic lupus erythematosus. J Rheumatol. 2002;29:292–297. [PubMed] [Google Scholar]
- 35.Johnson SR, Harvey PJ, Floras JS, Iwanochko M, Ibanez D, Gladman DD, Urowitz M. Impaired brachial artery endothelium dependent flow mediated dilation in systemic lupus erythematosus: preliminary observations. Lupus. 2004;13:590–593. doi: 10.1191/0961203304lu1072oa. [DOI] [PubMed] [Google Scholar]
- 36.Mak A, Liu Y, Ho RC. Endothelium-dependent but not endothelium-independent flow-mediated dilation is significantly reduced in patients with systemic lupus erythematosus without vascular events: a metaanalysis and metaregression. J Rheumatol. 2011;38:1296–1303. doi: 10.3899/jrheum.101182. [DOI] [PubMed] [Google Scholar]
- 37.Bozcali E, Babalik E, Himmetoglu S, Mihmanli I, Toprak S. [Omega]-3 fatty acid treatment in cardiac syndrome X: a double-blind, randomized, placebo-controlled clinical study. Coron Artery Dis. 2013;24:328–333. doi: 10.1097/MCA.0b013e32835f3005. [DOI] [PubMed] [Google Scholar]
- 38.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between Omega-3 fatty acid supplementation and risk of major cardiovascular disease events. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 39.Pownall JH, Brauchi D, Kilinc C, Osmundsen K, Pao Q, Payton-Ross C, et al. Correlation of serum triglyceride and its reduction by ω-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Arterioscler. 1999;143:285–297. doi: 10.1016/s0021-9150(98)00301-3. [DOI] [PubMed] [Google Scholar]
- 40.Harris WS, Ginsberg HN, Arunakul N, Shachter NS, Windsor SL, Adams M, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4:385–391. [PubMed] [Google Scholar]
- 41.Kiani AN, Post WS, Magder LS, Petri M. Predictors of progression in atherosclerosis over 2 years in systemic lupus erythematosus. Rheumatology. 2011;50:2071–2079. doi: 10.1093/rheumatology/ker285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lovaza® [package insert] GlaxoSmithKline. St. Petersburg, FL: 2010. Dec, [Google Scholar]
- 43.Contacos C, Barter PJ, Sullivan DR. Effect of pravastatin and omega-3 fatty acids on plasma lipids and lipoproteins in patients with combined hyperlipidemia. Arterioscler Thromb. 1993;13:1755–1762. doi: 10.1161/01.atv.13.12.1755. [DOI] [PubMed] [Google Scholar]
- 44.Suzukawa M, Abbey M, Howe PR, Nestel PJ. Effects of fish oil fatty acids on low density lipoprotein size, oxidizability, and uptake by macrophages. J Lipid Res. 1995;36:473–484. [PubMed] [Google Scholar]
- 45.Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD, Beilin LJ. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–1094. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 46.Campos H, Genest JJ, Jr, Blijlevens E, et al. Low density lipoprotein particle size and coronary artery disease. Arterioscler Thromb. 1992;12:187–195. doi: 10.1161/01.atv.12.2.187. [DOI] [PubMed] [Google Scholar]
- 47.Hahn BH, Grossman J, Ansell BJ, Skaggs BJ, McMahon M. Altered lipoprotein metabolism in chronic inflammatory states: proinflammatory high-density lipoprotein and accelerated atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2008;10:213. doi: 10.1186/ar2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 49.Cleland LG, James MJ, Neumann MA, D’Angelo M, Gibson RA. Linoleate inhibits EPA incorporation from dietary fish-oil supplements in human subjects. Am J Clin Nutr. 1992;55:395–399. doi: 10.1093/ajcn/55.2.395. [DOI] [PubMed] [Google Scholar]
- 50.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 51.Zonana-Nacach A, Barr SG, Magder LS, Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. 2000;43:1801–1808. doi: 10.1002/1529-0131(200008)43:8<1801::AID-ANR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]