Abstract
Background
Chronic congestive hepatopathy is known to cause hepatic fibrosis and portal hypertension in patients post-Fontan operation for single ventricle palliation. The clinical significance of these findings is not clear. We hypothesized that features of portal hypertension would be significantly related to major adverse events.
Methods
A retrospective review of 73 adult and pediatric post-Fontan patients referred for a liver evaluation from 2001-2011 was performed. The relationship between features of portal hypertension (VAST score ≥2, 1 point each for Varices, Ascites, Splenomegaly or Thrombocytopenia) and a major adverse event (death, need for transplant, or hepatocellular carcinoma) was examined using logistic regression.
Results
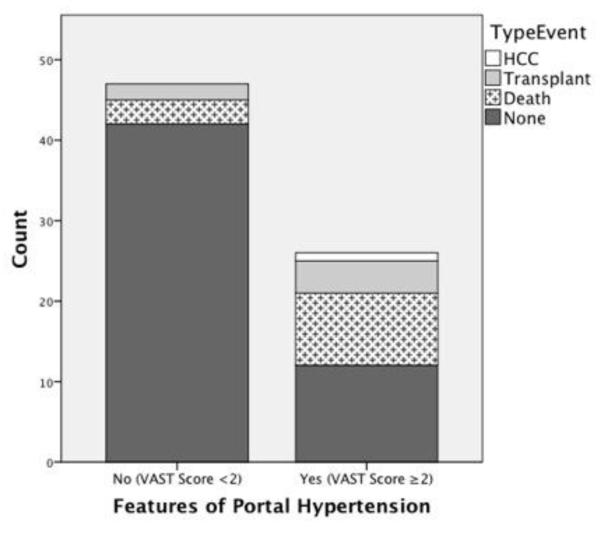
73 post-Fontan patients (30% female, 73% Caucasian, 66% systemic left ventricle (SLV), mean age 24 ±11 years, mean interval from Fontan 17 ±6 years) were included in analysis. Features of portal hypertension (VAST score ≥2) were present in 26 (36%), and there were 19 major adverse events: death (n=12), transplant (n=6), HCC (n=1). A significant relationship was found between VAST score ≥2 and major adverse events (OR=9.8, 95% CI [2.9-32.7]). After adjusting for time since Fontan, SLV, age, hemoglobin and type of failure, VAST score ≥2 remained significant (OR=9.1, 95% CI [1.4-57.6]).
Conclusion
Fontan patients with features of portal hypertension have a 9-fold increased risk for a major adverse event. Therapies targeted to manage clinical manifestations of portal hypertension, and early referral to heart transplant may help delay major adverse events. Future prospective studies are needed to confirm these findings.
Keywords: Fontan, liver disease, features of portal hypertension, congenital heart disease, adult congenital heart disease
Introduction
Over the last 40 years, the Fontan operation has become the standard of care for patients with congenital heart disease consisting of single ventricle physiology. While the operation improves oxygen saturation and short-term operative outcomes have improved substantially, the long-term complications are becoming increasing recognized. In the largest series of long-term follow up, including only the early operative survivors, 1 in 5 patients had died or received a heart transplant at 20 years post Fontan[1]. Indeed, some authors have referred to the physiology as the “failing Fontan[2].”
The mechanics of Fontan failure remain poorly understood. While clearly the failing hemodynamics are related to having a single systemic ventricle with passive blood flow through the lungs, the adult congenital patient with Fontan physiology has multi-system organ dysfunction—particularly involving the liver—that contributes to the pathophysiology[3].
Recent attention has focused on the pathology of the liver inherent to Fontan physiology that is presumably related to post-sinusoidal increased venous pressure. Numerous series have shown nodular changes and fibrosis that progress with time since the Fontan palliation[4, 5]. However, clinical markers of liver enzyme abnormalities, bilirubin, and even biopsy may not capture the extent of liver dysfunction and to date have not been correlated with clinical outcomes[6].
In this study, we examined a large group of both pediatric and adult Fontan patients to evaluate the relationship of clinical features of portal hypertension to major adverse events including hepatocellular carcinoma (HCC), need for transplant, or death.
Methods
Subjects
All children and adults palliated with a Fontan procedure and followed by Children’s Healthcare of Atlanta or Emory Adult Congenital Heart Center between 2001-2011 who had a hepatic evaluation were retrospectively reviewed (n=73). Approval was obtained from the Emory University IRB prior to data collection. Patients were screened, but not excluded, for known causes of hepatic injury (viral hepatitis or heavy alcohol use >2 drinks per day). Demographic, medical history, clinical, and imaging data at time of hepatic evaluation were collected via chart review.
Measurements
The primary outcome was a major adverse event, defined as having one of the following: death, heart transplant or listing for transplant, or HCC.
Hepatic imaging at the time of liver evaluation (MRI, CT, or abdominal ultrasound [US]) was reviewed. Data regarding nodular contour (yes or no), dilated hepatic veins (yes or no), texture (heterogenous or homogenous), splenomegaly (yes or no), and presence of varices including esophageal or gastric (yes or no) were collected. In case of discrepancy, order of imaging preference was as follows: MRI>CT>US.
Features of portal hypertension were determined using the author-developed VAST score. The score was calculated by the sum of clinical findings (Varices, Ascites, Splenomegaly, and Thrombocytopenia [platelets <150]). Varices were counted if present on any imaging modality. Ascites was considered present if seen either on imaging or clinical exam. Splenomegaly was counted if measured >13 cm in maximal diameter on any imaging modality. Scores ranged from 0 (no clinical findings) to 4 (all clinical findings present). A VAST score ≥2 was considered having features of portal hypertension.
Potential covariates of major adverse health outcomes and portal hypertension included demographics (gender, age and race), medical history, and cardiac variables. Medical history data included time since the Fontan operation (years), comorbidities (diabetes, hypertension, dyslipidemia, protein losing enteropathy, pulmonary arterial hypertension, arrhythmia), type of congenital heart disease (hypoplastic left heart syndrome, tricuspid atresia, double inlet left ventricle, pulmonary atresia/hypoplastic right heart, double outlet right ventricle, transposition of the great vessels, or other), type of Fontan (atriopulmonary, lateral tunnel, or extracardiac), and systemic ventricle (left or right).
The electrocardiogram was analyzed for rhythm and QRS duration. Echocardiographic data included the following: systemic ventricular function (normal/mild decreased vs. moderate/severely decreased), atrioventricular valve regurgitation (none/mild vs. moderate/severe), and IVC diameter (cm) measured at end exhalation. Ventricular function was determined by echocardiography using subjective assessment, Simpson’s method, or triplane three-dimensional reconstruction in multiple views.
Patients were classified as type of failure as follows: no failure (normal/mildly decreased ventricular function, no diuretics), heart failure (moderate/severely decreased ventricular function, on ≥1 diuretics) or Fontan failure (normal/mildly decreased function, on ≥1 diuretics).
Other clinical data included medications at time of liver evaluation, body mass index (BMI, kg/m2) and laboratory values (sodium, creatinine, total bilirubin, total protein, alkaline phosphatase (ALP), asparate aminotransferase (AST), alanine aminotransferase (ALT), hemoglobin, platelet count, and hepatitis serologies for A, B and C).
Statistical Methods
Differences between groups were determined using t-test and chi-square, and Fisher’s exact or Wilcoxon where appropriate. Bivariate correlations were used to determine any significant relationships between study variables and major adverse events, and between study variables and portal hypertension to determine potential confounders. Univariate and multivariate logistic regression were used to statistically evaluate the relationship between features of portal hypertension (VAST score ≥2) and major adverse events. As input to stepwise regression, variables were chosen that were either significant in univariate analysis at the p<0.20 level or chosen based on predictors of adverse events. In the final model, we included time since Fontan (years), age, and type of failure (heart failure vs. Fontan failure vs. no failure), all of which were chosen for univariate significance. Hemoglobin as a surrogate for cyanosis and systemic ventricle (left vs. right) were added to the model to account for suspected risk factors for outcomes. The data was analyzed using SAS 9.3, at an alpha level of 0.05.
Results
A total of 73 adult and pediatric Fontan patients were included in the analysis. The mean age of the cohort was 24 years (±11, range 9-54) and mean time since the Fontan operation was 16.9 years (±6.3 years). The majority were male (n=51, 70%), Caucasian (n=53, 73%), and had a systemic left ventricle (SLV, n=48, 66%). Twenty-six of the patients had a classic atriopulmonary Fontan as the primary procedure, 38 had a lateral tunnel Fontan, and 9 had an extracardiac Fontan. Six of the patients with an atriopulmonary Fontan had undergone a Fontan conversion to a lateral tunnel.
Anatomic features of the cohort were as follows: tricuspid atresia (n=27), hypoplastic left heart syndrome (n=13), double outlet right ventricle (n=10), double inlet left ventricle (n=10), pulmonary atresia/hypoplastic right heart (n=10) or other (n=3).
Of the entire cohort, 14 patients (19.2%) had varices, 25 (35.6%) had ascites, 23 (31.9%) had splenomegaly, and 32 (44.4%) had a platelet count <150K at the time of liver evaluation. Twenty-six patients (36%) had 2 or more of these findings. Nineteen patients (26%) experienced a major adverse event, none of which occurred in the pediatric cohort. Twelve patients died: 3 from congestive heart failure, 4 from Fontan failure with liver disease, 2 sudden deaths, and 3 related to surgical interventions including one patient 1 year following heart transplant, and 2 following revision to total cavopulmonary connection. A total of six patients were either listed for heart transplant or received transplant, and 1 patient developed HCC.
When compared to the 54 patients without a major adverse event, the group that experienced a major adverse event (death, need for transplant or HCC) was significantly more likely to have features consistent with portal hypertension defined as a VAST score ≥2 (OR=9.8, 95% CI [2.9-32.7], FIGURE 1). Those who suffered an event were significantly older (33.2 vs. 21.9 years, p<.0001), further out from Fontan operation (21.6 vs. 15.2 years, p<.0001) and more likely to show echocardiographic signs of dysfunction (decreased systemic ventricular function 47.4% vs. 7.7%, p=.0001) and elevated central venous pressure (IVC diameter 2.3 vs. 1.9 cm, p=.005) as compared to those without an event (TABLE 1). Adjusting for BSA, however, IVC diameter was no longer significantly related to adverse events (p=0.09). The event group had greater QRS prolongation compared to those without an event (128 vs. 105 ms, p=.006).
Figure 1. Comparison of major event by feature of portal hypertension (VAST≥2).
In univariate analysis, features of portal hypertension (VAST ≥2) were associated with a 9.8-fold increase (95% CI [2.9-32.7]) in risk of major adverse health outcomes.
Table 1.
| Variable | Overall n=73 |
No Event n=54 |
Event n=19 |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (mean, SD) | 24 (9-54) | 21.9 (9.8) | 33.2 (10.4) | <0.0001 |
| Female | 22 (30.1%) | 17 (31.5%) | 5 (26.3%) | 0.67 |
| Caucasian | 53 (72.6%) | 37 (68.5%) | 16 (84.2%) | 0.64 |
| Fontan Characteristics | ||||
| Years Post- Fontan (mean, SD) |
16.9 (6.3) | 15.2 (5.7) | 21.6 (5.5) | <0.0001 |
| Left Ventricle | 48 (65.8%) |
35 (64.8%) | 13 (68.4%) | 0.78 |
| Type of Fontan | 0.002 | |||
| Atriopulmonary | 26 (35.6%) | 13 (24.1%) | 13 (68.4%) |
|
| Lateral Tunnel | 38 (52.1%) | 32 (59.3%) | 6 (31.6%) |
|
| Extracardiac | 9 (12.3%) | 9 (16.7%) | 0 (0%) | |
| Fontan Revision | 6 (8.2%) | 5 (9.3%) | 1 (5.3%) | 1 |
| Pacemaker | 24 (32.9%) | 13 (24.1%) | 11 (57.9%) |
0.007 |
| QRS Duration msec (n=51) |
111.9 (27.7%) |
105.2 (18.5) | 128.0 (38.6) | 0.04 |
| Mod-severe decreased function (n=71) |
13 (18.3%) | 4 (7.7%) | 9 (47.4%) | <0.0001 |
| AV Regurgitation (Mod/Severe) (n=71) |
12 (16.9%) | 8 (15.4%) | 4 (21.1%) | 0.47 |
| Hepatic Imaging (MRI/CT/US) | ||||
| Nodular Contour | 40 (63.5%) | 26 (55.3%) | 14 (82.4%) | 0.05 |
| Dilated Hepatic Veins | 22 (34.9%) | 17 (36.2%) | 5 (29.4%) | 0.62 |
| Heterogeneous Texture | 56 (88.9%) | 40 (85.1%) | 16 (84.2%) | 0.67 |
AV = atrioventricular; SD = Standard deviation
Other significant variables in univariate analysis that were different between the two groups are depicted in TABLES 1-3 and included type of Fontan, presence of pacemaker, nodularity on liver imaging, medication usage, and laboratory values including sodium, creatinine, total protein, albumin, ALP. The event group was also more likely to be taking amiodarone, warfarin, ACE-inhibitors and multiple diuretics at the time of liver evaluation.
Table 3.
| Variable | Overall n=73 |
No Event n=54 |
Event n=19 |
p-value |
|---|---|---|---|---|
| Comorbidities | ||||
| Arrhythmia | 38 (52.1) | 25 (46.3) | 13 (72.2) | 0.06 |
| Hypertension | 6 (8.2) | 5 (9.3) | 1 (5.6) | 1 |
| Diabetes | 3 (4.1) | 2 (3.7) | 1 (5.6) | 0.74 |
| PLE | 7 (9.6) | 5 (9.3) | 2 (11.1) | 0.82 |
| PAH | 2 (2.7) | 1 (1.9) | 1 (5.6) | 0.44 |
| Dyslipidemia | 1 (1.4) | 0 (0) | 1 (5.6) | 0.25 |
| Heavy Alcohol Use | 1 (1.4) | 1 (1.9) | 0 (0) | 1 |
| Medications | ||||
| ACEI or ARB | 40 (55.6) | 34 (63.0) | 6 (33.3) | 0.05 |
| Beta-blocker | 33 (45.8) | 23 (42.6) | 10 (55.6) | 0.34 |
| Coumadin | 27 (37.5) | 16 (29.6) | 11 (61.1) | 0.02 |
| Amiodarone (n=41) | 9 (23.1) | 2 (9.1) | 7 (41.2) | 0.03 |
| ≥ 1 Diuretic | 36 (50.0) | 23 (42.6) | 13 (72.2) | 0.03 |
| Digoxin | 18 (25.0) | 15 (27.8) | 3 (16.7) | 0.53 |
| Lab Values* | ||||
| Sodium | 139.4 (2.9) | 139.8 (2.6) | 138.2 (3.6) | 0.04 |
| Hemoglobin | 15.2 (1.8) | 15.3 (1.6) | 14.7 (2.3) | 0.17 |
| Creatinine | .87 (.32) | .81 (.22) | 1.1 (.47) | 0.046 |
| Total Bilirubin | 1.1 (.74) | 1.0 (.76) | 1.3 (.61) | 0.14 |
| Total Protein | 7.5 (.86) | 7.6 (.76) | 6.9 (.95) | 0.003 |
| Albumin | 4.3 (.73) | 4.4 (.70) | 3.8 (.62) | 0.002 |
| Alkaline phosphatase | 141.5 (87.1) | 156.1 (91.3) | 97.9 (70.5) | 0.002 |
| AST | 35.3 (16.9) | 36.7 (14.2) | 31.0 (23.0) | 0.33 |
| ALT | 28.1 (13.7) | 27.5 (13.5) | 29.7 (14.7) | 0.57 |
| Platelets | 179.2 (70.9) |
189.7 (73.6) | 149.7 (54.4) | 0.03 |
| Hepatitis B (n=60) | 2 (2.8) | 1 (2.3) | 1 (7.1) | 0.43 |
| Hepatitis C (n=63) | 0 | 0% | 0% | 1 |
= <3% of data missing
PLE = protein losing enteropathy, PAH = pulmonary arterial hypertension
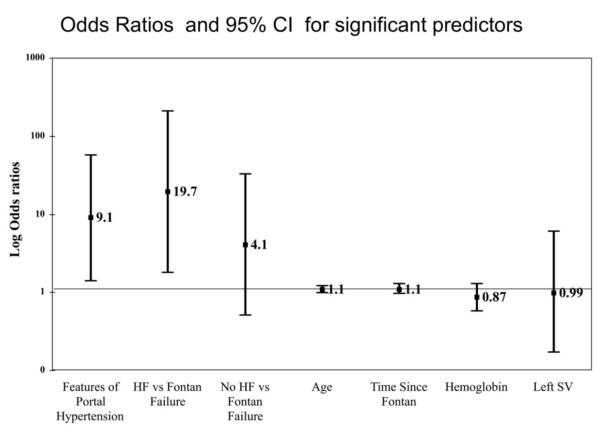
In multivariate analysis, adjusting for time since Fontan, SLV, age, hemoglobin level, and type of failure, features of portal hypertension remained significantly related to major adverse events. Patients with VAST ≥2 had 9 times the odds of experiencing an adverse event compared to those with VAST <2 (OR=9.1, 95% CI [1.4-57.6]). Type of failure was also significantly related to major adverse events. Compared to patients with Fontan failure, patients with heart failure had 19 times the odds of experiencing a major adverse event (OR=19.7, 95% CI [1.8-210.9]. The other variables tested in the model were not statistically significant FIGURE 2. The multivariate analysis was repeated excluding the two patients who died post-operatively following Fontan conversion, but this did not change the findings. VAST ≥2 remained significantly related to adverse events (OR 15.7 CI [1.8-135.1].
Figure 2. Odds Ratios and 95% CI for Multivariate Logistic Regression Analysis.
In multivariate analysis, features of portal hypertension (Portal HTN) and heart failure (HF) were significant predictors of major adverse events.
Among the groups of failure type, 7 (78%) of the 9 patients classified as heart failure with moderate to severe dysfunction experienced a major event. 25% of those with Fontan failure had a major event, and 15.8% of those patients without any form of failure experienced a major event (p=<.001) TABLE 4.
Table 4.
| Failure Type | Total n=71 |
No Event n=52 |
Event n=19 |
p-value |
|---|---|---|---|---|
| Heart Failure | 9 | 2 | 7 (78%) | <.001 |
| Fontan Failure | 24 | 18 | 6 (25%) | |
| No Failure | 38 | 32 | 6 (16%) |
Heart failure = moderate/severely decreased ventricular function, on ≥1 diuretics
Fontan failure = normal/mildly decreased function, on ≥1 diuretics
No failure = normal/mildly decreased ventricular function, no diuretics
Discussion
The Fontan operation, first described in 1971, is the final step in the surgical palliation of congenital cardiac patients with a single functional ventricle[7]. The procedure creates non-pulsatile flow via direct connection of the vena cavae to the pulmonary arteries and results in an immediate elevation in central venous (and thus hepatic venous) pressure.
Liver dysfunction among Fontan patients is prevalent and may be a significant cause of late morbidity and mortality in adults with congenital heart disease[3, 4]. To date, liver dysfunction has not been shown to correlate with clinical outcomes[6]. Among adults with Fontan physiology, non-invasive studies indicate that 57-90% show signs of liver dysfunction[8, 9]. In two invasive studies with biopsy, 17/18 and 7/12 adults had some form of cirrhosis[10, 11]. Early studies focused on laboratory data, showing mild abnormalities in coagulation profiles and liver enzymes. More recent studies have shown various radiographic changes, including surface nodularity, hepatic vein dilatation, increased paranchymal echogenecity, and the presence of collateral vessels[4, 12]. The extent of fibrosis, however, does not typically correlate with traditional liver function tests[6]. Histological and morphological changes may occur with only mildly abnormal coagulation and liver profiles and no clinical symptoms[11].
In this study, we examined a group of adult and pediatric Fontan patients referred for hepatic evaluation and determined predictors of major adverse events. As expected, on univariate analysis those with a major adverse event were more likely than controls to have moderate to severely decreased systemic ventricular function, longer QRS duration, older age at the time of event and longer duration since the time of Fontan. The group with events was sicker overall, as evidenced by the higher proportion with a pacemaker, use of Vaughn-Williams class III antiarrhythmics, and requiring diuretics. Laboratory values including sodium, creatinine, total protein, albumin and alkaline phosphatase were statistically different between groups, but were within the normal range for lab values and have limited clinical meaning.
There was a difference between the groups in the proportion of atriopulmonary Fontans, which comprised 68% of the event group as compared to 24% of the non-event group. Patients with an event were all adults and statistically older and further out from the time of Fontan surgery as compared to those with non-events, thus historically more likely to have a classic atriopulmonary Fontan. Previous work has suggested a number of potential advantages to Fontan conversion[13]. Although 6 of the patients underwent Fontan conversion, they were analyzed based on their original Fontan operation and thus no conclusions can be drawn regarding any potential advantage of conversion.
Definition of portal hypertension
We defined features of portal hypertension as having a VAST score of ≥2 of the following: Varices, Ascites, Splenomegaly, or Thrombocytopenia (each accounting for one point). This author-derived scoring system was used to address the challenge of characterizing liver disease in Fontan patients. The standard for evaluation of intrahepatic portal hypertension in patients with cirrhosis is assessment of the hepatic venous pressure gradient, where >10 mmHg is considered significant[14]. With regard to the definition of portal hypertension in the Fontan population, a gradient across the sinusoidal bed is not required to have portal hypertension[11]. Wedge pressure gradient is reflective of portal hypertension in sinusoidal disease, but not applicable in post sinusoidal hypertension (elevated hepatic venous pressures). The only true measure of portal venous pressure in that instance is direct measurement of the portal vein pressure, which is clinically not done with any regularity. Therefore surrogates were used for the clinical definition.
While other non-invasive scoring systems do exist, to our knowledge none have been used in this patient population. Each of the features of the VAST score has been associated with portal hypertension, and in our study was different between the event vs. non-event groups (while total platelet count was different between the groups, the dichotomized variable of <150 only trended toward significance; platelets <150 has been considered a marker for portal hypertension)[14]. In restricting the definition to ≥2 VAST features, we aimed to eliminate false positives.
Similar to pulmonary hypertension, which has been divided into groupings including pulmonary arterial hypertension and pulmonary venous hypertension, portal hypertension can also be grouped by anatomic level of the obstruction and may occur at multiple sites. Post-hepatic obstruction (post-sinusoidal), as occurs anatomically in the Budd-Chiari syndrome and physiologically in both right heart failure and the Fontan circulation, creates a picture of congestive hepatopathy. Long-standing congestive hepatopathy has been shown to lead to hepatic fibrosis, and ultimately cirrhosis[15]. The development of hepatic fibrosis may create intrahepatic pre-sinusoidal fibrosis, further obstructing portal venous inflow. These changes have been demonstrated in biopsy and postmortem studies of the liver in Fontan patients[10, 11]. In the late Fontan circulation in which clinical deterioration is noted with or without ventricular failure, the physiologic and neurohormonal changes that occur in the setting of elevated portal venous pressures likely contribute to circulatory dysfunction, renal hypoperfusion, and clinical deterioration.
Multivariate model
In the multivariate model, the strongest predictor of a major adverse event was heart failure as evidenced by moderate to severe dysfunction and requiring diuretics. There were only 9 patients with this degree of dysfunction, but they were 19 times more likely than patients with preserved ventricular function to experience an event.
Perhaps the more surprising finding is in regard to the influence of features of portal hypertension on major adverse events. Fontan patients with a VAST score of ≥2 had a 9-fold increased risk of experiencing a major adverse event as compared to those without. Over half of the patients with a major adverse event had preserved systolic function of the systemic ventricle, and the development of portal hypertension features may partially explain this phenomenon. Among those patients defined as Fontan failure with preserved ventricular function, 25% experienced an event. A VAST score ≥2 was a stronger predictor on multivariate analysis than age of the patient, morphologic left ventricle, or time since Fontan operation, criteria that might logically be associated with adverse outcomes in this group. These findings suggest that therapies may need to be tailored to the patient’s clinical situation and risk factors late after Fontan palliation.
The progressive liver impairment seen in many adult Fontan patients may be caused by chronic elevation of central venous pressure and hepatic ischemia related to chronic low cardiac output[2]. In non-congenital heart failure patients, cirrhotic cardiomyopathy has been recognized as a cause for heart failure associated with high cardiac output and low systemic vascular resistance[16]. While patients with cirrhotic cardiomyopathy share similarities with Fontan patients, there are important differences. Adults with Fontan physiology share a low systemic vascular resistance, but they have a relatively reduced cardiac index as compared to non-congenital patients (unpublished data). The additional insult of substantial hepatic injury could create a vicious feed-back loop and worsen cardiac function, effect pulmonary circulation (i.e. hepatopulmonary syndrome or portopulmonary hypertension), and further lower systemic vascular resistance with a limited ability to augment cardiac output. This could ultimately lead to poor perfusion of many organs, in particular the heart, kidneys, and liver.
Clinical implications
The significance of considering the physiologic consequences of elevated portal pressures in the Fontan circulation becomes relevant when considering the common occurrence of late clinical decompensation (“failing Fontan”) in the setting of normal ventricular function and “good Fontan pressures.” A better understanding of the physiologic changes that occur in the Fontan circulation will enable development of therapies to address the multifactorial physiologic changes leading to circulatory dysfunction.
In addition to better treating the late deterioration, an improved understanding the role of congestive hepatopathy and elevated portal pressures may have implications for preventing or delaying this late clinical deterioration. If elevated central venous pressure is causative of late hepatic fibrosis and cirrhosis, and features of portal hypertension are associated with major adverse events, this may have implications for the timing of the Fontan procedure. Previous work has shown in those with a Fontan physiology, one important risk factor for liver dysfunction is timing since Fontan, with risk increasing at 5 and 10 years post-operation[11]. If these late hepatic complications are based on time from surgery, and they limit life-expectancy, one could suggest delay in initial Fontan palliation when possible. Adults living today typically had the Fontan procedure as much as 10-20 years later than what occurs in contemporary clinical practice. Thus, if liver dysfunction is directly related to time since Fontan, the late morbidity and mortality in this population may begin to occur earlier, perhaps even in childhood. In our study, none of the major adverse events occurred in patients under 20 years of age. No patient less than 15 years old had features of portal hypertension, but there were 5 between the ages of 16-19 years that had a VAST score ≥2. Although time since Fontan was not significantly associated with major events in the multivariate analysis, this study may be underpowered to detect such a relationship.
The relationship of portal hypertension and poor clinical outcomes has a profound impact on how adult patients with Fontan physiology are managed. Cardiologists caring for older Fontan patients need to be aware of and screen for occult liver disease, evaluating for features of portal hypertension. To date, laboratory screening for liver enzyme abnormalities and even biopsy have not been correlated with clinical outcomes, suggesting synthetic function of the liver is relatively preserved. Given hepatic dysfunction and fibrosis after Fontan palliation, patients should be counseled to avoid hepatic toxins (e.g. alcohol) and employ preventative strategies for liver health (e.g. hepatitis vaccination). Medication may need to be tailored as the hemodynamic phenotype changes over time. For example, use of systemic vasodilators—routinely employed to treat heart failure of other etiologies—could potentially worsen circulatory dysfunction in those with advanced liver disease, potentially precipitating hepatorenal syndrome.
While cardiac transplant is a limited option for some failing adult Fontan patients, pre-operative evaluation must define the extent of liver disease. A few Fontan patients have gone on to combined heart and liver transplant, but data regarding outcomes is limited[17, 18]. Future efforts to manage this population should focus on tailoring therapy to the unique physiology in this patient population.
Study Limitations
Our study has a number of potential limitations. It is retrospective in nature, and included a selected, tertiary referral population with advanced symptoms. The results may not be as applicable to a “well” Fontan population. The study group is small, and the results and VAST score need to be validated in a larger population of Fontan patients. Features of the VAST score are often late findings in portal hypertension and thus may not detect early pathology in Fontan patients. Because of the small sample size, the multivariate model was not able to account for each of the univariate predictors of outcomes. We attempted to overcome this by creating a composite surrogate to incorporate univariate predictors by examining heart failure, Fontan failure vs. no evidence of failure.
Conclusions
In Fontan patients with advanced symptoms, those with features of portal hypertension (a VAST score ≥2) had a 9-fold increase in major adverse events including death, need for transplant, or HCC. Poor systemic ventricular function, though uncommon, was also significantly associated with events. Cardiologists need to be aware of the association of portal hypertension and related circulatory dysfunction with adverse events and should tailor medical therapy, including referral for cardiac transplantation, as appropriate.
Table 2.
| Variable | Overall n=73 |
No Event n=54 |
Event n=19 |
p-value |
|---|---|---|---|---|
| Features of portal hypertension |
26 (35.6) | 12 (22.2) | 14 (73.7) | <0.0001 |
| Varices | 14 (19.2) | 4 (7.4) | 10 (52.6) | <0.0001 |
| Ascites | 25 (35.6) | 13 (24.1) | 12 (63.2) | 0.004 |
| Splenomegaly | 23 (31.9) | 11 (20.4) | 12 (66.7) | <0.001 |
| Platelet Count < 150K | 32 (44.4%) | 21 (38.9) | 11 (57.9) | 0.18 |
Acknowledgments
Funding sources: NMM was partially funded by a T32NR012715 from the National Institute of Nursing Research and the Jonas Nursing Scholar Fund
Footnotes
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 - These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Disclosures or conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Khairy P, Fernandes SM, Mayer JE, Jr., Triedman JK, Walsh EP, Lock JE, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- [2].Rychik J. Forty years of the Fontan operation: a failed strategy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:96–100. doi: 10.1053/j.pcsu.2010.02.006. [DOI] [PubMed] [Google Scholar]
- [3].Rychik J, Veldtman G, Rand E, Russo P, Rome JJ, Krok K, et al. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol. 2012;33:1001–12. doi: 10.1007/s00246-012-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu FM, Ukomadu C, Odze RD, Valente AM, Mayer JE, Jr., Earing MG. Liver disease in the patient with Fontan circulation. Congenit Heart Dis. 2011;6:190–201. doi: 10.1111/j.1747-0803.2011.00504.x. [DOI] [PubMed] [Google Scholar]
- [5].Asrani SK, Asrani NS, Freese DK, Phillips SD, Warnes CA, Heimbach J, et al. Congenital heart disease and the liver. Hepatology. 2012;56:1160–9. doi: 10.1002/hep.25692. [DOI] [PubMed] [Google Scholar]
- [6].Guha IN, Bokhandi S, Ahmad Z, Sheron N, Cope R, Marshall C, et al. Structural and functional uncoupling of liver performance in the Fontan circulation. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.06.062. [DOI] [PubMed] [Google Scholar]
- [7].Fontan F, Mounicot FB, Baudet E, Simonneau J, Gordo J, Gouffrant JM. “Correction” of tricuspid atresia. 2 cases “corrected” using a new surgical technic. Ann Chir Thorac Cardiovasc. 1971;10:39–47. [PubMed] [Google Scholar]
- [8].Camposilvan S, Milanesi O, Stellin G, Pettenazzo A, Zancan L, D’Antiga L. Liver and cardiac function in the long term after Fontan operation. Ann Thorac Surg. 2008;86:177–82. doi: 10.1016/j.athoracsur.2008.03.077. [DOI] [PubMed] [Google Scholar]
- [9].Baek JS, Bae EJ, Ko JS, Kim GB, Kwon BS, Lee SY, et al. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart. 2010;96:1750–5. doi: 10.1136/hrt.2010.201772. [DOI] [PubMed] [Google Scholar]
- [10].Kendall TJ, Stedman B, Hacking N, Haw M, Vettukattill JJ, Salmon AP, et al. Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol. 2008;61:504–8. doi: 10.1136/jcp.2007.052365. [DOI] [PubMed] [Google Scholar]
- [11].Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward-Sadler H, et al. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–84. doi: 10.1136/hrt.2006.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bulut OP, Romero R, Mahle WT, McConnell M, Braithwaite K, Shehata BM, et al. Magnetic Resonance Imaging Identifies Unsuspected Liver Abnormalities in Patients after the Fontan Procedure. The Journal of pediatrics. 2013 doi: 10.1016/j.jpeds.2012.12.071. [DOI] [PubMed] [Google Scholar]
- [13].Dechert BE, Deal BJ. An integrated approach to the care of adult patients with prior atriopulmonary Fontan surgery. J Pediatr Health Care. 2008;22:246–53. doi: 10.1016/j.pedhc.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [14].Snowdon VK, Guha N, Fallowfield JA. Noninvasive evaluation of portal hypertension: emerging tools and techniques. Int J Hepatol. 2012;2012:691089. doi: 10.1155/2012/691089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schwartz MC, Sullivan LM, Glatz AC, Rand E, Russo P, Goldberg DJ, et al. Portal and Sinusoidal Fibrosis are Common on Liver Biopsy After Fontan Surgery. Pediatric cardiology. 2013;34:135–42. doi: 10.1007/s00246-012-0402-9. [DOI] [PubMed] [Google Scholar]
- [16].Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassell BW, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56:539–49. doi: 10.1016/j.jacc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- [17].Bernstein D, Naftel D, Chin C, Addonizio LJ, Gamberg P, Blume ED, et al. Outcome of listing for cardiac transplantation for failed Fontan: a multi-institutional study. Circulation. 2006;114:273–80. doi: 10.1161/CIRCULATIONAHA.105.548016. [DOI] [PubMed] [Google Scholar]
- [18].Horai T, Bhama JK, Fontes PA, Toyoda Y. Combined heart and liver transplantation in a patient with situs ambiguous. Ann Thorac Surg. 2011;91:600–1. doi: 10.1016/j.athoracsur.2010.07.078. [DOI] [PubMed] [Google Scholar]