Abstract
The simplest expression of episodic memory is the experience of familiarity, the isolated recognition that something has been encountered previously. Brain structures of the medial temporal lobe (MTL) make essential contributions to episodic memory, but the distinct contributions from each MTL structure to familiarity are debatable. Here we used specialized tests to assess recognition impairments and their relationship to MTL integrity in people with amnestic mild cognitive impairment (aMCI, n=19), people with probable Alzheimer's disease (AD; n=10), and age-matched individuals without any neurological disorder (n=20). Recognition of previously presented silhouette objects was tested in two formats—forced-choice recognition with four concurrent choices (one target and three foils) and yes/no recognition with individually presented targets and foils. Every foil was extremely similar to a corresponding target, such that forced-choice recognition could be based on differential familiarity among the choices, whereas yes/no recognition necessitated additional memory and decision factors. Only yes/no recognition was impaired in the aMCI group, whereas both forced-choice and yes/no recognition were impaired in the AD group. Magnetic resonance imaging showed differential brain atrophy, as MTL volume was reduced in the AD group but not in the aMCI group. Pulsed arterial spin-labeled scans demonstrated that MTL blood flow was abnormally increased in aMCI, which could indicate physiological dysfunction prior to the emergence of significant atrophy. Regression analyses with data from all patients revealed that regional patterns of MTL integrity were differentially related to forced-choice and yes/no recognition. Smaller perirhinal cortex volume was associated with lower forced-choice recognition accuracy, but not with lower yes/no recognition accuracy. Instead, smaller hippocampal volumes were associated with lower yes/no recognition accuracy. In sum, familiarity memory can be specifically assessed using the forced-choice recognition test, it declines later than other MTL-dependent memory functions as AD progresses, and it has distinct anatomical substrates.
Keywords: familiarity, episodic memory, recognition memory, amnestic mild cognitive impairment, Alzheimer's disease
1. Introduction
Episodic memory, the ability to consciously recognize and recall previously experienced events, critically depends on the medial temporal lobe (Scoville & Milner, 1957). These MTL regions include the hippocampus and adjacent cortical structures—entorhinal cortex and perirhinal cortex at the anterior end and parahippocampal cortex at the posterior end. The MTL is the most prominently affected brain area in Alzheimer's disease and first shows signs of disruption in the transitional stage known as mild cognitive impairment (MCI). Deficits at the MCI stage can impact one or more cognitive domains, often including episodic memory (amnestic subtype, aMCI). Patients with aMCI experience episodic memory deficits greater than those expected with healthy aging, but their cognitive deficits do not meet criteria for dementia (Petersen, 2007). Many individuals diagnosed with MCI never develop Alzheimer's disease, but all patients with Alzheimer's disease pass through an MCI stage.
In both AD and aMCI, histopathological studies have revealed increased neurofibrillary tangle density and neuron loss in MTL regions (Braak & Braak, 1991; Delacourte et al., 1999; Gomez-Isla et al., 1996; Guillozet, Weintraub, Mash, & Mesulam, 2003; Hyman, Van Hoesen, Damasio, & Barnes, 1984; Kordower et al., 2001; Mesulam, 1999), and various antemortem neuroimaging methods have indicated atrophy and reduced function in the MTL (Dickerson et al., 2001; Du et al., 2001; Jack et al., 2002; Kesslak, Nalcioglu, & Cotman, 1991; Killiany et al., 1993; Pennanen et al., 2004; Seab et al., 1988). Whereas pathological signs in postmortem brain tissue are needed for the formal diagnosis of Alzheimer's disease, typically there is no confirmation of pathology in patients under study — so the diagnosis given is “probable Alzheimer's disease” (here abbreviated as AD). Pathological and imaging studies have generally documented a greater extent of MTL damage in AD compared with aMCI.
A common assumption is that the progressive episodic memory deficits in aMCI and AD primarily arise from progressive MTL dysfunction. Yet, current theories suggest that episodic memory is not a unitary phenomenon. Thus, AD-related pathology may disrupt some phenomena more than others. The distinction between recollection and familiarity (Jacoby, 1991; Mandler, 1980; Yonelinas, 2002) may be particularly relevant. Recollection refers to the full-blown experience of recalling attended information and its contextual setting. Familiarity refers to the unsubstantiated sense that something has been experienced previously, without remembering associated contextual details. There is general agreement that both recollection and familiarity are disrupted in AD patients (e.g., Smith & Knight, 2002). In aMCI patients, recollection is typically disrupted but results have been mixed with regard to familiarity. Some studies reported preserved familiarity in aMCI (Anderson et al., 2008; Hudon, Belleville, & Gauthier, 2009; Serra et al., 2010; Westerberg et al., 2006), whereas others reported impaired familiarity in aMCI (Ally, Gold, & Budson, 2009; Wolk, et al., 2011; Wolk, Signoff, & Dekosky, 2008). Our aim is to further examine how aMCI and AD pathology may impact familiarity in unique ways.
A pervasive hypothesis common to many current memory models is that a significant contribution from the hippocampus is not necessary for familiarity (Aggleton & Brown, 1999; Davachi, 2006; Diana, Yonelinas, & Ranganath, 2007; Montaldi & Mayes, 2010; Norman & O'Reilly, 2003; Shimamura, 2010). Consistent with these theories, several studies have shown intact item recognition despite impaired recall in neurological patients with circumscribed hippocampal damage (Aggleton & Brown, 1999; Holdstock et al., 2002; Mayes, Holdstock, Isaac, Hunkin, & Roberts, 2002; Vargha-Khadem et al., 1997; Yonelinas et al., 2002), and some of these have confirmed that item familiarity was intact (see Montaldi & Mayes, 2010). Additionally, in fMRI studies with young healthy adults, a lack of apparent hippocampal activity but robust changes in perirhinal activity have been associated with item familiarity (Davachi, Mitchell, & Wagner, 2003; Montaldi, Spencer, Roberts, & Mayes, 2006; Ranganath, Heller, Cohen, Brozinsky, & Rissman, 2005; Staresina & Davachi, 2008). There is general agreement across these models that perirhinal cortex is sufficient to support item familiarity, whereas the role that entorhinal cortex and parahippocampal cortex may play in familiarity is somewhat unclear. Some investigators have speculated that parahippocampal cortex may be involved in contextual representations (Davachi, 2006; Diana et al., 2007), and Montaldi and Mayes (2010) have suggested it may mediate familiarity for context.
Nonetheless, an alternative view is that functional dissociations between MTL regions are not so clear-cut, and that a hippocampal contribution to familiarity can be operative (Smith, Wixted, and Squire, 2011; Song, Wixted, Hopkins, & Squire, 2011). In patients with circumscribed hippocampal damage, for example, recall and recognition were similarly impaired (Manns, Hopkins, Reed, Kitchener, & Squire, 2003; Wixted & Squire, 2004). In fMRI experiments, perirhinal cortex has been implicated in inter-item associative recognition, which presumably cannot be completed based on familiarity alone (Duzel et al., 2003; Kirwan & Stark, 2004; Tendolkar et al., 2007). Furthermore, Squire and colleagues argue that methods that purport to separate familiarity from other memory expressions fail to avoid confounding differences in memory strength (Squire, Wixted, & Clark, 2007). This view thus acknowledges possibilities for functional heterogeneity across MTL regions, but it argues against the strong position that familiarity memory can be highly localized to perirhinal cortex. These arguments underscore the fact that a key challenge for this research is in measuring valid deficits in familiarity memory independently from allied memory functions.
Important advantages for interpreting memory dysfunction can be achieved when the number of available strategies people can use to reach an accurate memory decision is small. Holdstock and colleagues (2002) took advantage of this view by using two recognition tests in which foils were highly similar to studied objects. One test entailed a four-alternative forced-choice format wherein the participant attempted to select the studied object from among four highly similar objects (Figure 1). The other test required standard yes/no decisions for studied objects and their similar foils, with one object presented at a time. Responding on the forced-choice but not the yes/no test can primarily rely on familiarity. For both the forced-choice and yes/no formats, recollecting conceptual information is unhelpful, given the high similarity among a target and its corresponding foils. For example, remembering a verbal label for a studied object will not yield accurate target-foil discrimination, nor will remembering contextual features from the study episode. Recollecting a specific visual feature can be helpful, but the large overlap in features present between the foils and corresponding targets makes it very difficult to recollect the critically distinguishing features. In the forced-choice format, when targets are grouped with their corresponding foils, recollecting distinguishing features may occasionally be effective, but a dominant strategy could be to determine the familiarity of each of the four highly similar choices and then select the one most familiar object. Familiarity levels can vary greatly across the set of all targets, but for each set of four choices in the forced-choice test (a target and three foils) familiarity levels will be very close. Accordingly, the strategy of selecting the most familiar object will be effective because the studied object is likely to be more familiar than the foil objects (Norman & O'Reilly, 2003). Such relative-memory comparisons are not easily achieved in a yes/no format, given that several studied objects and their corresponding foils are presented one at a time in a random order. Several factors make it difficult to determine a criterion above which positive responses should be given. When studied items have markedly different familiarity levels, some foils will be more familiar than some studied items. Also, prior test trials may influence familiarity on subsequent test trials with the same object class. Under these conditions, reliance on familiarity will yield performance that is often no better than chance. Holdstock et al. (2002) found that a patient with circumscribed hippocampal damage was impaired at yes/no but not forced-choice recognition using these special procedures, suggesting that familiarity was intact in this patient.
Figure 1.
Stimuli used for testing recognition. This is an example of one forced-choice recognition test trial. Only one of the four highly similar objects was previously studied.
Results from young healthy adults tested with the same forced-choice and yes/no tests used by Holdstock et al. (2002) support the view that forced-choice but not yes/no recognition can be based on familiarity (Migo, Montaldi, Norman, Quamme, & Mayes, 2009). In one condition, participants were trained to make test responses using a modified version of the remember-know procedure (Montaldi et al., 2006; Rajaram, 1993), such that know responses indicated that their answers were based only on familiarity. In a second condition, participants were not provided with any special test instructions, allowing responses to be based on any available strategy. The results showed that forced-choice recognition based primarily on familiarity in the former condition was equivalent to that in the condition in which no special instructions were given. On the other hand, yes/no recognition based primarily on familiarity was worse than when no special instructions were given. Therefore, relative familiarity comparisons were an effective strategy in the forced-choice but not the yes/no recognition test. Furthermore, the two test formats were found to be matched in difficulty in a separate group of control participants (Holdstock et al., 2002). Thus, although familiarity can readily support normal recognition in a similar-foils forced-choice test, it is not sufficient in the yes/no recognition test.
Previously we administered the same forced-choice and yes/no recognition tests to 8 aMCI patients, 8 AD patients, and 8 healthy older individuals (Westerberg et al., 2006). The aMCI patients were significantly impaired on standardized memory tests and on yes/no recognition, but not on forced-choice recognition. AD patients exhibited recognition impairments with either format. These findings suggest that recognition decisions based on familiarity may arise from neural mechanisms dissociable from those that underlie expressions of recognition supported by recollection and more complex decision processes.
In the present experiment, we tested forced-choice and yes/no recognition with highly similar targets and foils in aMCI patients, AD patients, and healthy older adults. Our goals were to verify the memory findings from our earlier study (Westerberg et al., 2006), and to assess relationships between MTL integrity and relative performance on the two different recognition tasks. We obtained two measures of MTL integrity, brain volume and blood flow. We assessed MTL volume by tracing the outline of each MTL structure on high-resolution structural MRI scans. Using this technique, MTL volume loss has been well documented in both AD and aMCI (e.g., Dickerson, et al., 2001; Du, et al., 2001). Accordingly, we expected moderate atrophy in aMCI patients and severe atrophy in AD patients. We obtained blood-flow measures for each MTL region using pulsed arterial spin-labeled (PASL) perfusion imaging (Golay, Hendrise, & Lim, 2004; Kim, 1995; Kwong et al., 1995; Parkes, Rashid, Chard, & Tofts, 2004). This relatively new method is preferable to traditional perfusion assessments, as it allows measurement of blood flow without the need for a radioactive tracer. Increased regional cerebral blood flow has been reported in the hippocampus using PASL perfusion in aMCI patients (Dai et al., 2009), although decreased perfusion in nearby regions has also been reported (Chao et al., 2010; Johnson et al., 2005). Perfusion in MTL regions is typically reduced in AD patients (Asllani et al., 2008; Bozzao, Floris, Baviera, Apruzzese, & Simonetti, 2001; Duara et al., 1986; Kogure et al., 2000; but see Alsop, Casement, de Bazelaire, Fong, & Press, 2008).
Given the emphasis on perirhinal cortex in many current theories of MTL contributions to episodic memory, one prediction is that perirhinal integrity will be a better predictor of forced-choice performance than hippocampal integrity. Consistent with this prediction, a recent study showed that the volume of MTL cortical regions (including entorhinal, perirhinal, and parahippocampal cortices), rather than the hippocampus, predicted familiarity memory in older adults, aMCI patients, and early AD patients (Wolk, Dunfee, Dickerson, Aizenstein, & DeKosky, 2011). However, familiarity was measured using a type of process-dissociation computation soundly criticized in the literature (e.g., Wixted, 2007). In another experiment, recognition based largely on familiarity correlated more strongly with entorhinal volume than with hippocampal volume in healthy older adults, although volumes of other MTL regions were not assessed (Yonelinas et al., 2007). To our knowledge, correlations between familiarity and MTL blood flow have not been reported previously, and none of the prior studies used a test as exquisitely sensitive to relative familiarity as the similar-foils forced-choice recognition test.
Beyond the MTL, dorsolateral prefrontal cortex (DLPFC) has also been implicated in episodic memory in both the neuroimaging (Fletcher & Henson, 2001) and neuropsychological literatures (Ranganath & Knight, 2003). It is thought that this region may influence memory judgments through monitoring of retrieved information (Henson, Rugg, Shallice, & Dolan, 2000; Henson, Shallice, & Dolan, 1999; Rugg, Fletcher, Chua, & Dolan, 1999; Rugg, Henson, & Robb, 2003) or through the flexible engagement of retrieval processes based on task demands (Ranganath, Heller, & Wilding, 2007). Given reports of possible damage and dysfunction in this region in aMCI and AD (e.g., Buckner et al., 2005; Chang et al., 2010), DLPFC volumes were measured to assess possible relationships with recognition.
2. Method
2.1 Participants
We recruited 10 AD patients (4 male), 20 aMCI patients (6 male), and 20 healthy controls (5 male) from the Northwestern University Alzheimer's Disease Center memory disorders research clinic. All participants received monetary compensation for participation. Data from one aMCI patient were excluded after a tumor was discovered on her MRI scan, leaving 19 aMCI patients in the aMCI group. The aMCI group did not differ from controls in age (aMCI mean = 73.6, control mean = 74.6, p=.7) or education level (aMCI mean = 15.4 years, control mean = 15.7 years, p=.9), nor did the AD group (AD mean age = 75.0, p=.9; AD mean education = 13.9 years, p=.2). The aMCI group and the AD group also did not differ in either age (p=.5) or education level (p=.2). We used t-tests rather than ANOVA here and in other analyses reported below because our primary hypotheses concerned pairwise group differences. Exclusion criteria included history of central neurological disease, DSM-IV criteria for major psychiatric disorder, alcohol or substance abuse, serious medical illness (thyroid disorder; renal, hepatic, cardiac, or pulmonary insufficiency; unstable diabetes; uncontrolled high blood pressure; cancer), and chronic psychoactive drug use.
AD patients met DSM-IV criteria for dementia and research diagnostic criteria for probable AD (McKhann et al., 1984). Diagnosis of aMCI followed current guidelines (Petersen, 2004). None of the aMCI patients showed impairments in daily living activities as assessed with the Functional Assessment Questionnaire (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982) and the Informant Questionnaire on Cognitive Decline in the Elderly (Jorm, 1994), nor did any aMCI patient reach clinical criteria for dementia. Fifteen of the aMCI patients had scores of 1.5 or more standard deviations below the mean for individuals of comparable age, gender, and education level in neuropsychological tests of declarative memory. Ten of these 15 also showed impairment in other cognitive domains (amnestic MCI multiple domain; Petersen, 2007). The four remaining aMCI patients did not show objective cognitive impairments but were considered aMCI based on subjective memory complaints and clinical assessment; prior reports suggest that such individuals may show similar patterns of medial temporal damage consistent with incipient AD (Dickerson et al., 2001). Table 1 shows mean neuropsychological test scores for each participant group.
Table 1.
Average scores from neuropsychological testing for each group.
| Control | aMCI | AD | |
|---|---|---|---|
| MMSE (max=30) (Folstein, Folstein, & McHugh, 1975) | 29.1 | 27.6 | 18.8 |
| CERAD category fluency (Morris et al., 1989) | 21.4 | 18.7 | 9.8 |
| Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983) | 95% | 88% | 74% |
| Trail Making A (max=150 s) (Reitan, 1992) | 33.6 s | 45.6 s | 97.7 s |
| Trail Making B (max=300 s) (Reitan, 1992) | 71.1 s | 134.4 s | N/A |
| WMS-R Logical Memory II (Wechsler, 1987) | 46% | 33% | 5% |
Note. Max = maximum score
2.2 Procedure
When participants arrived at the imaging facility, informed consent was obtained and participants were screened for MRI safety. Participants were then taken to a quiet room where two memory tests were completed. Immediately following testing, participants received a series of MRI scans.
2.3 Memory Tests
2.3.1 Stimuli
We used a set of silhouette images of 24 common object classes (half living, half nonliving). Each object class contained four highly similar versions of a nameable object, as shown in Figure 1. The object classes were divided into two sets of 12. A target object was randomly selected from each object class, and the remaining three objects within a class served as foils. Previous testing ensured that the similarity between objects within each class did not differ between the two sets (Holdstock et al., 2002).
2.3.2 Procedure
Participants completed a yes/no recognition test and a forced-choice recognition test. The order of tests and the set of objects used for each test were counterbalanced across participants. Each test comprised a learning phase, a 1-min break wherein participants completed simple arithmetic problems, and a recognition phase.
The learning phase was identical for the yes/no and forced-choice tests. The 12 targets appeared evenly spaced on two pages. A cardboard mask with a viewing window controlled by the experimenter ensured that participants viewed one target at a time for 3 s per viewing. First, participants viewed each target while verbally responding whether it was natural or man-made. Then, during a second presentation of each target, participants remained silent but were instructed to study the details of each object.
For the yes/no test, participants viewed objects on small cards presented one at a time in a random order. They were asked to respond “yes” if the object was identical to an object that was studied during the learning phase and “no” if the object was not. Test objects included all 12 targets and the 36 corresponding foils. Each foil was presented once. Four targets were presented one time, four other targets were presented two times, and the remaining four targets were presented three times; these additional presentations were included to minimize the usefulness of basing a decision on responses made earlier in the test. Only responses from the first presentation of a target were included in analyses.
For the forced-choice test, participants viewed a page for each of the 12 trials containing a target and its three corresponding foils (as in Figure 1). Participants were instructed to point to the one object they had previously studied. Trials were randomly ordered and the location of the target on each trial was randomly assigned.
2.4 Imaging
MRI data were collected on a Siemens Trio 3T scanner at the Northwestern University Feinberg School of Medicine. Two separate scanning protocols were implemented to achieve a high-resolution structural image and an ASL perfusion image. The high-resolution structural scan was collected first, using a 3D MPRAGE T1-weighted sequence (176 axial slices, voxel size = 1 × 1 × 1 mm3). Quantitative PASL data were acquired using PICORE (Wong, Buxton, & Frank, 1997) variant with QUIPSS II (Wong, Buxton, & Frank, 1998) modification, which improves accuracy of perfusion quantification. Alternating control and tag images were acquired using echo-planar imaging with the following parameters: echo time = 23ms, repetition time = 2s, voxel size = 3.1 × 3.1 × 5 mm3. Five slices were prescribed to cover the MTL region. A total of fifty pairs of control and tag images were acquired in each PASL scan. An additional single-shot EPI scan centered at the ventricles was acquired after a 30-s delay to provide an estimate for the equilibrium magnetization of cerebral spinal fluid, which is required for PASL quantification.
2.4.1 Volume Analyses
Medial temporal regions of interest (ROIs) were drawn on the high-resolution structural scans using AFNI software (Cox, 1996), and included the hippocampus and entorhinal, perirhinal, and parahippocampal cortices bilaterally. Prior to drawing, the images were re-sampled to a coronal slice thickness of 1.6 mm and re-oriented perpendicularly to the long-axis of the hippocampus, as recommended for optimally locating the rostral edge of the entorhinal cortex (Goncharova, Dickerson, Stoub, & deToledo-Morrell, 2001). Boundaries of all regions were defined based on anatomical landmarks described elsewhere (Insausti et al., 1998) by a single rater who was blinded to group status (C.W.). To minimize operator error and to avoid confusion in the entorhinal/perirhinal boundary due to variability in the depth of the collateral sulcus, the lateral border of the entorhinal cortex stopped at the medial edge of the collateral sulcus, and the perirhinal cortex began at the fundus of the collateral sulcus. This method for defining the entorhinal cortex was initially described by Goncharova and colleagues (2001) and was shown to yield highly similar estimates of entorhinal volume to the method described by Insausti and colleagues (2001) in which collateral sulcus depth was taken into account.
To ensure reliability of the measurements, a second rater (J.C.) independently defined each MTL region in 3 controls, 3 aMCI patients, and 2 AD patients, randomly selected. The second rater was also blinded to group status. Intraclass correlation coefficients (ICCs) were subsequently calculated [ICC(3); Shrout & Fleiss, 1979], and revealed high agreement across all regions (right hippocampus=.98, left hippocampus=.97, right entorhinal=.88, left entorhinal=.91, right perirhinal=.84, left perirhinal=.85, right parahippocampal=.92, left parahippocampal=.90).
Volumes of the dorsolateral prefrontal cortex (DLPFC) and calcarine cortex were also measured. Calcarine cortex volume was measured to confirm the regional specificity of correlations with memory found in other brain regions, as this region does not exhibit pathological changes until the advanced stages of AD (Braak & Braak, 1991). For these ROIs, images were re-sampled to a coronal slice thickness of 1.5 mm and oriented perpendicularly to the plane passing through the anterior and posterior commissures and boundaries of each region were defined based on previously published reports (Raz et al., 1997; 2004). The DLPFC included the gray matter from the most dorsomedial point of the superior frontal gyrus to the dorsal edge of the lateral occipital sulcus, on slices comprising the rostral 40% of the distance between the tip of the frontal pole and the genu of the corpus callosum. The calcarine cortex included the gray matter of the calcarine sulcus, appearing in the rostral 50% of the distance between the midpoint of the vermis and the occipital pole.
To account for individual differences in head size, all ROIs were normalized by dividing the raw volume of each ROI (mm3) by intracranial volume (mm3) and multiplying by mean intracranial volume (mm3) across all participants. Intracranial volume was measured by tracing the inner table of the cranium on 5-mm sagittal slices across the entire brain (Stoub et al., 2006), and did not differ across groups (control vs. aMCI patients, p=.9; control vs. AD, p=.5; aMCI vs. AD, p=.5).
2.4.2 Perfusion Analyses
To obtain medial temporal perfusion measures, the PASL timeseries was motion corrected to the first image using a six-parameter rigid body spatial transformation. Pairwise subtraction between control and tag images was used to isolate the perfusion signal. Difference images were averaged over time to improve signal to noise ratio, and converted to physiological units of ml/100g/min using a previously published single blood compartment model (Buxton, 2005). The medial temporal regions defined for the volume analysis on the T1 images were then co-registered with the perfusion maps. The MTL regions were then used as a mask to extract mean perfusion values for each ROI.
3. Results
3.1 Memory Performance
The expected impairments in recognition memory were clearly observed across mean group scores (hit rates and false-alarm rates), as shown in Table 2. Accuracy was highest in the control group, intermediate in the aMCI group, and lowest in the AD group. To compare performance across yes/no and forced-choice tests, hit and false-alarm rates were used to estimate recognition sensitivity (d′) on each test for each participant (Macmillan & Creelman, 2005). All proportions were corrected by adding 0.5 to each frequency and dividing by N+1, where N = number of trials, to avoid complications with transforming extreme values (0, 1) to z-space.
Table 2.
Hit and false-alarm rates and recognition sensitivity estimates (d′) on the yes/no and forced-choice recognition tests for controls, aMCI patients, and AD patients with standard error of the means in parentheses.
| Yes/No | Forced-choice | ||||
|---|---|---|---|---|---|
| Group | Hits | False Alarms | d ′ | Hits | d ′ |
| Control | .83 (.03) | .40 (.04) | 1.21 (.14) | .63 (.04) | 1.27 (.13) |
| aMCI | .78 (.03) | .53 (.03) | .69 (.10) | .56 (.04) | 1.02 (.11) |
| AD | .82 (.06) | .69 (.07) | .36 (.21) | .28 (.04) | .12 (.15) |
Sensitivity values were submitted to a 2 × 3 ANOVA, with test format as the within-subjects variable (yes/no, forced-choice) and participant group as the between-subjects variable (control, aMCI, AD). There was a significant main effect of group, F(2,46)=16.2, p<.01, as controls (1.2) performed better than aMCI patients (0.86), and aMCI patients performed better than AD patients (0.24). The main effect of format was not significant, p =.6, but the group × format interaction trended near significance, F(2,46)=2.7, p =.08, suggesting that performance declines for aMCI and AD patients were not equivalent for the two test formats, as was observed in our previous study using the same paradigm (Westerberg et al., 2006). Planned pairwise comparisons between groups confirmed that aMCI patients were impaired relative to controls on the yes-no test [t(37)= 3.0, p<.05] but not on the forced-choice test (p=.2), whereas AD patients were impaired on both tests relative to controls [yes-no: t(28)= 3.2, p<.01; forced-choice: t(37)= 5.5, p<.001]. AD patients performed significantly worse than aMCI patients on the forced-choice test [t(27)= 4.5, p<.001] but not on the yes/no test (p=.2). Indeed, these patterns of group differences paralleled those we previously observed (Westerberg et al., 2006).
To confirm that the d′ transformation did not distort the pattern of forced-choice results, a hit-rate analysis was conducted. As expected, hit rate in the forced-choice test differed across the three groups [F(2,46)=15.8, p <.001], was significantly lower in AD patients relative to controls [t(28)= 5.7, p<.001] and relative to aMCI patients [t(27)= 4.8, p<.001], and did not differ between aMCI patients and controls (p=.2).
As the aMCI group included four individuals who received the diagnosis based on memory complaints and clinical assessment, without objective memory impairments relative to age-based norms, additional d′ analyses were conducted to characterize the group excluding these individuals. For the yes/no test, results replicated those from the full aMCI group. Yes/no d′ was impaired relative to that in controls [t(34)= 2.8, p<.01] and not significantly different from that in AD patients (p=.3). However, forced-choice d′ was impaired relative to controls [t(34)= 2.0, p<.05], suggesting that preserved forced-choice recognition in aMCI patients in our previous study (Westerberg et al., 2006) and in the analysis with the full aMCI group may be primarily driven by individuals at the earliest stages of the disease. Nonetheless, forced-choice d′ in the subgroup of 15 aMCI patients was still superior to that in AD patients [t(14)= 4.2, p<.001].
As the 15 individuals in the aMCI group who exhibited objective memory impairments included a mix of multiple- and single-domain patients, additional analyses were completed to determine if patterns of memory performance differed based on this factor. Neither forced-choice nor yes/no recognition differed between the single- and multiple-domain patients (p>.1 and p>.2, respectively), although it should be noted that only 5 patients were classified as single domain. A larger sample size is likely necessary to determine if any important differences exist between the two patient types.
Memory deficits on the yes/no test were also examined via recognition bias (c), hit rate, and false-alarm rate (Table 2). All participant groups exhibited a bias to respond “old” (–.32 for control group, –.42 for aMCI group, and –.81 for AD group). Pairwise group differences were nonsignificant [controls vs. aMCI, p=.4; aMCI vs. AD, p=.1; control vs. AD, t(28)= 2.0, p=.06]. Hit rates were high in all participants and did not differ significantly across groups (p values >.6). On the other hand, false-alarm rates did differ across groups [F(2,46)= 10.9, p <.001]. False-alarm rates were higher in AD patients than controls [t(28)= 3.3, p<.01] and higher in aMCI patients than controls [t(37)= 2.9, p<.05]. AD patients had a marginally higher false-alarm rate than aMCI patients [t(27)= 1.6, p=.06]. Thus, in the yes/no test the most sensitive measure of patients’ memory decline relative to controls was their higher tendency to false alarm. This outcome makes sense in light of the high hit rates and strong bias to respond “old.” Patients may have had difficulty retaining detailed perceptual information about the studied objects, and the greater the difficulty the greater the reliance on general object features, producing more false alarms for similar foils.
3.2 Volume
3.2.1 Medial Temporal Lobe
Normalized ROI volumes for all medial temporal regions are depicted in Figure 2. Volume reductions were clearly evident in the AD group but not in the aMCI group. Volumes were collapsed across right and left hemispheres, as preliminary analyses did not reveal noteworthy changes to the main patterns of results based on hemisphere, and right and left total MTL volumes did not significantly differ across all participants (p values >.8).
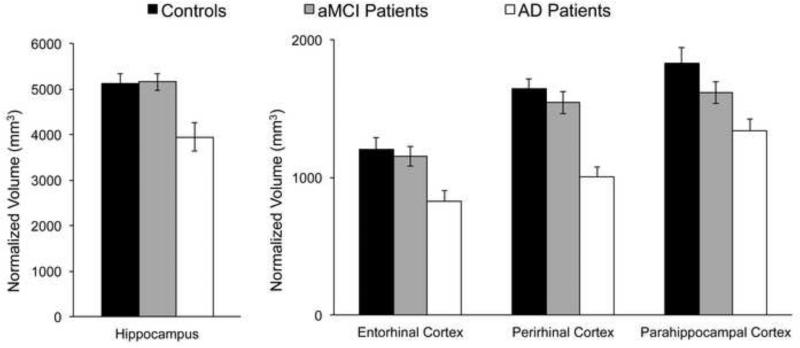
Figure 2.
Volume measurements for each medial temporal region of interest. Results normalized for individual variations in intracranial volume are shown separately for the control group (n=20), the amnestic Mild Cognitive Impairment group (aMCI, n=19), and the probable Alzheimer's disease group (AD, n=10).
To analyze group effects, total MTL volume was compared across groups. Total MTL volume in AD patients was significantly smaller than in controls [t(28)=4.6, p<.001] and than in aMCI patients [t(27)=4.0, p<.001]. However, aMCI and control volumes did not significantly differ (p=.3). Within the aMCI group, total MTL volume did not differ between multiple- and single-domain patients (p>.4), nor did volumes of any individual MTL regions (p values >.3).
To uncover regional patterns of atrophy, percent volume loss measures were computed for each patient by dividing each volume by the mean control volume, and converting the result into percentage loss (or gain). For the aMCI group, volume loss was significant only in the parahippocampal cortex [12%, t(18)=2.4, p<.05; all other regions p values>.2]. For the AD group, volume loss was significant in the hippocampus [16%, t(9)=3.3, p<.005], perirhinal [28%, t(9)=8.6, p<.001], entorhinal [21%, t(9)=−4.5, p<.001], and parahippocampal regions [19%, t(9)=5.5, p<.001]. In summary, volume loss in aMCI was only apparent in the parahippocampal cortex, whereas volume loss in AD was prevalent in all MTL regions.
3.2.2 Other brain regions
DLPFC measures were numerically smaller in aMCI patients relative to controls (15,490 mm3 vs. 15,800 mm3 respectively), but this difference did not approach significance (p=.6). On the other hand, AD patients showed significant DLPFC volume loss (13,412 mm3) relative to both controls [t(28)=3.5, p<.01] and aMCI patients [t(27)=2.7, p<.05]. As expected, calcarine cortex volume was not reduced in either aMCI patients or AD patients relative to controls (2922mm3, 3287mm3, and 3127 mm3, respectively; p values >.4), nor did calcarine cortex differ between the two patient groups (p=.2).
3.3 Perfusion
Mean results are shown in Figure 3 for the aMCI and control groups. Perfusion data were not analyzed for AD patients, as seven out of the ten were unable to tolerate remaining in the scanner for the additional time necessary to obtain perfusion measures. Technical difficulties disrupted image acquisition in one control participant and in 3 aMCI patients, leaving final group sizes of n=19 controls and n=16 aMCI patients. Data from left and right hemispheres were collapsed, as preliminary analyses indicated no significant differences to the main pattern of results based on hemisphere.
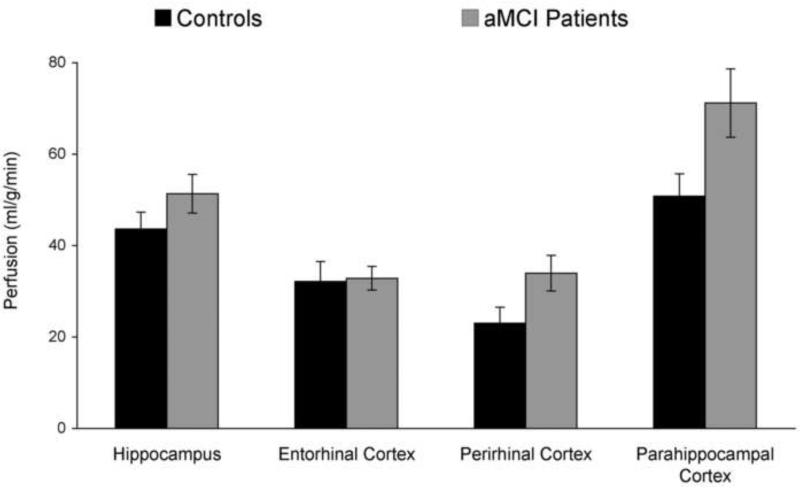
Figure 3.
Perfusion estimates for each medial temporal region for the control group and the aMCI group.
Averaged across all MTL regions, aMCI patients showed higher perfusion rates than controls [47.3 vs. 37.4 ml/g/min, respectively; t(33)=2.1, p<.05]. An examination of individual MTL regions indicated that perfusion rates were greater for aMCI patients than for controls in parahippocampal cortex [t(33)=2.3, p<.05] and in perirhinal cortex [t(33)=2.2, p<.05] but not in the hippocampus (p=.2) or in entorhinal cortex (p=1.0). Within the aMCI group, total MTL perfusion rates for multiple-domain patients did not significantly differ from rates in single-domain patients (p>.2), nor did perfusion rates differ in any individual MTL region (p values >.09).
3.4 Volume-Memory Relationships
To maximize statistical power and span a wide range both for the memory and atrophy variables, AD and aMCI patients were considered together. The two chief memory measures used in these analyses were hit rates for the forced-choice test and false-alarm rates for the yes/no test. Regression analyses using forced-choice d′ repeated the pattern of results using hit rates. Regression analyses using yes/no d′ did not reveal any significant relationships.
3.4.1 Medial Temporal Lobe
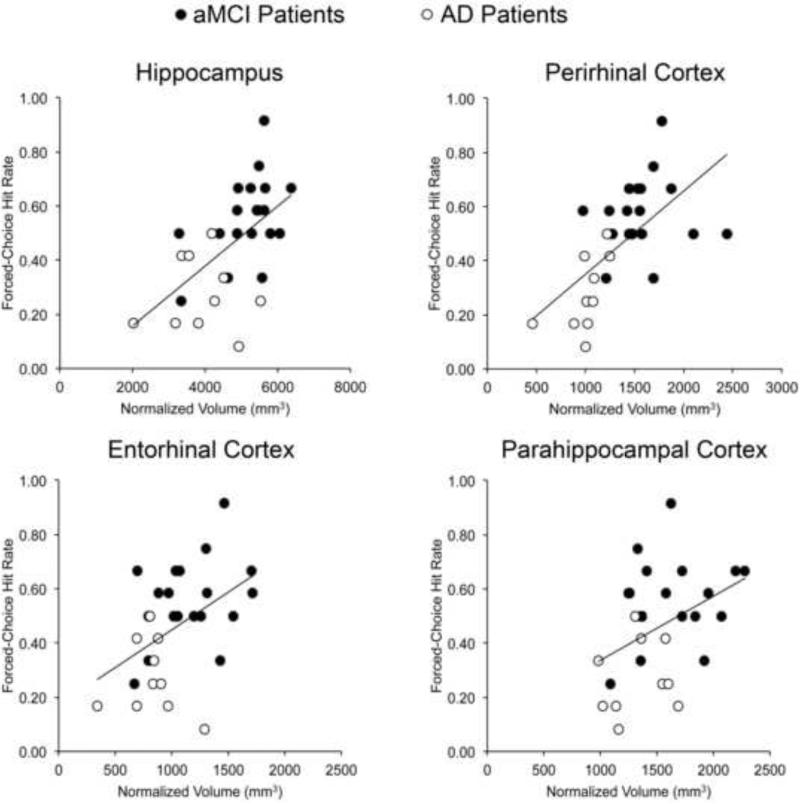
Given a priori hypotheses regarding the role of perirhinal cortex in familiarity (e.g., Bowles et al., 2007; Martin, Bowles, Mirsattari, & Köhler, 2011), we examined whether a relationship between perirhinal cortex volume and forced-choice hit rate was present. In the patient group, a positive correlation was observed (r=.62, p<.01). Additional forced-choice correlations were completed with total MTL volume as well as with other individual MTL regions, but because these were not predicted in advance, a stringent significance level of p <.01 was adopted for these analyses. Correlations were apparent with total MTL volume (r=.65, p<.001) and other individual MTL regions (Table 3). Correlations were significant for hippocampus (p<.01) and showed trends (p values<.05) for parahippocampal cortex and entorhinal cortex. Scatterplots for these four regions (Figure 4) show relationships with forced-choice performance across individual MTL regions.
Table 3.
Pearson correlation coefficients (r values) for correlations between individual medial temporal regions and recognition performance in the two tests.
| Forced-Choice Hits | Yes/No False Alarms | |||||
|---|---|---|---|---|---|---|
| Combined Patient Group | aMCI Group | Control Group | Combined Patient Group | aMCI Group | Control Group | |
| Perirhinal Cortex | .62* | .27 | .01 | −.26 | .31 | .08 |
| Hippocampus | .57* | .46† | .04 | −.41† | .16 | .25 |
| Entorhinal Cortex | .45† | .35 | .09 | −.41† | −.18 | .14 |
| Parahippocampal Cortex | .40† | .42 | −.11 | .08 | .29 | .39 |
Note.
p<.01
p<.05.
Figure 4.
Relationships between individual MTL regional volumes and forced-choice hit rate for the combined patient group.
Although volume measures for multiple MTL regions were correlated with forced-choice performance, the strong inter-region volume correlations (Table 4) place limitations on conclusions that can be drawn from these results. A multiple regression analysis was performed in which all regional volumes were entered simultaneously. The model was significant [F(4,24)=4.86, p<.01], but no individual MTL region emerged as a significant predictor (perirhinal: t=1.6, p=.12; hippocampus: t=0.7, p=.47; entorhinal: t=0.8, p=.39; parahippocampal t=0.5, p=.63). Because total MTL as well as some individual MTL regional volumes were significantly correlated with forced-choice performance, but none of the MTL regional correlations were significant when shared variance was accounted for in the simultaneous regression, this shared variance was likely a strong driving force behind the regional correlations. Thus, the prediction that perirhinal volume would be a strong predictor of forced-choice performance was supported in this patient group, but the results cannot discriminate between two possible explanations for the other regional correlations — that these other regions also play a role in forced-choice recognition or that these other regions are merely correlated with recognition because MTL regions tend to atrophy in parallel during early stages of AD.
Table 4.
Pearson correlation coefficients (r values) and significance levels (p values) for correlations between individual medial temporal region volumes for all patients.
| Entorhinal Cortex | Perirhinal Cortex | Parahippocampal Cortex | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Hippocampus | .554 | <.005 | .665 | <.001 | .464 | <.05 |
| Entorhinal Cortex | .466 | <.05 | .056 | n.s. | ||
| Perirhinal Cortex | .560 | <.005 | ||||
Note. n.s. = not significant.
As important differences in structure/function relationships may exist as an individual progresses from aMCI to AD, the above analyses were also completed in aMCI patients only. Total MTL volume, perirhinal volume, and other individual regional volumes were positively related with forced-choice hit rate (Table 3), although these comparisons did not reach significance, likely owing to reduced variance of both memory and volume measures with this smaller sample.
Given prior reports of the involvement of the hippocampus in recollection (Aggleton & Shaw, 1996; Skinner & Fernandez, 2007), hippocampal volumes were correlated with yes/no false-alarm rates. In the combined aMCI/AD group, a significant correlation was present (r=−.41, p<.05). As with the forced-choice analyses, additional yes/no correlations were completed with total MTL volume and with other individual MTL regions, using a stringent significance level of p <.01 for these analyses. No relationship was found between yes/no false-alarm rate and total MTL volume (p>.05), nor did correlations with other individual MTL regions (perirhinal, entorhinal, and parahippocampal cortices) reach significance (Table 3). Whereas forced-choice recognition was most strongly dependent on perirhinal cortex volume, this was clearly not the case for yes/no recognition. A direct test carried out using Williams’ Formula (Steiger, 1980) showed that the correlation between perirhinal volume and forced-choice recognition was significantly stronger than the correlation between perirhinal volume and false-alarm rate in the yes/no test (p = .036).
An additional analysis was completed to examine how relative recognition deficits in the patient group were related to MTL volume. If familiarity is mediated by MTL regions that do not play a large role in yes/no recognition, volumes of these regions should be associated with the degree to which forced-choice recognition is preserved in relation to yes/no impairments. Each patient's yes/no d′ value was subtracted from the mean control yes/no d′ value and divided by the value's standard deviation in the control group to account for control variability to obtain a yes/no deficit score, and likewise for forced-choice d′ values. A relative deficit score was then calculated by subtracting the yes/no deficit score from the forced-choice deficit score. A negative relative deficit score indicated a larger deficit in yes/no than in forced-choice recognition (most patients had negative relative deficit scores). A significant negative correlation was present when relative deficit scores were regressed against perirhinal volume (r=−.53, p<.01), indicating that patients who were most impaired at yes/no relative to forced-choice recognition showed the largest perirhinal volumes. Additional correlations in the same direction were present with total MTL volume (r=−.56, p<.01), and hippocampus (r=−.50, p<.01), with trends for relationships with entorhinal (r=−.37, p=.05) and parahippocampal cortex (r=−.34, p=.07). A simultaneous regression with relative deficit score as the dependent measure was also significant [F(4,24)=2.9, p<.05], but no individual region reached significance (p values>.2). As in the above analyses, these analyses support the conclusion that the relative preservation of forced-choice performance depends on perirhinal volume, leaving open the possibility that processing across multiple MTL regions is relevant.
Correlations between MTL volume and memory were also completed for the control group. Individual MTL regions did not show relationships with forced-choice hit rate (p values>.6) or yes/no false-alarm rate (p values>.09), nor did total MTL volume show relationships with these memory measures (p=.6 and p=.1, respectively).
3.4.2 Other Brain Regions
DLPFC volume did not correlate with any of the memory measures in patients (p values>.1) or controls (p values>.4). Likewise, calcarine volume did not correlate with memory measures in patients (p values>.7) or controls (p values>.5).
3.5 Perfusion-Memory Relationships
Medial temporal perfusion rates for aMCI patients were subjected to a parallel regression analysis. Overall MTL perfusion did not correlate with the recognition measure from either test (p values>.1). Among the individual regions, hippocampal perfusion correlated with yes/no false-alarm rate (r=.51, p<.05). As perfusion rate increased, false-alarm rate also increased. No other relationships between memory and perfusion were observed (p values> .5).
A regression analysis was also run in the control group. Overall MTL perfusion did not correlate with either of the memory measures (p values>.05).. No other relationships between memory and perfusion were observed (p values>.2).
As blood flow measurements were taken at the voxel level and averaged across all voxels within each MTL region, hippocampal blood flow measurements may have been more stable than measurements for other MTL regions due to the larger size of the hippocampus. Given the small size of individual MTL cortical regions, we also computed a combined MTL cortical perfusion measure (entorhinal, perirhinal, and parahippocampal cortex). MTL cortical perfusion did not show significant relationships with memory performance in the aMCI group (p values>.3) or in the control group (p values>.4).
A final set of analyses was completed to determine if MTL perfusion was related to MTL volume. In the patient group, there was a trend for a positive relationship between MTL perfusion and total MTL volume, but this relationship was not significant (p=.09). Notably, analyses with individual MTL regions revealed a trend for a positive relationship between the two measures in the hippocampus (r=.49, p=.05), but not in other regions (p values>.15). In the control group, no relationship was present between overall MTL perfusion and total MTL volume (p=.4), nor in any individual regions (p values>.2).
4. Discussion
Despite their pronounced memory decline on standard neuropsychological tests, patients in the aMCI group exhibited preserved forced-choice recognition when target silhouette objects were grouped with highly similar foils. When the same sorts of stimuli were tested in a yes/no format, recognition was impaired. These findings replicate our previous results (Westerberg et al., 2006), and provide a window into the impact of age-related neuropathology on different types of episodic memory. Whereas aMCI patients showed only modest MTL damage, extensive MTL damage was present in AD patients, who exhibited severe impairments in both recognition tests. Thus, additional MTL damage sustained by AD patients appears to disrupt additional memory functions. Importantly, relationships between MTL integrity and recognition depended on test format, and the pattern of these relationships suggests that familiarity-based recognition in the forced-choice test is mediated at least in part by processing within perirhinal cortex whereas recognition in the yes/no test does not reflect this same type of MTL specificity.
Preserved recognition in the aMCI group in this unique forced-choice test format is consistent with previous reports of intact familiarity in aMCI (Algarabel et al., 2009; Anderson et al., 2008; Hudon, Belleville, & Gauthier, 2009; Serra et al., 2010; Westerberg et al., 2006). In the forced-choice format, when four choices were viewed together (one target and three corresponding foils), correct recognition could be achieved through a relative familiarity comparison, due to covariance of familiarity levels for a target and its three corresponding foils. Yes/no recognition decisions, on the other hand, benefited minimally if at all from relative familiarity comparisons. Instead, accurate yes/no recognition critically required recollection of specific details, which could support a recall-to-reject strategy, or compartmentalizing information from prior test trials and the study phase. In addition, yes/no recognition presumably relied on applying a decision criterion on each trial based on level of memory strength, comparison with prior test trials, and related decision factors.
Memory scores varied considerably within each patient group. Indeed, memory disruptions in aMCI generally range from negligible to moderate, and when analyses excluded four aMCI patients likely at the earliest stage of the disease, forced-choice recognition was impaired in this aMCI subgroup relative to controls. Likewise, other studies have reported impaired familiarity in aMCI patients (Ally, Gold, & Budson, 2009; Wolk, et al., 2011; Wolk, Signoff, & Dekosky, 2008). Whereas recollective processes may be disrupted in early stages of the disease, familiarity may remain intact until later stages when it becomes progressively abnormal; familiarity is evidently quite impaired in patients diagnosed with AD. Whether or not there is a familiarity impairment in any particular study would thus depend on the degree of disease progression in the specific patients tested. Future research may benefit to the extent that independent measures of disease progression can be identified and related to recognition abilities. The silhouette recognition test used in the present experiment may be particularly well suited for measuring the specific recognition function of familiarity with visual objects. Here, we confirmed that aMCI patients can achieve highly accurate recognition based on relative familiarity judgments for silhouette objects, but that familiarity begins to diminish when the memory defect becomes more severe in the progression from aMCI to AD.
Several contemporary accounts of episodic memory suggest that the hippocampus is not necessary for familiarity and that perirhinal cortex is central for accurate item familiarity-based recognition (Aggleton & Brown, 1999; Davachi, 2006; Diana et al., 2007; Montaldi & Mayes, 2010; Norman & O'Reilly, 2003). Yet, this hypothesis has faced intense scrutiny (Smith, Wixted, & Squire, 2011; Song, Wixted, Hopkins, & Squire, 2011; Squire & Wixted, 2011; Squire, Wixted, & Clark, 2007). The current experimental approach provides new evidence relevant to this controversy, suggesting that perirhinal cortex makes a significant contribution to familiarity for silhouette objects, although the specificity of this relationship and how much it generalizes to other kinds stimuli and testing situations deserves further investigation.
By virtue of the variability in memory and atrophy across patients, results showed that patients with relatively higher forced-choice recognition scores also had relatively larger MTL volumes. A degree of MTL specificity was evident, as forced-choice recognition was correlated with MTL volume but not DLPFC or calcarine volume. Perirhinal cortex in isolation also showed a positive relationship with forced-choice performance, and the magnitude of preserved forced-choice recognition relative to deficient yes/no recognition was most strongly related to perirhinal volume. Other MTL regions also showed such relationships, but high inter-correlations among individual MTL regions necessitated additional analyses of whether perirhinal plays a privileged role in forced-choice recognition. A simultaneous regression model indicated that no MTL region made a significant contribution, which means that the correlations can be attributed to shared variance among MTL regions. These findings lend additional support to the hypothesis that perirhinal cortex plays a role in familiarity (Aggleton & Brown, 1999; Davachi, 2006; Diana, et al., 2007; Montaldi & Mayes, 2010; Norman & O'Reilly, 2003), but at the same time they leave open the possibility that other MTL regions also make contributions to familiarity. In particular, the significant hippocampal correlation could reflect a role for the hippocampus in familiarity or it could merely reflect disease progression whereby perirhinal atrophy and hippocampal atrophy occur in tandem. Whereas other data suggest that the hippocampus does not make a necessary contribution to familiarity (Aggleton & Brown, 1999; Mayes, Holdstock, Isaac, Hunkin, & Roberts, 2002; Vargha-Khadem et al., 1997; Yonelinas et al., 2002), some findings cast doubt on this conclusion (Squire & Wixted, 2011; Squire, et al., 2007). The potential hippocampal contribution to familiarity must thus remain an open topic for further investigation.
Patterns of MTL volume relationships were different for yes/no recognition than for forced-choice recognition, supporting the conclusion that dissociable neural mechanisms support forced-choice and yes/no recognition. When volumes of individual MTL regions were separately regressed with yes/no recognition, higher false-alarm rates in the patient group were associated with smaller hippocampal volumes and weakly with smaller entorhinal volumes. This finding is consistent with a study of older adults showing a relationship between false alarms in an associative memory task and volumes of the dentate gyrus and CA3 and CA4 subfields of the hippocampus in particular (Shing et al., 2011). Associations between the hippocampus and entorhinal cortex and recognition are also consonant with the literature describing the memory functions of these regions (Aggleton & Shaw, 1996; Skinner & Fernandes, 2007).
Results from the perfusion analyses also support the conclusion that dissociable neural mechanisms support forced-choice and yes/no recognition. In aMCI patients, increased hippocampal blood flow was associated with higher yes/no false-alarm rates. As suggested by Dai and colleagues (2009), increased blood flow may be an early sign of cellular dysfunction and may reflect a compensatory response, as more blood flow is required to maintain function after some cells are damaged. Therefore, it is possible that hippocampal dysfunction in aMCI patients may disrupt once-reliable memory signals, forcing a strategy shift to reliance on familiarity, which would result in more “yes” responses to stimuli that shared similarities with studied items, resulting in a higher false-alarm rate. This idea is consistent with findings of an abnormally liberal response bias in AD (Beth, Budson, Waring, & Ally, 2009; Budson, Wolk, Chong, & Waring, 2006). The pattern of false alarms across the AD, aMCI, and control groups supports the idea that degraded memory signals result in a strategy shift whereby the number of yes responses to similar foils increase. However, the possibility that differences in vasculature between the hippocampus and cortical regions may explain the failure to find significant relationships between perfusion in MTL cortical regions cannot be ruled out. Therefore, it is unclear whether the failure to find relationships with blood flow in other MTL regions reflected different functions for MTL cortex or differences inherent to the vascular system.
Collectively, the brain-behavior relationships observed here argue that neural processes in the MTL that support familiarity differ from those that support the type of processing required for yes/no recognition. The perirhinal cortex appears to make a strong contribution to familiarity although it is possible other MTL regions are involved as well, whereas with respect to yes/no recognition, the hippocampus appears to be particularly relevant. These results are consistent with current theories that suggest that MTL processes supporting familiarity differ from those that are needed to support full-blown recollection, which can of course also support recognition judgments (Aggleton & Brown, 1999; Davachi, 2006; Diana, Yonelinas, & Ranganath, 2007; Montaldi & Mayes, 2010; Norman & O'Reilly, 2003; Shimamura, 2010).
In contrast to the presence of striking relationships between MTL volume and recognition in the patient group, no relationships between MTL volume and recognition were observed in the control group. There are multiple possible explanations for these null results. One possibility is that memory/volume relationships do not emerge until MTL damage reaches a certain threshold. Van Petten (2004) reported findings consistent with this idea from a meta-analysis of studies on relationships between hippocampal volume and memory ability. Volume differences in controls likely reflect individual differences to a greater extent than the effects of degenerative changes. It should also be noted that variance in both the memory and volume measures was larger in the patient group than in the control group (forced-choice hit rate SD: patients=.22, controls=.18; total MTL volume SD: patients=1.12, controls=.80), and these differences in within-group variability likely increased our ability to detect relationships in the patient group compared with the control group. Nonetheless, there was strong support for the hypothesis that hippocampal volume is related to memory in neurological patients.
In both the patient and control group, neither forced-choice nor yes/no recognition was related to DLPFC volume. Although prior reports have indicated an involvement of this region in recognition memory (e.g., Fletcher & Henson, 2001; Ranganath, et al., 2007), variations in DLPFC volume did not appear highly relevant for variations in performance on these specific recognition tests. One reason for this may be that DLPFC damage was not as extensive as MTL damage. In keeping with studies reporting that the MTL is the first and most extensively damaged area in AD (e.g., Braak & Braak, 1991), average percent volume loss from the control mean for the AD patients was only 15% for DLPFC compared with 26% for the MTL. Alternatively, DLPFC may not play a large role in recognition decisions relevant for the tests used in this paradigm.
Our results also provided data relevant to characterizing the progression of AD-related pathology in individual MTL regions. Although MTL volume measures in aMCI and AD patients have frequently been reported, all four regions (hippocampus, entorhinal, perirhinal, and parahippocampal cortex) are seldom quantified within the same experiment. Here, we found that volumes of all MTL structures were much smaller in the AD group compared with the control and aMCI groups. This finding replicates several findings of significant MTL volume reductions in AD (Dickerson et al., 2001; Jack, Petersen, O'Brien, & Tangalos, 1992; Jack et al., 1997; Kesslak et al., 1991; Killiany et al., 1993; Petersen et al., 2000; Seab et al., 1988), and underscores the devastating damage incurred to this region in AD. Although MTL volumes in aMCI patients were slightly smaller than control volumes, only the parahippocampal cortex was significantly reduced in the aMCI group relative to controls. Additional analyses comparing degree of atrophy across regions indicated that atrophy in this region was not reliably more prominent than other regions, although future research will be necessary to determine if this pattern of atrophy is typical in aMCI. Overall, the small amount of volume loss in aMCI patients observed here was somewhat surprising, given several previous reports of MTL volume reductions in aMCI patients relative to controls (deToledo-Morrell et al., 2004; Du et al., 2001; Jack et al., 1999; Jack et al., 2005; Pennanen et al., 2004; Stoub, Rogalski, Leurgans, Bennett, & deToledo-Morrell, 2010). However, not all studies have found MTL volume reductions in aMCI patients (Dickerson et al., 2005; Laakso et al., 1998; Soininen et al., 1994), and the discrepant results across studies likely reflect the heterogeneous nature of aMCI patients. Studies that failed to find significant volume reductions may have predominantly included individuals at the earliest stages of the disease process or those who will not ultimately convert to AD, whereas studies that did find reductions may have sampled individuals closer to AD conversion. In line with this possibility, our aMCI group included four individuals who did not exhibit objective memory impairments but were nonetheless diagnosed with aMCI based on clinical assessment, with self and informant reports of memory decline. Patients located very early along the continuum from healthy aging to AD may have predominated in our sample compared with other studies. Another factor contributing to our lack of MTL volume differences, given that numerical decreases were present, may be statistical power. Our aMCI sample was relatively small (n=19) compared with other studies that did find differences (e.g., n=72 in Jack et al., 2005; n=65 in Pennanen et al., 2004).
Despite only minor MTL volume differences, our sample of aMCI patients did show modestly increased perfusion rates in some MTL regions relative to controls. Dai and colleagues (2009) also found this pattern of results using the ASL technique, and further demonstrated that hippocampal blood flow is reduced in AD patients. As discussed above, they hypothesized that increased MTL perfusion in aMCI reflects a compensatory mechanism at the initial stages of the disease before significant atrophy is present and blood flow levels decrease. The current results support this hypothesis, as our sample of aMCI patients did not exhibit large amounts of MTL volume loss but did show increased MTL blood flow. Also in line with this hypothesis, other studies have shown fMRI activation increases in aMCI patients relative to controls during memory encoding (despite activation decreases in AD patients), especially aMCI patients at the earliest stages of the disease (Dickerson et al., 2004; Dickerson, et al., 2005). This has prompted the suggestion that MTL activation patterns in fMRI experiments also follow a nonlinear trajectory along the healthy-aging-to-AD continuum (Sperling, 2007). Although fMRI measures blood oxygenation while an individual is actively completing a task whereas ASL perfusion in our study measured blood flow in the absence of specific task requirements, there is a striking similarity in the pattern of results across the two methodologies. Collectively, these findings suggest that methods examining functional brain changes in aMCI may be more sensitive than structural methods with respect to the MTL, especially in the earliest stages of the disease. Future research should be directed at how these functional changes may relate to memory decline.
In summary, our results demonstrate that MTL substrates of forced-choice recognition of confusable silhouettes are distinct from those of yes/no recognition. Perirhinal cortex showed the strongest relationship with forced-choice recognition, though it is unclear whether contributions from other MTL regions might also be relevant. A different pattern of MTL regional volumes was associated with yes/no recognition, without as much of a contribution from perirhinal cortex. Understanding how different memory experiences may be affected early in the course of AD and how this relates to the underlying pathology could have important implications for designing treatments aimed at maximizing memory function along different points of the disease spectrum. Preserved familiarity signals in patients may reflect largely intact perirhinal functioning associated with minimal volume loss and somewhat enhanced perfusion in this region.
Highlights.
Recognition memory and medial temporal integrity were assessed in three groups
Amnestic MCI patients showed intact familiarity on a special forced-choice test
Patients with early Alzheimer's disease showed atrophy in medial temporal regions
Amnestic MCI patients showed minimal atrophy and medial temporal hyperperfusion
Medial temporal atrophy in patients was associated with impairments linked to familiarity
Acknowledgements
We thank Nondas Leloudas for assistance with MRI data acquisition, Olivia Marczuk for assistance with data analyses, and Travis Stoub, Leyla deToledo-Morrell, Michael J. Bailey, Naftali Raz, and Satoru Suzuki for advice with analysis strategies.
Role of the Funding Source
This work was supported by a grant from the Illinois Department of Public Health Alzheimer's Disease Research Fund, NIMH NRSA fellowship F32 MH073247, and NIA grant P30 AG13854.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–444. [PubMed] [Google Scholar]
- Aggleton JP, Shaw C. Amensia and recognition memory: A re-analysis of psychometric data. Neuropsychologia. 1996;34:51–62. doi: 10.1016/0028-3932(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Algarabel S, Escudero J, Mazon JF, Pitarque A, Fuentes M, Peset V, et al. Familiarity-based recognition in the young, healthy elderly, mild cognitive impaired and Alzheimer's patients. Neuropsychologia. 2009;47:2056–2064. doi: 10.1016/j.neuropsychologia.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer's disease and mild cognitive impairment using receiver operating characteristics. Brain and Cognition. 2009;69:504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Casement M, de Bazelaire C, Fong T, Press DZ. Hippocampal hyperperfusion in Alzheimer's disease. Neuroimage. 2008;42:1267–1274. doi: 10.1016/j.neuroimage.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22:177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Asllani I, Habeck C, Scarmeas N, Borogovac A, Brown TR, Stern Y. Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer's disease. Journal of Cerebral Blood Flow and Metabolism. 2008;28:725–736. doi: 10.1038/sj.jcbfm.9600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beth EH, Budson AE, Waring JD, Ally BA. Response bias for picture recognition in patients with Alzheimer disease. Cognitive and Behavioral Neurology. 2009;22:229–235. doi: 10.1097/WNN.0b013e3181b7f3b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, et al. Impaired familiarity with preserved recollection after anterior temporal lobe resection that spares the hippocampus. Proceedings of the National Academy of Sciences USA. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzao A, Floris R, Baviera ME, Apruzzese A, Simonetti G. Diffusion and perfusion MR imaging in cases of Alzheimer's disease: correlations with cortical atrophy and lesion load. American Journal of Neuroradiology. 2001;22:1030–1036. [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer's disease: separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Quantifying CBF with arterial spin labeling. Journal of Magnetic Resonance Imaging. 2005;22:723–726. doi: 10.1002/jmri.20462. [DOI] [PubMed] [Google Scholar]
- Chang YL, Jacobson MW, Fennema-Notestine C, Hagler DJ, Jr., Jennings RG, Dale AM, et al. Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cerebral Cortex. 2010;20:1305–1313. doi: 10.1093/cercor/bhp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, et al. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Disease and Associated Disorders. 2010;24:19–27. doi: 10.1097/WAD.0b013e3181b4f736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–866. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinions in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proceeding of the National Academy of Sciences USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiology of Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiology of Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, et al. Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duara R, Grady C, Haxby J, Sundaram M, Cutler NR, Heston L, et al. Positron emission tomography in Alzheimer's disease. Neurology. 1986;36:879–887. doi: 10.1212/wnl.36.7.879. [DOI] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. Journal of Neuroscience. 2003;23:9439–9444. doi: 10.1523/JNEUROSCI.23-28-09439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Topics in Magnetic Resonance Imaging. 2004;15:10–27. doi: 10.1097/00002142-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr., Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. Journal of Neuroscience. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova II, Dickerson BC, Stoub TR, deToledo-Morrell L. MRI of human entorhinal cortex: a reliable protocol for volumetric measurement. Neurobiology of Aging. 2001;22:737–745. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Archives of Neurology. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. Journal of Cognitive Neuroscience. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999;122(Pt 7):1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O'Reilly RC, et al. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Hudon C, Belleville S, Gauthier S. The assessment of recognition memory using the Remember/Know procedure in amnestic mild cognitive impairment and probable Alzheimer's disease. Brain and Cognition. 2009;70:171–179. doi: 10.1016/j.bandc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychological Medicine. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology. 1991;41:51–54. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Albert MS, Sandor T, Tieman J, Jolesz F. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Archives of Neurology. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magnetic Resonance in Medicine. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, et al. Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. Journal of Nuclear Medicine. 2000;41:1155–1162. [PubMed] [Google Scholar]
- Kwong KK, Chesler DA, Weisskoff RM, Donahue KM, Davis TL, Ostergaard L, et al. MR perfusion studies with T1-weighted echo planar imaging. Magnetic Resonance in Medicine. 1995;34:878–887. doi: 10.1002/mrm.1910340613. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Annals of Neurology. 2001;49:202–213. [PubMed] [Google Scholar]
- Laakso MP, Soininen H, Partanen K, Lehtovirta M, Hallikainen M, Hanninen T, et al. MRI of the hippocampus in Alzheimer's disease: sensitivity, specificity, and analysis of the incorrectly classified subjects. Neurobiology of Aging. 1998;19:23–31. doi: 10.1016/s0197-4580(98)00006-2. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User's Guide. 2nd ed. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2005. [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Martin CB, Bowles B, Mirsattari SM, Köhler S. Selective familiarity deficits after left anterior temporal-lobe removal with hippocampal sparing are material specific. Neuropsychologia. 2011;49:1870–1880. doi: 10.1016/j.neuropsychologia.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12:325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Migo E, Montaldi D, Norman KA, Quamme J, Mayes A. The contribution of familiarity to recognition memory is a function of test format when using similar foils. Quarterly Journal of Experimental Psychology. 2009;62:1198–1215. doi: 10.1080/17470210802391599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magnetic Resonance Medicine. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiology of Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment: current research and clinical implications. Seminars in Neurology. 2007;27:22–31. doi: 10.1055/s-2006-956752. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Jr., Xu YC, Waring SC, O'Brien PC, Smith GE, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: Two means of access to the personal past. Memory & Cognition. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15:997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Heller AS, Wilding EL. Dissociable correlates of two classes of retrieval processing in prefrontal cortex. Neuroimage. 2007;35:1663–1673. doi: 10.1016/j.neuroimage.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Knight RT. Prefrontal cortex and episodic memory: integrating findings from neuropsychology and event-related functional neuroimaging. In: Wilding A, Bussey T, editors. The Cognitive Neuroscience of Memory Encoding and Retrieval. Psychology Press; Philadelphia: 2003. pp. 83–99. [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerbral cortex: replicability of regional differences in volume. Neurobiology of Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: Manual for Administration and Scoring. Reitan Neuropsychology Laboratory; Tucson, AZ: 1992. [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RN, Robb WG. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seab JP, Jagust WJ, Wong ST, Roos MS, Reed BR, Budinger TF. Quantitative NMR measurements of hippocampal atrophy in Alzheimer's disease. Magnetic Resonance Medicine. 1988;8:200–208. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- Serra L, Bozzali M, Cercignani M, Perri R, Fadda L, Caltagirone C, et al. Recollection and familiarity in amnesic mild cognitive impairment. Neuropsychology. 2010;24:316–326. doi: 10.1037/a0017654. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Hierarchical relational binding in the medial temporal lobe: the strong get stronger. Hippocampus. 2010;20:1206–1216. doi: 10.1002/hipo.20856. [DOI] [PubMed] [Google Scholar]
- Shing YL, Rodrigue KM, Kennedy KM, Fandakova Y, Bodammer N, Werkle-Bergner M, et al. Hippocampal subfield volumes: age, vascular risk, and correlation with associative memory. Frontiers in Aging Neuroscience. 2011;3:1–8. doi: 10.3389/fnagi.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia. 2007;45:2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Smith CN, Wixted JT, Squire LR. The hippocampus supports both recollection and familiarity when memories are strong. Journal of Neuroscience. 2011;31:15693–15702. doi: 10.1523/JNEUROSCI.3438-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Knight RG. Memory processing in Alzheimer's disease. Neuropsychologia. 2002;46:666–682. doi: 10.1016/s0028-3932(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- Song Z, Wixted JT, Hopkins R, Squire LR. Impaired capacity for familiarity after hippocampal damage. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9655–9660. doi: 10.1073/pnas.1107247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since h.m. Annual Review of Neuroscience. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer's disease. Proceedings of the National Academy of Sciences USA. 2006;103:10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, deToledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: relation to memory function. Neurobiology of Aging. 2010;31:1089–1098. doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendolkar I, Arnold J, Petersson KM, Weis S, Anke B-D, van Eijndhoven P, et al. Probing the neural correlates of associative memory formation: a parametrically analyzed event-related functional MRI study. Brain Research. 2007;1142:159–168. doi: 10.1016/j.brainres.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wechsler A. The Wechsler Memory Scale-Revised Manual. Psychological Corporation; New York: 1987. [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, et al. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology. 2006;20:193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. Recall and recognition are equally impaired in patients with selective hippocampal damage. Cognitive, Affective, and Behavioral Neuroscience. 2004;4:58–66. doi: 10.3758/cabn.4.1.58. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal detection theory of recognition memory. Psychological Review. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, DeKosky ST. A medial temporal lobe division of labor: insights from memory in aging and early Alzheimer disease. Hippocampus. 2011;21:461–466. doi: 10.1002/hipo.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, Dekosky ST. Recollection and familiarity in amnestic mild cognitive impairment: a global decline in recognition memory. Neuropsychologia. 2008;46:1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR in Biomedicine. 1997;10:237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magnetic Resonance in Medicine. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1134–1140. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]