Abstract
Pregnancy in placental mammals offers exceptional comprehensive benefits of in utero protection, nutrition, and elimination of metabolic waste for the developing fetus. However, these advantages also require durable strategies to mitigate maternal rejection of fetal tissue expressing foreign paternal antigens. Since the initial postulate of expanded maternal immune tolerance by Sir Peter Medawar 60 years ago, an amazingly elaborate assortment of molecular and cellular modifications acting both locally at the maternal placental interface and systemically have been shown to silence potentially detrimental maternal immune responses. In turn, simultaneously maintaining host defense against the infinite array of potential pathogens during pregnancy is equally important. Fortunately, resistance against most infections is preserved seamlessly throughout gestation. On the other hand, recent studies on pathogens with unique predisposition for prenatal infection have uncovered distinctive holes in host defense associated with the reproductive process. Using these infections to probe the response during pregnancy, the immune suppressive regulatory subset of maternal CD4 T cells has been increasingly shown to dictate the inter-workings between prenatal infection susceptibility and pathogenesis of ensuing pregnancy complications. Herein, the recent literature suggesting a necessity for maternal regulatory T cells in pregnancy induced immunological shifts that sustain fetal tolerance is reviewed. Additional discussion is focused on how expansion of maternal regulatory T cell suppression may become exploited by pathogens that cause prenatal infection, and the perilous potential of infection induced immune activation that may mitigate fetal tolerance and inadvertently inject hostility into the protective in utero environment.
Keywords: Immunology, Pregnancy, Infection
Introduction
Pregnancy in eutherian placental mammals requires expanded maternal tolerance to encompass paternal antigens expressed by the developing fetus. This vital process initiated at the earliest stages of pregnancy with invasion of fetal trophoblast cells into the uterine lining prevents recognition and rejection of foreign fetal cells. Given the essential nature of reproduction for species survival, it is not surprising numerous non-overlapping immune evasion strategies have been identified that together reinforce protection for the developing fetus. This includes sharply reduced or extinguished expression of immune recognition major histocompatibility antigens on trophoblast cells (Hunt, et al. 1987, Mattsson 1998, Ozato, et al. 1985, Sunderland, et al. 1981, Zuckermann and Head 1986), tryptophan catabolism that prevents maternal T cell activation (Mellor and Munn 2004, Munn, et al. 1998), selective expression of galectin-1 that moderates T cell differentiation or Crry that prevents complement deposition (Blois, et al. 2007, Xu, et al. 2000), entrapment of antigen presenting cells within the uterus (Collins, et al. 2009), and blunted chemokine expression by decidual stromal cells (Nancy, et al. 2012). By coalescing these potent immune suppressive features where they are most needed at the maternal-fetal interface, responsiveness that maintains host defense against most pathogens systemically and within non-reproductive tissue would be predicted to be preserved.
On the other hand, since low level dissemination of fetal cells into maternal blood and non-reproductive tissue also occurs during pregnancy (Guetta, et al. 2003, Khosrotehrani, et al. 2004, Liegeois, et al. 1981), systemic immune modifications may also be needed to reinforce fetal tolerance. Here, multiple non-overlapping strategies are likely to be simultaneously at work. One is the selective elimination of maternal T cells with high affinity to fetal antigen through apoptotic death in early gestation (Erlebacher, et al. 2007). However, this process is incomplete both temporally and for maternal T cells that recognize fetal antigen with lower affinity, suggesting other ways to prevent activation of maternal immune cells with fetal specificity also exist. In this regard, the selective silencing of immune effector cells with specificity to non-self paternal antigens during pregnancy can be viewed as an example of peripheral immune tolerance. By contrast, even with substantial overlap between maternal and fetal antigens, central tolerance that eliminates developing T cells with self specificity within the thymus is less operational since maternal thymectomy does not diminish fertility, and in cases of autoimmunity may improve the outcomes of pregnancy (Griesemer, et al. 2010, Hoff, et al. 2007, Stritesky, et al. 2012, Visser, et al. 2004). Thus, immune components that sustain peripheral tolerance in other contexts, (e.g. commensal microbes in tissue with direct contact to the external environment or self-antigen for immune cells that escape central tolerance) are likely to play expanded roles in maintaining fetal tolerance during pregnancy. Since the pregnancy associated immune modifications that sustain fetal tolerance have been recently summarized in a very comprehensive fashion both in general terms and from more distinctive perspectives including the maternal-fetal interface, lymphoid organs that drain this compartment, antigen presenting dendritic cells, and how immunological shifts impact local susceptibility to viral pathogens (Bizargity and Bonney 2009, Erlebacher 2013a, b, Moffett and Loke 2006, Mold and McCune 2012, Mor and Cardenas 2010, Munoz-Suano, et al. 2011, Taglauer, et al. 2010), we will use this opportunity to focus more specifically on evidence for systemic immune modifications, and how the dynamic cross regulation between immunological shifts required for sustaining fetal tolerance dictates susceptibility to prenatal infection and the potential immune pathogenesis of ensuing pregnancy complications.
Maternal regulatory T cells and fetal tolerance
The Foxp3+ subset of CD4 T cells called regulatory T cells (Tregs) have potent immune suppressive properties and play essential roles in sustaining peripheral immune tolerance (Josefowicz, et al. 2012, Littman and Rudensky 2010, Wing and Sakaguchi 2010). Spontaneous foxp3 defects result in fatal systemic and organ-specific autoimmunity within the first 6 months of life described as the immunedysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome (Bennett, et al. 2001, Wildin, et al. 2001). In turn, similar mortal symptoms arise in mice with naturally occurring or targeted disruptions in foxp3 (Fontenot, et al. 2003, Khattri, et al. 2003). The importance of maternal Tregs in fetal tolerance was first suggested by their progressive expansion in healthy human pregnancy, and blunted expansion in cases of spontaneous compared with induced abortion (Sasaki, et al. 2004, Somerset, et al. 2004). At the same time, pioneering studies in mice showed paralleled levels of maternal Treg accumulation throughout gestation, whereas the selective elimination of these cells caused fetal wastage and resorption (Aluvihare, et al. 2004).
Within the next few years after these seminal findings, the critical necessity for maternal Tregs in sustaining fetal tolerance have been reinforced by numerous other studies characterizing these cells in human and animal pregnancy. For example, significantly reduced levels of maternal Treg expansion have been shown repeatedly for women with preeclampsia or recurrent spontaneous abortion (Prins, et al. 2009, Santner-Nanan, et al. 2009, Sasaki, et al. 2007, Toldi, et al. 2008, Winger and Reed 2011, Yang, et al. 2008). Along with these quantitative reductions, qualitative diminutions in suppressive function among maternal Tregs on a per cell basis have also recently been described for preterm compared with term human pregnancies (Gomez-Lopez and Laresgoiti-Servitje 2012, Schober, et al. 2012). Here, it is important to point out blunted maternal Treg expansion may not be universally associated with all cases of preeclampsia since normal Treg accumulation has also been reported (Hu, et al. 2008, Paeschke, et al. 2005). However, the interpretation of these isolated negative findings is somewhat moderated by the wide variation in peripheral lymphocyte numbers and Treg percentages among individuals. Nevertheless, consistent reductions in maternal Treg suppression among multiple seemingly unrelated clinical pregnancy complications underscore the potential importance of preserving fetal tolerance by these cells in healthy pregnancy.
The necessity of maternal Tregs in maintaining pregnancy has been more definitively addressed in animal pregnancy models where experimental manipulation allows the cause and effect relationship of Treg manipulation on pregnancy outcomes to be investigated. During mouse allogeneic pregnancy, maternal Treg depletion or reconstituting T cell deficient mice exclusively with non-Treg effector cells each triggers significantly increased rates of fetal resorption with reciprocal loss of live pups (Aluvihare, et al. 2004, Rowe, et al. 2011, Samstein, et al. 2012, Shima, et al. 2010). Furthermore, the accumulation of activated effector T cells within the decidua and systemic expansion of maternal effector T cells with fetal specificity with maternal Treg ablation each reinforce a critical protective role for these cells in sustaining fetal tolerance (Rowe, et al. 2011, Samstein, et al. 2012). Along with these findings illustrating the necessity for maternal Tregs after the near complete ablation of these cells, the specific requirement for expanded maternal Foxp3+ cells has also been described. The latter using mice where the high affinity human diphtheria toxin receptor is co-expressed with Foxp3 and exploiting the X-linked inheritance of foxp3 where female mice heterozygous for the diphtheria toxin receptor transgene contain an equal ratio of Tregs that are either susceptible or resistant to ablation found even partial transient ablation to pre-pregnancy levels triggers fetal resorption and fractures fetal tolerance (Rowe, et al. 2011). Therefore, despite considerable variation in the overall magnitude of fetal resorption between individual studies that likely reflect differences in the timing and efficiency of Treg depletion using unique experimental approaches (Aluvihare, et al. 2004, Rowe, et al. 2011, Samstein, et al. 2012, Shima, et al. 2010), these studies in mice collectively illustrate the importance of maternal Tregs, and the sustained expansion of these cells in maintaining pregnancy.
Other complementary data highlighting the importance of Tregs in maternal-fetal tolerance are evolutionary conserved genetic elements required for selective Foxp3 expression unique to humans and other eutherian placental mammals that are conspicuously absent in marsupials and egg laying monotremes (Andersen, et al. 2012, Samstein, et al. 2012). This includes the foxp3 enhancer conserved noncoding sequence (CNS)-1 required for peripheral induced Treg differentiation among non-Treg CD4 T cells (Zheng, et al. 2010). Pregnancies in mice with targeted defects in CNS-1 show significantly increased rates of fetal resorption with more pronounced decidual inflammation and abnormal spiral artery remodeling consistent with the pathological features of preeclampsia in human pregnancy (Samstein, et al. 2012). This apparent requirement for induced Treg differentiation parallels the efficiency whereby trophoblast cells and high-level progesterone each induce Foxp3 expression among non-Treg CD4 T cells (Lee, et al. 2012, Ramhorst, et al. 2012). On the other hand, using adoptively transferred Foxp3 depleted cells to show the pre-existing pool of peripheral Foxp3+ CD4 T cells also contributes significantly to the overall accumulation of maternal Tregs during pregnancy may explain the considerably reduced magnitude of fetal resorption after the selective elimination of induced Tregs based on CNS-1 deficiency compared with bulk Tregs based on Foxp3 expression (Rowe, et al. 2011, Rowe, et al. 2012c, Samstein, et al. 2012).
Additional investigation exclusive to animal pregnancy has uncovered other interesting facets on the fundamental biology whereby maternal Tregs respond to fetal antigen stimulation. For example, using the wealth of defined inbred strains in mice, maternal Tregs have been shown to accumulate to higher levels during allogeneic (when MHC discordant parents are used for breeding) compared with syngeneic (between MHC identical parents) pregnancy where the only potential source of antigen heterogeneity is that encoded by the Y chromosome (Kahn and Baltimore 2010, Rowe, et al. 2011). Reciprocally, fetal resorption triggered by maternal Treg ablation is consistently reduced in syngeneic compared with allogeneic pregnancy (Aluvihare, et al. 2004, Rowe, et al. 2012c, Samstein, et al. 2012, Shima, et al. 2010). Accordingly, the degree of mis-match between maternal and paternal-(fetal) MHC alloantigen appears to dictate the necessity for expanded maternal Tregs during pregnancy. In this regard, the natural heterogeneity between maternal and paternal MHC antigens in human pregnancy is more fully recapitulated in mouse allogeneic pregnancy. However, given the selective loss of male offspring with partial maternal Treg ablation during syngeneic pregnancy, these cells also likely mitigate mis-match between minor alloantigens encoded on the Y chromosome as well (Kahn and Baltimore 2010). Together, these findings suggest a critical role for maternal Treg differentiation and the sustained expansion of these cells in maintaining tolerance to paternal non-self antigens expressed by the developing fetus during pregnancy.
Pregnancy induced shifts in systemic immune responsiveness and maternal Tregs
In addition to these protective roles in maintaining fetal tolerance, the sustained accumulation of immune suppressive maternal Tregs has also been linked with notable shifts in non-fetal responses outside female reproductive tissues throughout gestation. A remarkable example of this is the amelioration of many autoimmune disorders during human pregnancy. For example, significant reductions in disease severity or complete remission occur for women with rheumatoid arthritis during pregnancy (Barrett, et al. 1999, Da Silva and Spector 1992, de Man, et al. 2009, Ostensen and Villiger 2007). The protective benefits are most likely conferred by maternal Tregs given the inverse correlation between disease severity and circulating levels of these cells during pregnancy and after parturition, and the protective capacity of maternal Tregs from mice with pregnancy induced remission from collagen induced arthritis after adoptive transfer into naive recipients (Forger, et al. 2008, Munoz-Suano, et al. 2012). Similarly for multiple sclerosis, pregnancy induced amelioration of weakness and clinical disease exacerbations has been directly linked with the progressive expansion of maternal Tregs (Confavreux, et al. 1998, Iorio, et al. 2009, Sanchez-Ramon, et al. 2005). In turn, paralleled disease remission also occurs for Graves’ disease and autoimmune hepatitis during pregnancy (Buchel, et al. 2002, Colle and Hautekeete 1999, Weetman 2010). Together, these findings suggest immune tolerance that expands during pregnancy is not restricted only to paternal antigen expressed by the developing fetus, but extends to maternal responsiveness to pathological “self” antigen that cause autoimmunity as well. Moreover, these unambiguous examples of autoimmunity remission in the joints, thyroid, liver, and central nervous system highlight that although immune modifications at the fetal interface are clearly important for sustaining pregnancy, systemic alterations in immune responsiveness also become engaged during pregnancy.
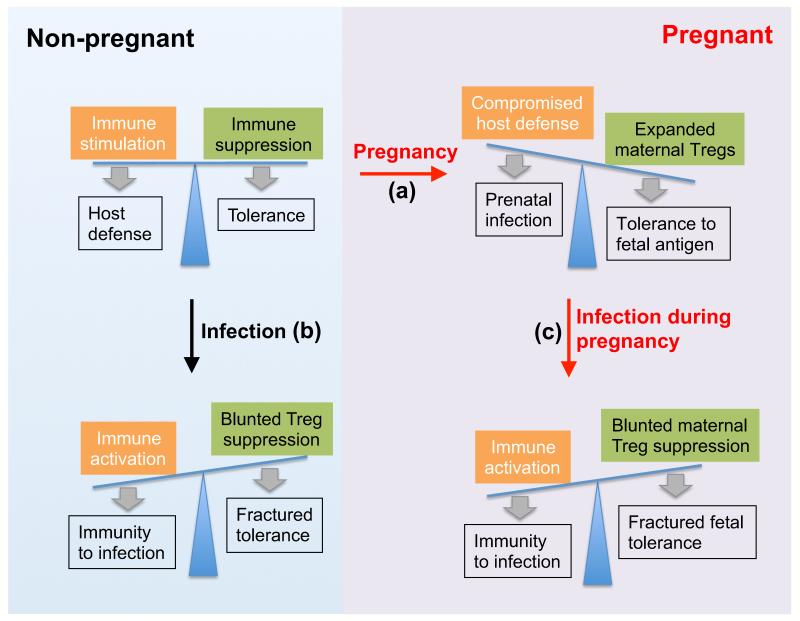
With these pregnancy induced protective benefits that clearly extend beyond fetal tolerance, it may be interesting to consider why the physiological set point for Tregs is not more consistently adjusted to the higher levels found during allogeneic pregnancy. Here, our recent studies suggest susceptibility to infection offsets the protective benefits of expanded tolerance from pregnancy induced maternal Treg accumulation (Rowe, et al. 2011). Intriguingly however, since resistance against most pathogens is not significantly deteriorated with pregnancy, these holes in host defense are likely limited only to those that share the common theme of intracellular survival and placental cell invasion (Robbins and Bakardjiev 2012). Perhaps the best example of this is the markedly increased susceptibility to disseminated infection with the intracellular bacterium Listeria monocytogenes during human pregnancy that extends to mice and other rodents (Bakardjiev, et al. 2006, Redline and Lu 1987, 1988, Rowe, et al. 2011, Schuchat, et al. 1991, Silver 1998). Here, pregnancy induced Treg expansion likely dictates innate susceptibility because inducing the accumulation of Foxp3+ CD4 T cells in non-pregnant mice to levels comparable to that found during allogeneic pregnancy confers paralleled infection susceptibility (Rowe, et al. 2011). On the other hand, maternal Treg depletion restores resistance, but at the insufferable cost of fractured fetal tolerance and wastage. Similarly, for Salmonella typhimurium representing another intracellular bacterium that causes more severe disseminated infection during pregnancy, resistance is restored with maternal Treg ablation (Chattopadhyay, et al. 2010, Pejcic-Karapetrovic, et al. 2007, Rowe, et al. 2011). Thus, susceptibility to a relatively small handful of prenatal pathogens that exploit the physiological hole in host defense created by the reproductive process is likely the unfortunate by-product of the greater good served by expanded maternal Tregs that sustain fetal tolerance (Figure 1a).
Figure 1.
Shifts in the balance between immune stimulation and suppression during pregnancy or following infection may dictate the immune pathogenesis of pregnancy complications after prenatal infection. (a) The expansion of immune suppressive maternal Tregs essential for sustaining fetal tolerance during pregnancy can also compromise host defense against pathogens that cause prenatal infection. (b) Infection or inflammation induced reductions in Treg suppression unleash the activation of immune components required for optimal protection against infection. (c) However, with maternal infection during pregnancy, reductions in Treg suppression that unleash the activation of protective immune components can also fracture fetal tolerance with ensuing immune mediated pregnancy complications.
Another interesting example is increased susceptibility to influenza A virus during pregnancy. Pregnant women have significantly higher rates of hospitalization, morbidity, and mortality after infection with either pandemic or seasonal influenza A strains (Neuzil, et al. 1998, Pierce, et al. 2011, Siston, et al. 2010). Here, the newly recommended use of inactivated vaccine formulations during pregnancy provides an exceptional opportunity to characterize how pregnancy impacts systemic immune responsiveness. Consistent with the notion of systemic immune moderation during pregnancy, reductions in vaccine induced antibody titers among pregnant women compared with non-pregnant controls with both seasonal and pandemic inactivated influenza A vaccine have been described (Bischoff, et al. 2013, Schlaudecker, et al. 2012). Similarly, reduced antibody responses were found among pregnant women after administration of the live attenuated vaccine for yellow fever virus (Nasidi, et al. 1993). However, it is important to also highlight that despite reductions in vaccine induced influenza A antibody titers during pregnancy, these diminished responses nevertheless provide significant protection for both the mother and infant against respiratory infection, especially in resource limited environments where influenza A is highly endemic (Zaman, et al. 2008). These protective benefits of influenza A vaccination during pregnancy for subsequent infection and possibly fetal death have also been recently confirmed in more developed settings where influenza A is less prevalent (Haberg, et al. 2013). Together, these examples where immune responsiveness to self-antigen, pathogens, and vaccination are each consistently dampened during pregnancy underscores the exceptional latent systemic immune modulatory properties that become unleashed with gestation. At face value, these findings imply systemic shifts in the balance between immune suppression that averts autoimmunity and immune stimulation required for optimal host defense against infection beyond fetal tolerance become engaged with the accumulation of maternal Tregs during pregnancy.
Along with these quantitative shifts between Tregs and non-Treg effector cells, modifications in suppressive potency for Tregs on a per cell basis can also occur (Sakaguchi 2003). These qualitative shifts enable Tregs to more efficiently fine-tune the delicate balance between immune activation and suppression by rapidly responding to environmental cues. This feature is likely especially critical in the early stages after infection when the race between pathogen replication and immune activation play decisive impacts on the eventual outcome of infection. At the molecular level, this is consistent with drastic shifts in Treg suppressive potency induced by microbial ligands that stimulate cells through conserved pattern recognition receptors such as Toll-like receptors. For example, purified LPS or flagellin each stimulate increased Treg suppressive potency (Caramalho, et al. 2003, Crellin, et al. 2005), whereas CpG oligonucleotide or the bacterial lipoprotein Pam3Cys-SK4 each prime reductions in suppressive potency (Liu, et al. 2006, Peng, et al. 2005). Importantly, these shifts in Treg suppression after stimulation with individual microbial ligands in vitro parallel similar changes in suppressive potency after in vivo infection with intact pathogen reflecting the cumulative response to multiple microbial ligands and the ensuing immune response (Ertelt, et al. 2011, Johanns, et al. 2010, Minigo, et al. 2009).
Applied to the unique physiological situation of pregnancy where sustained immune tolerance to fetal antigens must be sustained, fractured tolerance stemming from disruptions in maternal Treg suppression may explain why fetal resorption occurs after systemic LPS administration, especially in mice lacking the immune regulatory cytokine IL-10 (Robertson, et al. 2007, Robertson, et al. 2006). Here, a specific role for maternal Tregs is supported by the protective properties of purified CD4 T cells that differentiate into Tregs with LPS induced preterm delivery (Bizargity, et al. 2009). Interestingly, since mice lacking all T and B cells (Rag1−/−) are more susceptible to LPS induced preterm delivery (Bizargity, et al. 2009), inflammation induced activation of innate immune components likely also contribute to pregnancy complications, especially in the absence of Tregs. The extension of Treg suppression in this context is consistent with the increasingly appreciated role Foxp3+ cells play in moderating the activation of more prototypical innate immune cells such as neutrophils and natural killer cells (D’Alessio, et al. 2009, Gasteiger, et al. 2013a, Gasteiger, et al. 2013b, Murphy, et al. 2005). Thus, dampening maternal Treg suppression that unleashes the activation of innate or adaptive immune response pathways for optimal protection against infection has the potential to unravel the fine-tuned shifted balance between immune suppression and stimulation that maintains fetal tolerance during pregnancy. In this regard, infection induced disruption of maternal Treg suppression that fracture fetal tolerance may represent an underappreciated, but perhaps more unifying pathway to explain the fundamental biology whereby pregnancy complications occur with clinically apparent or asymptomatic infection.
Infection induced shifts in Treg suppression and pregnancy complications
Although the cause and effect relationship between maternal infection and unfortunate complications in human pregnancy including spontaneous abortion, stillbirth, and preterm labor have been described (Andrews, et al. 2000, Goldenberg, et al. 2008, McClure, et al. 2010), establishing the mechanistic basis whereby maternal infection triggers these complications have lagged behind. One important limitation has been the lack of representative animal models to specifically investigate pregnancy outcomes after infection. In particular, while pregnancy outcomes have been extensively characterized using rodent infection models, the discordance in pregnancy kinetics and immune cell development between rodents and humans limits the translational relevance of these findings, especially for complications related to birth timing (Bezold, et al. 2013, Mold and McCune 2012). These limitations are somewhat bypassed in larger mammals including elegant descriptions of pregnancy in horses, sheep, and non-human primates (Antczak 2012, Barry, et al. 2006, Jobe 2005, Noronha and Antczak 2010). However, the significantly more prolonged gestation time, relative lack of immunological tools and defined inbred strains, differences in placental architecture, and exponentially higher experimental costs impose other restrictions that are perhaps even more formidable. Furthermore, while human gestational cells and tissues have become more widely used to characterize the pathogenesis of prenatal infection (Robbins, et al. 2010, Robbins, et al. 2012, Zeldovich, et al. 2011), these in vitro models neither recapitulate the dynamic cross talk between maternal and fetal cells nor the immune response to infection that each likely play critical roles in the pathogenesis of pregnancy complications. Therefore, we propose combining the most salient aspects of individual models may represent the most efficient way to uncover the fundamental biology whereby pathogens cause prenatal infection and pregnancy complications.
The additive and potential synergistic value is illustrated by recent complementary studies describing infection and pregnancy outcomes using the intracellular bacterium Listeria monocytogenes. Using this bacterium to investigate the underlying pathogenesis of prenatal infection is clearly important given the ubiquitous presence of this pathogen in our food supply, colonization within the gastrointestinal tract, propensity for disseminated infection during pregnancy, and alarming rate of morbidity and mortality associated with human prenatal infection (Gellin and Broome 1989, Iida, et al. 1998, MacGowan, et al. 1991, Mylonakis, et al. 2002, Silver 1998, Southwick and Purich 1996). Here, a somewhat surprising degree of resistance to L. monocytogenes infection for placental cells has been described using human organ cultures (Abrahams, et al. 2006, Koga and Mor 2008, Robbins and Bakardjiev 2012). In particular, human syncytiotrophoblasts that line the placental surface where nutrient and gas exchange occurs with direct exposure to maternal blood are highly resistant to bacterial invasion and intercellular spread (Robbins, et al. 2010). Placental invasion instead primarily occurs through a substantially smaller subset of extravillous trophoblasts cells that anchor the placenta in the uterine lining. However, even after invasion into extravillous trophoblasts cells, profound defects in L. monocytogenes escape from the endocytic vacuole and intracellular replication remain (Robbins, et al. 2012). Interestingly, these protective properties of trophoblasts are not limited to L. monocytogenes, but have also been shown for a variety of other bacterial, parasitic and viral pathogens (Abrahams, et al. 2006, Koga and Mor 2008, Robbins and Bakardjiev 2012, Zeldovich, et al. 2011). Thus, placental cells provide a critical protective barrier to fetal infection, at least in vitro without the additional constraints imposed by the ensuing inflammatory response and maternal-fetal tolerance.
In light of these innate cellular barriers to infection, it is interesting to reconsider the basic physiology that confers susceptibility to disseminated infection during pregnancy, and the underlying mechanism whereby maternal infection triggers pregnancy complications. With regards to maternal susceptibility to disseminated infection, the prior dogma that placental and fetal tissue represent expanded target tissue susceptible to invasion seem less likely given the finding that placental cells are actually very resistant to infection (Abrahams, et al. 2006, Koga and Mor 2008, Robbins and Bakardjiev 2012). Similarly, the notion that pregnancy induced dampening of CD4 helper type 1 (Th1) responses required for protection against intracellular pathogens is questionable given the unimpeded innate and early adaptive immune response in MHC class II CD4 T cell deficient mice (Barber, et al. 2005, Sun and Bevan 2003). Instead, given the necessity for maintaining fetal tolerance through sustained expansion of immune suppressive maternal Tregs, we propose active suppression by these cells may also play critical roles in conferring maternal susceptibility to disseminated infection (Figure 1a). This notion is supported by aforementioned epidemiological and experimental data showing increased susceptibility to systemic infection during pregnancy in humans and mice, and non-pregnant mice with expanded Tregs, whereas Treg ablation restores resistance for each group (Redline and Lu 1987, 1988, Rowe, et al. 2011, Schuchat, et al. 1991, Silver 1998). Interestingly however, since immunity against most infections is preserved during pregnancy, these do not appear to be drastic defects in global host defense, and are instead more isolated holes that become exploited by pathogens with an established predisposition for infection during pregnancy. Furthermore, given the striking consistency in pathogens that cause prenatal infection in humans and other mammalian species (Givens and Marley 2008, Robbins and Bakardjiev 2012), these host defense defects associated with the reproductive process are apparently widely conserved.
Perhaps more intriguing are recent findings using allogeneic pregnancy in mice to investigate the pathogenesis of pregnancy complications triggered by disseminated maternal infection. Here, related studies using L. monocytogenes as a model to dissect the basic immunology whereby protective T cells are primed after in vivo infection need to be carefully considered in parallel. Unlike the necessity for distinct cell intrinsic stimulation signals including the T cell receptor (signal 1), co-stimulation (signal 2), and inflammatory cytokines (signal 3) for T cell activation shown using elegantly simplistic in vitro models (Curtsinger and Mescher 2010, Curtsinger, et al. 2005), CD8 T cells responsive to heterologous antigens expressed by recombinant L. monocytogenes expand and become activated even when all known inflammatory cytokine third signals have been eliminated (Ertelt, et al. 2010, Orgun, et al. 2008, Way, et al. 2007). Instead, transient reductions in Treg suppression that unleash the activation of protective immune effector cells following infection likely circumvent the need for some, but not all, more classical T cell intrinsic activation signals (Ertelt, et al. 2013, Ertelt, et al. 2011). The importance of overriding Treg suppression for immune activation parallels the robust expansion of protective CD8 effector T cells in mice transiently ablated of Tregs after stimulation with purified peptide (Ertelt, et al. 2011). Thus, infection or inflammation induced reductions in Treg suppression may represent a more fundamental signal zero for stimulating the activation of protective T effector cells (Rowe, et al. 2012a) (Figure 1b).
Applied to pregnancy when sustained expansion of maternal Tregs is essential for maintaining fetal tolerance, infection or inflammation induced reductions in Treg suppression have the critical potential to fracture fetal tolerance with ensuing pregnancy complications (Figure 1c). This has been most definitively shown with a highly informative mating strategy using transgenic male mice that ubiquitously express defined model antigens to establish allogeneic pregnancy with non-transgenic female mice that transforms model antigens into surrogate fetal antigens (Erlebacher, et al. 2007, Moldenhauer, et al. 2009, Taglauer, et al. 2009). After L. monocytogenes infection, the normally blunted accumulation of maternal T effector cells with fetal specificity is overturned (Rowe, et al. 2012b). In turn, the robust expansion and activation of maternal T effector cells with fetal specificity with maternal infection recapitulates the quantitative accumulation and qualitative activation of these cells with maternal Treg ablation during pregnancy (Rowe, et al. 2011). Intriguingly, while fetal resorption with reciprocal loss of live pups each occur in a dose dependent fashion that parallels infection induced reductions in maternal Treg suppression, bacteria could not be recovered from the majority of resorbed placental-fetal units after infection with low or intermediate dosages of virulent L. monocytogenes (Rowe, et al. 2012b). These more recent findings that suggest a threshold inoculum is required for establishing sustained systemic infection are consistent with prior studies illustrating dose-dependent rates of placental invasion after intravenous L. monocytogenes inoculation (Redline and Lu 1987). Here, it is important to highlight even attenuated L. monocytogenes that cannot invade the placental fetal unit due to targeted defects in ActA required for intercellular spread can also induce fetal wastage with disruptions in fetal tolerance and loss of live pups (Bakardjiev, et al. 2005, Le Monnier, et al. 2007, Rowe, et al. 2012b). Thus, L. monocytogenes infection induced pregnancy complications do not require in utero pathogen invasion, and may instead be due to reductions in maternal Treg suppression with ensuing disruptions in fetal tolerance (Figure 2a).
Figure 2.
Proposed model for how pregnancy complications may occur with or without direct pathogen invasion of the placental-fetal unit. Infection induced reductions in maternal Treg suppression that unleash the activation of protective immune components fracture fetal tolerance unmasking normally tolerized fetal antigen. In turn, inflammation occurs at the maternal fetal interface containing the highest concentration of exposed fetal cells. With low dosage infection (a), circulating pathogen is eradicated, but pregnancy complications ensue from damage caused by activated maternal immune effector cells. With higher dosage infection (b), the persistence of circulating pathogen and inflammation at the maternal-fetal interface provides a conduit for invasion overriding the normally protective placental barrier.
By showing pregnancy complications can occur without direct bacterial invasion of the placental-fetal unit, the high frequency of pregnancy complications associated with L. monocytogenes infection during human pregnancy despite placental cell resistance to direct bacterial invasion can be potentially reconciled (Gellin and Broome 1989, Mylonakis, et al. 2002, Robbins and Bakardjiev 2012, Silver 1998). On the other hand, since a majority of newborn infants are infected when born to mothers with disseminated infection (Mylonakis, et al. 2002), there are also presumably ways to bypass the protective placental barrier with infection in vivo. We hypothesize inflammation at the maternal-placental interface induced by disrupted fetal tolerance may provide a more direct conduit drawing circulating bacteria into otherwise resistant placental cells. Thereafter, previously described local immune suppression at the maternal-placental interface and high bacterial concentrations within the infected placenta likely provide a repository for continuous re-infection (Bakardjiev, et al. 2006, Redline and Lu 1987, 1988). Based on these findings with perinatal L. monocytogenes infection, we propose a model whereby (1) the expansion of immune suppressive maternal Tregs required for maintaining fetal tolerance compromises innate protection against disseminated infection, (2) the normally protective blunting of Treg suppression that unleashes activation of protective immune components to eradicate infection disrupts fetal tolerance with fetal wastage caused by attack from maternal immune cells with infection during pregnancy, and (3) inflammation at the maternal-placental interface with fractured fetal tolerance draws circulating pathogen through the normally protected placental cell barrier promoting fetal invasion (Figure 2b).
The more general applicability of this model now requires analogous studies with other pathogens that cause prenatal infection and pregnancy complications. In this regard, some clues for pathogens known to cause more severe systemic infection during pregnancy are already in place. For example, Salmonella typhimurium infection during pregnancy triggers catastrophic fetal wastage with exaggerated immune responses within the placenta, and ~2-fold reductions in Treg suppressive potency especially in the later stages of persistent infection (Chattopadhyay, et al. 2010, Johanns, et al. 2010, Pejcic-Karapetrovic, et al. 2007). Similarly, quantitative reductions in peripheral Tregs have been described in early stages after systemic murine cytomegalovirus and intestinal Toxoplasma gondii infection (Li, et al. 2008, Oldenhove, et al. 2009). If similar reductions in maternal Treg suppression were to occur with infection during pregnancy, disruptions in fetal tolerance with ensuing immune mediated fetal wastage would be predicted.
It is also important to point out a potentially interesting exception for Plasmodium infection that results in placental localization and robust inflammatory changes leading to intrauterine fetal demise (Poovassery and Moore 2009, Poovassery, et al. 2009). Unlike most other acute infections where Treg suppression is reduced, increased proportions of activated Tregs with higher suppressive potency are found after Plasmodium infection; and these shifts are likely to minimize inflammatory sequelae at the expense of parasite replication (Minigo, et al. 2009, Walther, et al. 2005). Therefore for this infection, other molecules such as chondroitin sulfate providing placental adhesion may provide a more direct conduit for fetal invasion without the need for over turning maternal Treg suppression (Duffy and Fried 2003, Fried and Duffy 1996). Nevertheless, given the necessity for sustained maternal Treg expansion in fetal tolerance, infection-induced qualitative or quantitative shifts in Treg suppression highlight a potentially more unifying pathway whereby prenatal infection may trigger pregnancy complications.
Summary
Reproduction and averting infection are arguably the two most dominant fundamental driving forces in nature. Adaptations that simultaneously improve both reproductive fitness and host defense are most ideal, and should be strongly enriched for through positive selection. On the other hand, adaptations that favor only one must be counterbalanced by concurrent adaptations that promote or at least sustain the other. In regards to host defense against infection, diversity in immune recognition MHC molecules between individuals is clearly advantageous for species survival. However, in placental mammals that also require the benefits of more prolonged in utero protection for the fetus, the same antigenic diversity beneficial for host defense is potentially detrimental for reproductive fitness, unless foreign antigens associated with reproduction can be discriminated against those that cause infection. Fortunately, humans and other mammalian species are endowed with many such discriminatory mechanisms that act at the maternal-fetal interface and more systemically to ensure protection for the developing fetus. Using animal pregnancy models and investigating complications in human pregnancy, we have uncovered some, but certainly not all, the key players in this meticulously orchestrated process.
Based on what we currently know, an elaborate plethora of molecular and cellular changes occur at the maternal placental interface to silence the local activation potentially detrimental maternal immune cells. However, these local modifications alone are insufficient as the systemic expansion of immune suppressive maternal regulatory CD4 T cells is also essential. Although Tregs have been shown to impair optimal host defense against a wide variety of potential human pathogens in non-pregnancy models, the expansion of these cells during pregnancy does not confer increased susceptibility to most. In fact, the detrimental impacts on host defense for expanded maternal Treg during pregnancy across mammalian species seem to be limited to a remarkable small handful of microbes that share the common features of residence within infected cells and placental colonization. Thus, prenatal pathogens exploit a naturally occurring hole in host defense created by the reproductive process.
As biologists, we can marvel at this apparent intricate regulation that allows protection against the vast array of potential human pathogens and reproduction to occur efficiently enough as we exponentially approach a population of 7 billion individuals. However, as pediatricians and parents, our struggle is to uncover ways to optimize the outcomes of every last pregnancy. It is through the latter perspective that we have focused on why pregnancy confers susceptibility and the pathogenesis of pregnancy complications after infection with the prototypical prenatal pathogen, Listeria monocytogenes. The most critical data to support increased infection susceptibility during pregnancy and ensuing pregnancy complications linked with maternal Tregs are discussed. Based on these results, we propose critically important next step are to investigate if other microbes that cause prenatal infection either systemically or locally by ascending through the birth canal utilize similar pathogenesis pathways. If so, therapeutic strategies that focus on the moderating the immune response to infection, as well as pathogen eradication, may help improve the outcomes of pregnancy. Furthermore, given the remarkable heterogeneity among Tregs both in terms of antigen specificity and cell intrinsic molecules utilized for suppression, establishing and reinforcing the protective maternal Treg features that sustain pregnancy and dissociating them from others that compromise host defense also represent areas with critical implications for improving maternal and infant health.
Acknowledgements
Given space limitations, we apologize for not being able to discuss in a more in-depth fashion or cite the findings from numerous other important prior studies. This work was supported by the NIH-NIAID through awards R01AI100934 and R01AI087830 (SSW), and training grants from NIH-NIDDK F30DK084674 (JHR). SSW holds an Investigator in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund.
References
- Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Aldo PB, Romero R, Wira CR, Mor G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I : C) Hum Reprod. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Andersen KG, Nissen JK, Betz AG. Comparative Genomics Reveals Key Gain-of-Function Events in Foxp3 during Regulatory T Cell Evolution. Front Immunol. 2012;3:113. doi: 10.3389/fimmu.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17:357–365. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
- Antczak DF. T-cell tolerance to the developing equine conceptus. Reprod Domest Anim. 2012;47(Suppl 4):376–383. doi: 10.1111/j.1439-0531.2012.02101.x. [DOI] [PubMed] [Google Scholar]
- Bakardjiev AI, Stacy BA, Portnoy DA. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J Infect Dis. 2005;191:1889–1897. doi: 10.1086/430090. [DOI] [PubMed] [Google Scholar]
- Bakardjiev AI, Theriot JA, Portnoy DA. Listeria monocytogenes traffics from maternal organs to the placenta and back. PLoS Pathog. 2006;2:e66. doi: 10.1371/journal.ppat.0020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber EM, Fazzari M, Pollard JW. Th1 cytokines are essential for placental immunity to Listeria monocytogenes. Infect Immun. 2005;73:6322–6331. doi: 10.1128/IAI.73.10.6322-6331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JH, Brennan P, Fiddler M, Silman AJ. Does rheumatoid arthritis remit during pregnancy and relapse postpartum? Results from a nationwide study in the United Kingdom performed prospectively from late pregnancy. Arthritis Rheum. 1999;42:1219–1227. doi: 10.1002/1529-0131(199906)42:6<1219::AID-ANR19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Barry PA, Lockridge KM, Salamat S, Tinling SP, Yue Y, Zhou SS, Gospe SM, Jr., Britt WJ, Tarantal AF. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J. 2006;47:49–64. doi: 10.1093/ilar.47.1.49. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Bezold KY, Karjalainen MK, Hallman M, Teramo K, Muglia LJ. The genomics of preterm birth: from animal models to human studies. Genome Med. 2013;5:34. doi: 10.1186/gm438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff AL, Folsgaard NV, Carson CG, Stokholm J, Pedersen L, Holmberg M, Bisgaard A, Birch S, Tsai TF, Bisgaard H. Altered Response to A(H1N1)pnd09 Vaccination in Pregnant Women: A Single Blinded Randomized Controlled Trial. PLoS One. 2013;8:e56700. doi: 10.1371/journal.pone.0056700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizargity P, Bonney EA. Dendritic cells: a family portrait at mid-gestation. Immunology. 2009;126:565–578. doi: 10.1111/j.1365-2567.2008.02918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizargity P, Del Rio R, Phillippe M, Teuscher C, Bonney EA. Resistance to lipopolysaccharide-induced preterm delivery mediated by regulatory T cell function in mice. Biol Reprod. 2009;80:874–881. doi: 10.1095/biolreprod.108.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Buchel E, Van Steenbergen W, Nevens F, Fevery J. Improvement of autoimmune hepatitis during pregnancy followed by flare-up after delivery. Am J Gastroenterol. 2002;97:3160–3165. doi: 10.1111/j.1572-0241.2002.07124.x. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A, Robinson N, Sandhu JK, Finlay BB, Sad S, Krishnan L. Salmonella enterica serovar Typhimurium-induced placental inflammation and not bacterial burden correlates with pathology and fatal maternal disease. Infect Immun. 2010;78:2292–2301. doi: 10.1128/IAI.01186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle I, Hautekeete M. Remission of autoimmune hepatitis during pregnancy: a report of two cases. Liver. 1999;19:55–57. doi: 10.1111/j.1478-3231.1999.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T, Pregnancy in Multiple Sclerosis Group Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva JA, Spector TD. The role of pregnancy in the course and aetiology of rheumatoid arthritis. Clin Rheumatol. 1992;11:189–194. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- de Man YA, Hazes JM, van der Heide H, Willemsen SP, de Groot CJ, Steegers EA, Dolhain RJ. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 2009;60:3196–3206. doi: 10.1002/art.24914. [DOI] [PubMed] [Google Scholar]
- Duffy PE, Fried M. Plasmodium falciparum adhesion in the placenta. Curr Opin Microbiol. 2003;6:371–376. doi: 10.1016/s1369-5274(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013a;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013b;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt JM, Buyukbasaran EZ, Jiang TT, Rowe JH, Xin L, Way SS. B7-1/B7-2 blockade overrides the activation of protective CD8 T cells stimulated in the absence of Foxp3+ regulatory T cells. J Leukoc Biol. 2013 doi: 10.1189/jlb.0313118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt JM, Johanns TM, Rowe JH, Way SS. Interleukin (IL)-21-independent pathogen-specific CD8+ T-cell expansion, and IL-21-dependent suppression of CD4+ T-cell IL-17 production. Immunology. 2010;131:183–191. doi: 10.1111/j.1365-2567.2010.03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt JM, Rowe JH, Mysz MA, Singh C, Roychowdhury M, Aguilera MN, Way SS. Foxp3+ regulatory T cells impede the priming of protective CD8+ T cells. J Immunol. 2011;187:2569–2577. doi: 10.4049/jimmunol.1100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Forger F, Marcoli N, Gadola S, Moller B, Villiger PM, Ostensen M. Pregnancy induces numerical and functional changes of CD4+CD25 high regulatory T cells in patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:984–990. doi: 10.1136/ard.2007.075283. [DOI] [PubMed] [Google Scholar]
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med. 2013a;210:1179–1187. doi: 10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger G, Hemmers S, Firth MA, Le Floc’h A, Huse M, Sun JC, Rudensky AY. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013b;210:1167–1178. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellin BG, Broome CV. Listeriosis. Jama. 1989;261:1313–1320. [PubMed] [Google Scholar]
- Givens MD, Marley MS. Infectious causes of embryonic and fetal mortality. Theriogenology. 2008;70:270–285. doi: 10.1016/j.theriogenology.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Laresgoiti-Servitje E. T regulatory cells: regulating both term and preterm labor? Immunol Cell Biol. 2012;90:919–920. doi: 10.1038/icb.2012.48. [DOI] [PubMed] [Google Scholar]
- Griesemer AD, Sorenson EC, Hardy MA. The role of the thymus in tolerance. Transplantation. 2010;90:465–474. doi: 10.1097/TP.0b013e3181e7e54f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetta E, Gordon D, Simchen MJ, Goldman B, Barkai G. Hematopoietic progenitor cells as targets for non-invasive prenatal diagnosis: detection of fetal CD34+ cells and assessment of post-delivery persistence in the maternal circulation. Blood Cells Mol Dis. 2003;30:13–21. doi: 10.1016/s1079-9796(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Haberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, Skrondal A, Cappelen I, Engeland A, Aavitsland P, Madsen S, Buajordet I, Furu K, Nafstad P, Vollset SE, Feiring B, Nokleby H, Magnus P, Stoltenberg C. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368:333–340. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff JM, Daltveit AK, Gilhus NE. Myasthenia gravis in pregnancy and birth: identifying risk factors, optimising care. Eur J Neurol. 2007;14:38–43. doi: 10.1111/j.1468-1331.2006.01538.x. [DOI] [PubMed] [Google Scholar]
- Hu D, Chen Y, Zhang W, Wang H, Wang Z, Dong M. Alteration of peripheral CD4+CD25+ regulatory T lymphocytes in pregnancy and pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:190–194. doi: 10.1080/00016340701823991. [DOI] [PubMed] [Google Scholar]
- Hunt JS, Andrews GK, Wood GW. Normal trophoblasts resist induction of class I HLA. J Immunol. 1987;138:2481–2487. [PubMed] [Google Scholar]
- Iida T, Kanzaki M, Nakama A, Kokubo Y, Maruyama T, Kaneuchi C. Detection of Listeria monocytogenes in humans, animals and foods. J Vet Med Sci. 1998;60:1341–1343. doi: 10.1292/jvms.60.1341. [DOI] [PubMed] [Google Scholar]
- Iorio R, Frisullo G, Nociti V, Patanella KA, Bianco A, Marti A, Mirabella M, Tonali PA, Batocchi AP. T-bet, pSTAT1 and pSTAT3 expression in peripheral blood mononuclear cells during pregnancy correlates with post-partum activation of multiple sclerosis. Clin Immunol. 2009;131:70–83. doi: 10.1016/j.clim.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol. 2005;25(Suppl 2):S31–35. doi: 10.1038/sj.jp.7211317. [DOI] [PubMed] [Google Scholar]
- Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 2010;6:e1001043. doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. Jama. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- Koga K, Mor G. Expression and function of toll-like receptors at the maternal-fetal interface. Reprod Sci. 2008;15:231–242. doi: 10.1177/1933719108316391. [DOI] [PubMed] [Google Scholar]
- Le Monnier A, Autret N, Join-Lambert OF, Jaubert F, Charbit A, Berche P, Kayal S. ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes. Infect Immun. 2007;75:950–957. doi: 10.1128/IAI.01570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lydon JP, Kim CH. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol. 2012;42:2683–2696. doi: 10.1002/eji.201142317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YN, Zhou YF, Shu SN, Zhu DD, Yang ZF, Fang F. [Effects of acute and chronic murine cytomegalovirus infections on the ratio of regulatory T cells and expression of Th1/Th2 transcription factors T-bet/GATA-3] Zhonghua Yi Xue Za Zhi. 2008;88:2999–3002. [PubMed] [Google Scholar]
- Liegeois A, Gaillard MC, Ouvre E, Lewin D. Microchimerism in pregnant mice. Transplant Proc. 1981;13:1250–1252. [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGowan AP, Marshall RJ, MacKay IM, Reeves DS. Listeria faecal carriage by renal transplant recipients, haemodialysis patients and patients in general practice: its relation to season, drug therapy, foreign travel, animal exposure and diet. Epidemiol Infect. 1991;106:157–166. doi: 10.1017/s0950268800056521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson R. The non-expression of MHC class II in trophoblast cells. Am J Reprod Immunol. 1998;40:383–384. doi: 10.1111/j.1600-0897.1998.tb00422.x. [DOI] [PubMed] [Google Scholar]
- McClure EM, Dudley DJ, Reddy UM, Goldenberg RL. Infectious causes of stillbirth: a clinical perspective. Clin Obstet Gynecol. 2010;53:635–645. doi: 10.1097/GRF.0b013e3181eb6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182:8080–8093. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, AL Mellor. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- Munoz-Suano A, Kallikourdis M, Sarris M, Betz AG. Regulatory T cells protect from autoimmune arthritis during pregnancy. J Autoimmun. 2012;38:J103–108. doi: 10.1016/j.jaut.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 2002;81:260–269. doi: 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasidi A, Monath TP, Vandenberg J, Tomori O, Calisher CH, Hurtgen X, Munube GR, Sorungbe AO, Okafor GC, Wali S. Yellow fever vaccination and pregnancy: a four-year prospective study. Trans R Soc Trop Med Hyg. 1993;87:337–339. doi: 10.1016/0035-9203(93)90156-k. [DOI] [PubMed] [Google Scholar]
- Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- Noronha LE, Antczak DF. Maternal immune responses to trophoblast: the contribution of the horse to pregnancy immunology. Am J Reprod Immunol. 2010;64:231–244. doi: 10.1111/j.1600-0897.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgun NN, Mathis MA, Wilson CB, Way SS. Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12p40 and type I IFNs sustains protective CD8 T cells. J Immunol. 2008;180:4109–4115. doi: 10.4049/jimmunol.180.6.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29:185–191. doi: 10.1007/s00281-007-0072-5. [DOI] [PubMed] [Google Scholar]
- Ozato K, Wan YJ, Orrison BM. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985;82:2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeschke S, Chen F, Horn N, Fotopoulou C, Zambon-Bertoja A, Sollwedel A, Zenclussen ML, Casalis PA, Dudenhausen JW, Volk HD, Zenclussen AC. Pre-eclampsia is not associated with changes in the levels of regulatory T cells in peripheral blood. Am J Reprod Immunol. 2005;54:384–389. doi: 10.1111/j.1600-0897.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- Pejcic-Karapetrovic B, Gurnani K, Russell MS, Finlay BB, Sad S, Krishnan L. Pregnancy impairs the innate immune resistance to Salmonella typhimurium leading to rapid fatal infection. J Immunol. 2007;179:6088–6096. doi: 10.4049/jimmunol.179.9.6088. [DOI] [PubMed] [Google Scholar]
- Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. Bmj. 2011;342:d3214. doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovassery J, Moore JM. Association of malaria-induced murine pregnancy failure with robust peripheral and placental cytokine responses. Infect Immun. 2009;77:4998–5006. doi: 10.1128/IAI.00617-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovassery JS, Sarr D, Smith G, Nagy T, Moore JM. Malaria-induced murine pregnancy failure: distinct roles for IFN-gamma and TNF. J Immunol. 2009;183:5342–5349. doi: 10.4049/jimmunol.0901669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, Erwich JJ. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28:300–311. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- Ramhorst R, Fraccaroli L, Aldo P, Alvero AB, Cardenas I, Leiros CP, Mor G. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol. 2012;67:17–27. doi: 10.1111/j.1600-0897.2011.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW, Lu CY. Role of local immunosuppression in murine fetoplacental listeriosis. J Clin Invest. 1987;79:1234–1241. doi: 10.1172/JCI112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW, Lu CY. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988;140:3947–3955. [PubMed] [Google Scholar]
- Robbins JR, Bakardjiev AI. Pathogens and the placental fortress. Curr Opin Microbiol. 2012;15:36–43. doi: 10.1016/j.mib.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010;6:e1000732. doi: 10.1371/journal.ppat.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JR, Zeldovich VB, Poukchanski A, Boothroyd JC, Bakardjiev AI. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect Immun. 2012;80:418–428. doi: 10.1128/IAI.05899-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod. 2007;76:738–748. doi: 10.1095/biolreprod.106.056143. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177:4888–4896. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Way SS. Foxp3(+) regulatory T cells, immune stimulation and host defence against infection. Immunology. 2012a;136:1–10. doi: 10.1111/j.1365-2567.2011.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. Listeria monocytogenes Cytoplasmic Entry Induces Fetal Wastage by Disrupting Maternal Foxp3(+) Regulatory T Cell-Sustained Fetal Tolerance. PLoS Pathog. 2012b;8:e1002873. doi: 10.1371/journal.ppat.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012c;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Control of immune responses by naturally arising CD4+ regulatory T cells that express toll-like receptors. J Exp Med. 2003;197:397–401. doi: 10.1084/jem.20030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ramon S, Navarro AJ, Aristimuno C, Rodriguez-Mahou M, Bellon JM, Fernandez-Cruz E, de Andres C. Pregnancy-induced expansion of regulatory T-lymphocytes may mediate protection to multiple sclerosis activity. Immunol Lett. 2005;96:195–201. doi: 10.1016/j.imlet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J, Saito S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149:139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Schlaudecker EP, McNeal MM, Dodd CN, Ranz JB, Steinhoff MC. Pregnancy modifies the antibody response to trivalent influenza immunization. J Infect Dis. 2012;206:1670–1673. doi: 10.1093/infdis/jis592. [DOI] [PubMed] [Google Scholar]
- Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90:935–944. doi: 10.1038/icb.2012.33. [DOI] [PubMed] [Google Scholar]
- Schuchat A, Swaminathan B, Broome CV. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Silver HM. Listeriosis during pregnancy. Obstet Gynecol Surv. 1998;53:737–740. doi: 10.1097/00006254-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Tabony L, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. Jama. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick FS, Purich DL. Intracellular pathogenesis of listeriosis. N Engl J Med. 1996;334:770–776. doi: 10.1056/NEJM199603213341206. [DOI] [PubMed] [Google Scholar]
- Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland CA, Naiem M, Mason DY, Redman CW, Stirrat GM. The expression of major histocompatibility antigens by human chorionic villi. J Reprod Immunol. 1981;3:323–331. doi: 10.1016/0165-0378(81)90048-6. [DOI] [PubMed] [Google Scholar]
- Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2010;54:421–430. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol. 2009;80:12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldi G, Svec P, Vasarhelyi B, Meszaros G, Rigo J, Tulassay T, Treszl A. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet Gynecol Scand. 2008;87:1229–1233. doi: 10.1080/00016340802389470. [DOI] [PubMed] [Google Scholar]
- Visser J, Klatter F, Hillebrands JL, Jansen A, Vijfschaft L, Rozing J. Thymectomy should be the first choice in the protection of diabetes-prone BB rats for breeding purposes. Lab Anim. 2004;38:371–375. doi: 10.1258/0023677041958936. [DOI] [PubMed] [Google Scholar]
- Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF, Bejon P, Thompson F, Dunachie SJ, Edele F, de Souza JB, Sinden RE, Gilbert SC, Riley EM, Hill AV. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman AP. Immunity, thyroid function and pregnancy: molecular mechanisms. Nat Rev Endocrinol. 2010;6:311–318. doi: 10.1038/nrendo.2010.46. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- Winger EE, Reed JL. Low circulating CD4(+) CD25(+) Foxp3(+) T regulatory cell levels predict miscarriage risk in newly pregnant women with a history of failure. Am J Reprod Immunol. 2011;66:320–328. doi: 10.1111/j.1600-0897.2011.00992.x. [DOI] [PubMed] [Google Scholar]
- Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- Zeldovich VB, Robbins JR, Kapidzic M, Lauer P, Bakardjiev AI. Invasive extravillous trophoblasts restrict intracellular growth and spread of Listeria monocytogenes. PLoS Pathog. 2011;7:e1002005. doi: 10.1371/journal.ppat.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann FA, Head JR. Expression of MHC antigens on murine trophoblast and their modulation by interferon. J Immunol. 1986;137:846–853. [PubMed] [Google Scholar]